Abstract
Objective:
To examine whether probable REM sleep behavior disorder (pRBD) was associated with increased risk of developing stroke in a community-based cohort.
Methods:
The study included 12,003 participants (mean age 54.0 years) of the Kailuan Study, free of stroke, cancer, Parkinson disease, dementia, and head injury at baseline (2012). We determined pRBD using a validated REM sleep behavior disorder (RBD) questionnaire in 2012. Incident stroke cases were confirmed by review of medical records. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke according to pRBD status, adjusting for several sleep measures (i.e., insomnia, daytime sleepiness, sleep duration, snoring, and use of hypnotics) and other potential confounders.
Results:
During 3 years of follow-up, we documented 159 incident stroke cases. Relative to participants without pRBD at the baseline, those with pRBD had a 157% higher risk (95% CI 59%–313%) of developing stroke. Presence of pRBD was associated with increased risk of both stroke types—the adjusted HR was 1.93 (95% CI 1.07–3.46) for ischemic stroke and 6.61 (95% CI 2.27–19.27) for hemorrhagic stroke.
Conclusions:
Presence of pRBD was associated with a higher risk of developing stroke, including both ischemic and hemorrhagic types. Future studies with clinically confirmed RBD and a longer follow-up would be appropriate to further investigate this association.
REM sleep behavior disorder (RBD) is associated with synucleinopathies, and a large proportion of individuals with RBD may already have or later develop Parkinson disease (PD), dementia with Lewy bodies, or multiple system atrophy.1–3 Synucleinopathies were often associated with symptoms of autonomic dysfunction, such as impaired blood pressure control and reduced heart rate variability.4,5 Consistently, individuals with idiopathic RBD experienced worse autonomic function, including cardiovascular, gastrointestinal, and urinary domains, relative to control participants.6,7 The autonomic nervous system may have a role in the prepathologic state or contribute to the precipitating factors of the acute phase of stroke, such as atherosclerosis and sympathetic hyperactivity.8 Some case reports suggested that stroke may cause RBD.9–11 In a previous cross-sectional study, we also found that individuals with probable RBD (pRBD) had higher odds of several concurrent stroke risk factors, such as diabetes and hyperlipidemia.12 However, whether RBD predicts subsequent stroke risk remains unknown to date. RBD might have a different effect on different stroke types. For example, RBD-related α-synuclein deposition could be more relevant to cerebral amyloid angiopathy, which is established as a major cause of primary intracerebral hemorrhage, particularly for the lobar hematomas.2,13 On the other hand, RBD-related autonomic dysfunction could lead to atherosclerosis, which is the leading cause of ischemic stroke.8 We hypothesized that presence of RBD might be associated with high risk of both ischemic and intracerebral hemorrhagic stroke. To test this hypothesis, we expanded our previous analyses to prospectively examine whether individuals with pRBD at baseline (2012) had a higher risk of stroke during 3 years of follow-up among ∼12,000 Chinese adults.
METHODS
Participants.
As detailed elsewhere,12 the current community-based study included 12,990 Chinese adults (10,725 men and 2,265 women) free of PD and dementia in 2012 (i.e., baseline) who were a subset of the Kailuan Study, an ongoing Chinese cohort.14,15 We further excluded participants with a history of stroke, cancer, or head injury at baseline, leaving 12,003 participants (mean age 54 years) in the current analysis.
Standard protocol approvals, registrations, and patient consents.
The study was approved jointly by the Ethics Committee of the Kailuan General Hospital and the Human Subjects Committee at Brigham and Women's Hospital/Harvard Medical School.
Assessment of incident stroke.
The outcome was the first occurrence of stroke, either the first nonfatal stroke event or stroke death without a proceeding nonfatal event. Ascertainment of incident stroke was described previously.14,15 Briefly, all participants were linked to the Municipal Social Insurance Institution database and Hospital Discharge Register for incidence of stroke, which cover all the Kailuan study participants. We used the ICD-10 for the identification of potential stroke cases (I61 for intracerebral hemorrhagic stroke and I63 for ischemic stroke). Additional information regarding medical history of stroke was collected via questionnaire biennially in the Kailuan Study since 2006 (the cohort baseline). Deaths were collected from provincial vital statistics offices. For potential stroke cases identified by the ICD code or questionnaire, a panel of 3 physicians, consisting of neurologists, cardiologists, and radiologists, reviewed the medical records, blind to exposure status. A nonfatal stroke was defined as the sudden onset of a focal neurologic deficit with a vascular mechanism lasting >24 hours. Fatal stroke was confirmed by medical records, autopsy reports, and death certificates with stroke listed as the underlying cause. Stroke was diagnosed according to the WHO criteria16 and either brain CT or MRI for confirmation. In the current study, we included 2 main stroke types: ischemic and intracerebral hemorrhagic.
Assessment of pRBD.
In 2012, we collected information regarding RBD symptoms using a validated RBD questionnaire–Hong Kong (RBDQ-HK). The RBDQ-HK is a Chinese version of a screening tool for diagnosis of RBD, including 13 questions pertaining to various clinical features of RBD, which are screened on scales of lifetime occurrence and recent 1-year frequency.17 A previous validation study in the general Chinese population and individuals with PD and obstructive sleep apnea showed robust sensitivity (82%–85%), specificity (81%–87%), internal consistency, and test-retest reliability of the RBDQ-HK, relative to polysomnography-based diagnosis.17,18 The cutoff point to estimate RBD for the total scale (score range 0–100) was >18, and the alternative cutoff point based on 7 subgroup behavioral factors including sleep talking, shouting, limb movements, and sleep-related injuries (range 0–70) was >7.17
Assessment of sleep parameters.
Information on several sleep parameters including insomnia, daytime sleepiness, sleep duration, snoring, and use of hypnotics was collected in 2012, as detailed below.
Insomnia.
We assessed insomnia status of the participants in the last month using the validated Chinese version of the Athens Insomnia Scale (AIS),19 which was validated in the Chinese population.20 The AIS is a self-administered questionnaire comprising 8 questions on sleep features.19 The cutoff to determine insomnia for the total scale was ≥6.19
Daytime sleepiness.
Daytime sleepiness was determined on the basis of the Chinese version of the Epworth Sleepiness Scale (ESS).21 The higher the total score, the higher the chance of falling asleep while engaged in specific situations of daily life. Total score ≥10 indicates excessive daytime sleepiness.21 A validation study of the ESS among Chinese individuals has demonstrated good test-retest reliability (ρ = 0.74).22
Sleep duration and snoring status.
In 2012, we collected sleep duration and snoring status in a typical night by questionnaire, and further categorized the answers into 4 groups for sleep duration: <6, 6, 7, and ≥8 h/d, and 3 groups for snoring: never/rare, occasional, and frequent snoring with breathing stops.
In the 2014 survey, we included the STOP-BANG questionnaire and measured neck circumference to the nearest centimeter using a tape measure by trained field workers to screen individuals at high risk of obstructive sleep apnea (OSA).23 The questionnaire consists of 8 dichotomous items, including snoring, daytime tiredness, observed apneas, blood pressure, body mass index (BMI) (>35 kg/m2), age (>50 years), neck circumference (>40 cm), and sex (men). Individuals are considered at intermediate or high risk of OSA with 3 or more of the 8 items scored positive and at low risk otherwise.23 The STOP-BANG questionnaire has been validated among different populations, including Chinese, with a high sensitivity (91%–94%) to detect OSA.24 There were 9,311 participants (78%) who completed the STOP-BANG questionnaire. We used multiple imputation for these missing values based on a fully conditional method that has been described.25 In total, 2,311 participants were considered at intermediate or high risk of OSA (STOP-BANG score ≥3).23
Assessment of other potential covariates.
Information regarding education level, income level, occupation, physical activity, smoking status, and alcohol intake was collected in 2012 via questionnaires.12 Weight and height were measured by trained field workers during the 2012 interview. BMI was calculated as body weight (kg) divided by the square of height (m2). Systolic and diastolic blood pressures were measured twice from the seated position using a mercury sphygmomanometer. We used the average of the 2 readings for analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of antihypertensive medications in the last 2 weeks regardless of blood pressure status. Prehypertension was classified as systolic blood pressure between 120 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. Myocardial infarction cases were confirmed by review of medical record, as detailed elsewhere.14 Atrial fibrillation cases were identified by biennial resting ECG since 2006. Fasting blood samples were collected. Concentrations of glucose, triglyceride, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and uric acid were assessed using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. Diabetes was defined as a concentration of fasting blood glucose ≥7.0 mmol/L or use of oral hypoglycemic agent or active treatment with insulin, and prediabetes was defined as a concentration of fasting blood glucose between 5.6 and 6.9 mmol/L.
Statistical analyses.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Formal hypothesis testing was 2-sided with a significance level of 0.05. Person-years for each participant were calculated from the date the 2012 questionnaire was completed to the diagnosed date of stroke or death, or December 31, 2014, whichever came first. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of stroke based on pRBD status.
We fit 3 multivariate models: model 1 adjusted for age and sex; model 2 further adjusted for education level (primary, middle, or college and higher), income level (<500, 500–1,000, or >1,000 renminbi [RMB]/mo), occupation (blue collar/white collar), physical activity (never, <4 times/wk, or ≥4 times/wk), smoking status (never, past, or current smoker), alcohol status (never, past drinker, current drinker: <2, 2–4, or ≥5 servings/d), hypertension (no, prehypertension, or hypertension), diabetes (no, prediabetes, or diabetes), BMI (<24, 24–28, or ≥28 kg/m2), atrial fibrillation (yes/no), myocardial infarction (yes/no), and plasma concentrations of triglycerides (quartiles), LDL-C (quartiles), HDL-C (quartiles), and urate (quartiles); model 3 further adjusted for sleep time (<6, 6–7, 7–8, 8–9, or ≥9 h/d), daytime sleepiness (yes/no), insomnia (yes/no), hypnotics use (yes/no), and snoring status (never/rare, occasionally, or frequently snoring).
To test the robustness of our observations, we conducted a sensitivity analysis by using the alternative cutoff point (range 0–70, >7) for determining pRBD based on 7 subgroup behavioral factors of RBDQ-HK, including sleep talking, shouting, limb movements, and sleep-related injuries. To minimize the potential effect of RBD mimics on the observed association between pRBD and stroke, we conducted several sensitivity analyses by excluding participants with other sleep disorders (including excessive daytime sleepiness, insomnia, and frequently snoring), or those using hypnotics or alcohol at baseline, separately. We also conducted a sensitivity analysis by excluding those 2,311 participants with intermediate or high risk of OSA, based on the 2014 STOP-BANG questionnaire.
We examined potential interactions of presence of pRBD (yes/no) with age (< or ≥60 years), sex (women/men), overweight (yes/no, based on BMI ≥25 kg/m2), hypertension (yes/no), and smoking status (never/ever), in relation to stroke risk, by including multiplicative terms in the Cox models, with adjustment for other potential confounders in model 3.
RESULTS
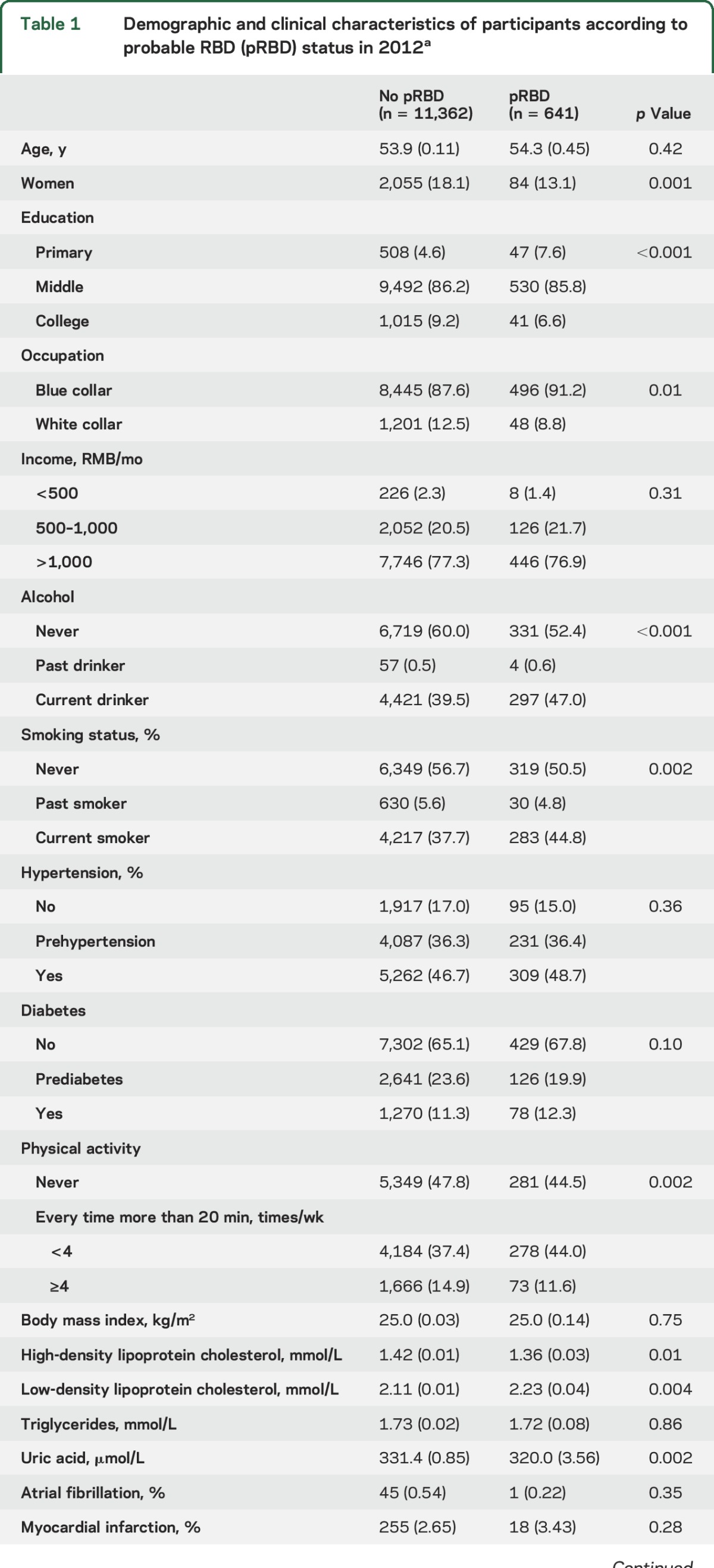
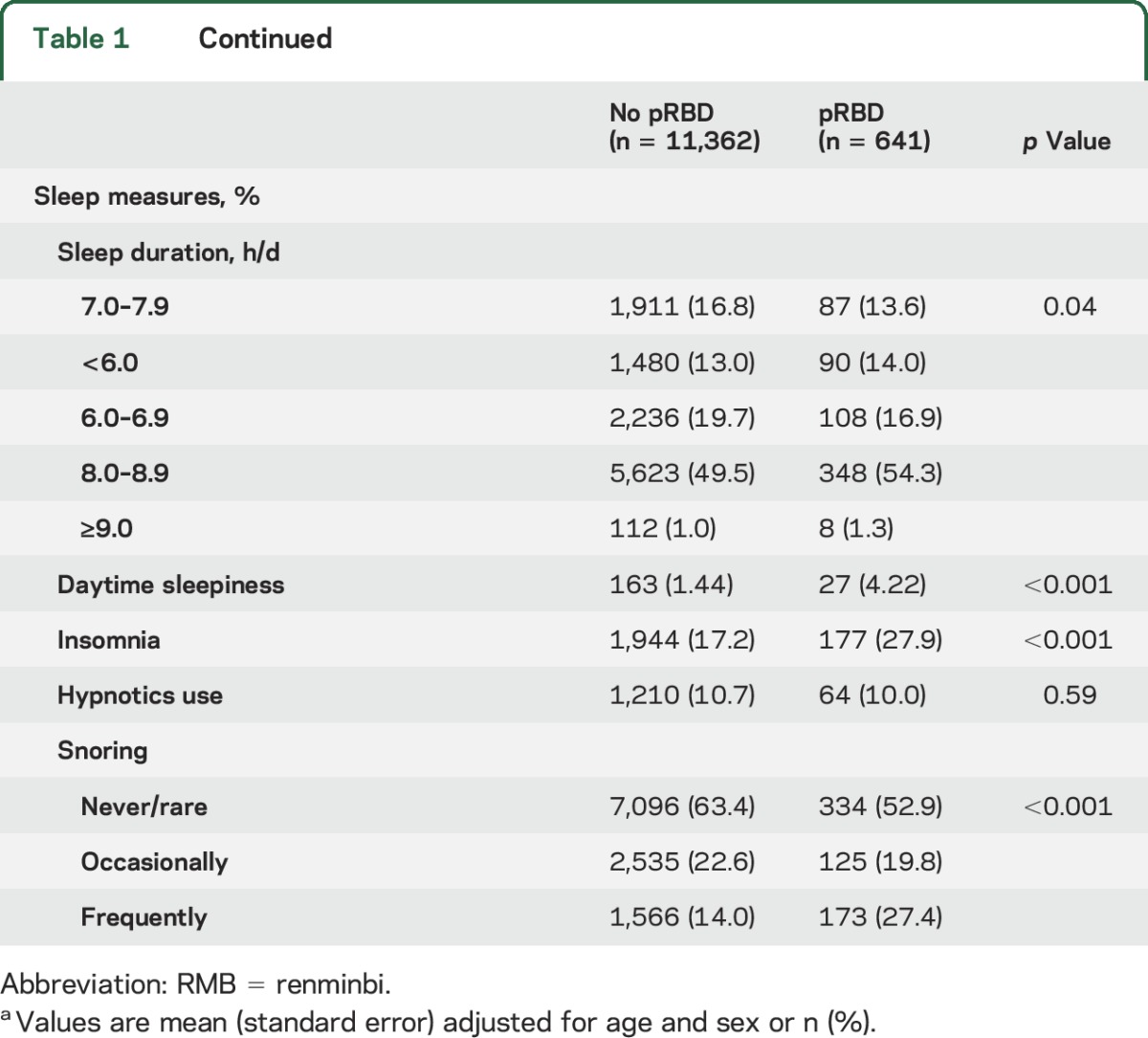
Compared to participants without pRBD, participants with pRBD were more likely to be men, blue collar (compared to white collar occupation), alcohol drinkers, smokers, at a lower education and physical activity level, and to have a higher LDL-C and lower HDL-C and uric acid concentration (table 1). Abnormal sleep measures, such as daytime sleepiness, insomnia, and snoring, were also significantly associated with higher odds of having pRBD (table 1).
Table 1.
Demographic and clinical characteristics of participants according to probable RBD (pRBD) status in 2012a
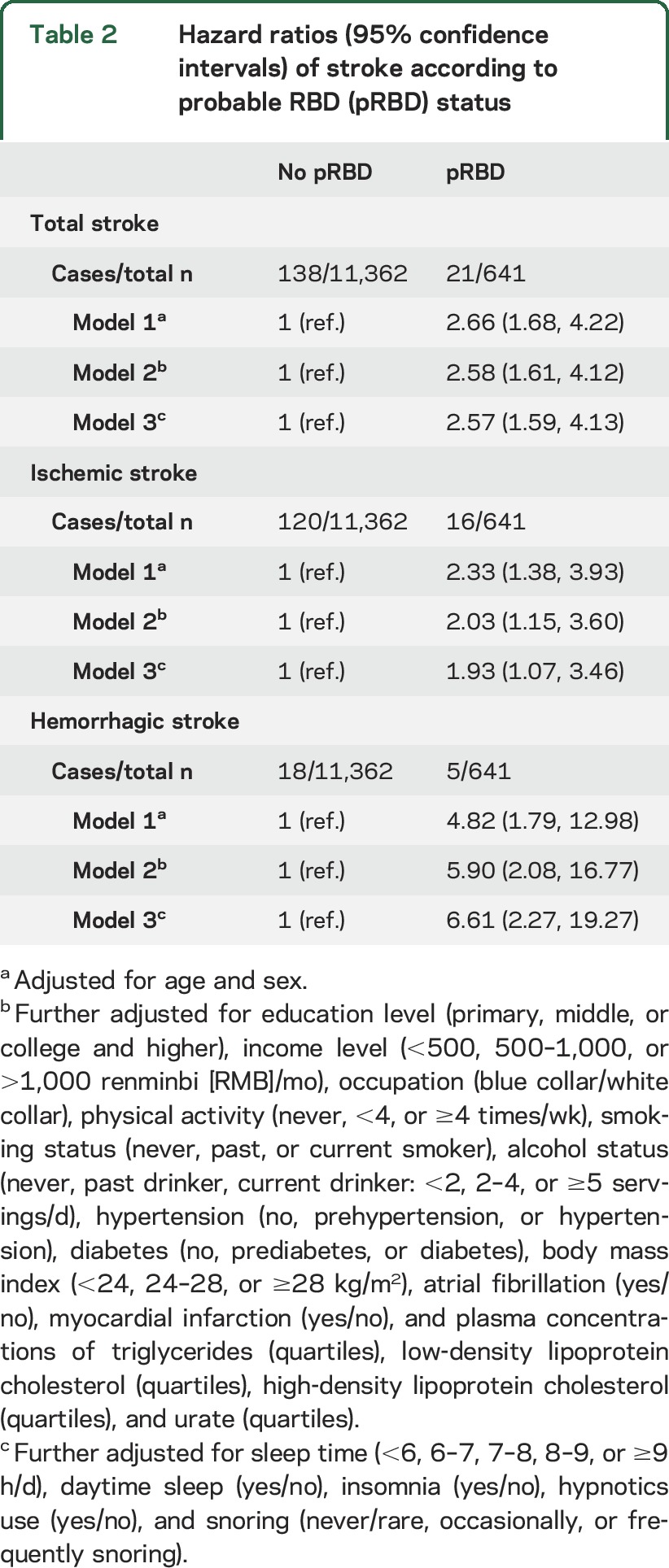
During 3 years of follow-up, we documented 159 incident stroke cases. Relative to participants without pRBD, those with pRBD had a 157% higher risk (95% CI 39%–313%) of developing stroke during the follow-up, after adjusting for age, sex, education level, income level, occupation, physical activity, smoking status, alcohol status, hypertension, diabetes, BMI, plasma concentrations of lipids and uric acid, atrial fibrillation, myocardial infarction, and other sleep measures (table 2). Presence of pRBD was associated with increased risk of both stroke types and the association was stronger for hemorrhagic stroke, relative to ischemic stroke (table 2).
Table 2.
Hazard ratios (95% confidence intervals) of stroke according to probable RBD (pRBD) status
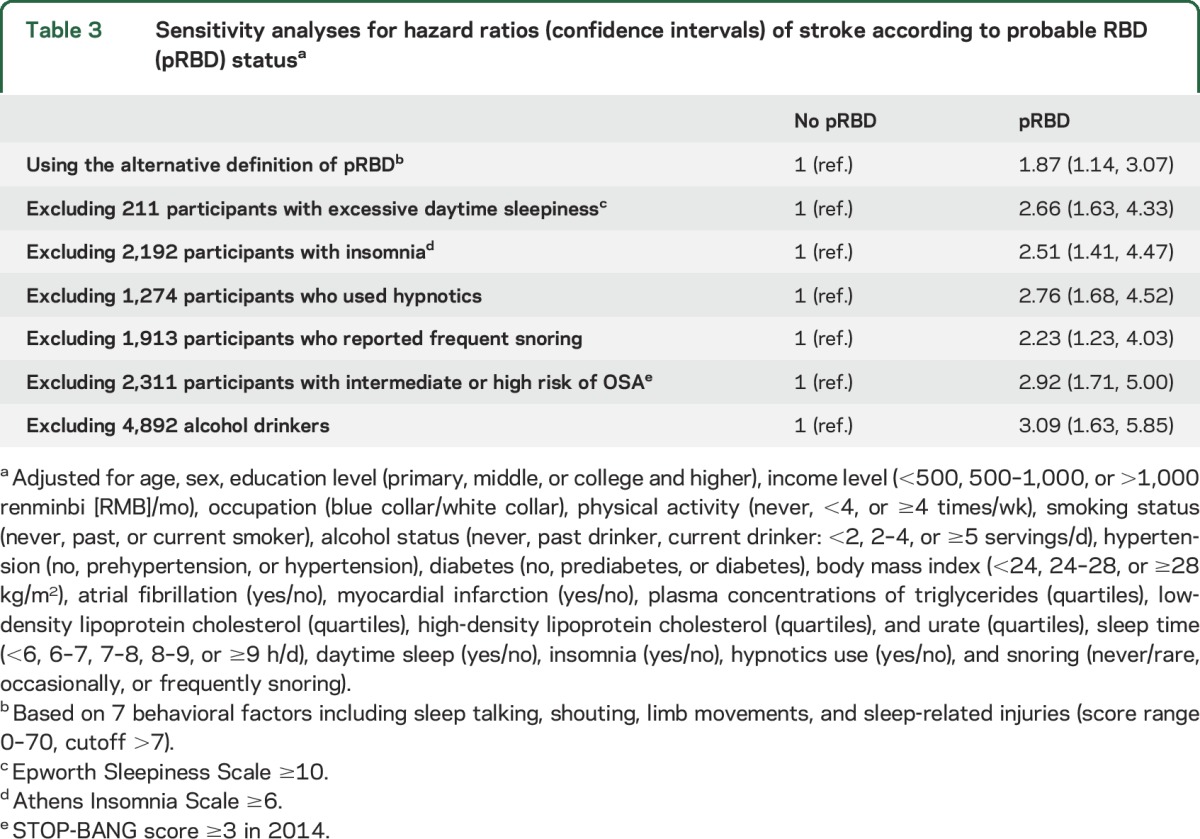
Results did not change materially in the sensitivity analyses by using alternate pRBD definition and excluding RBD mimics (table 3). Further adjustment for systolic and diastolic blood pressure did not materially change the observed association between pRBD and stroke (data not shown). The interactions between pRBD and other stroke risk factors (older age, male sex, overweight, hypertension, and smoking) were not significant (p for interaction ≥0.1 for all) (table e-1 at Neurology.org).
Table 3.
Sensitivity analyses for hazard ratios (confidence intervals) of stroke according to probable RBD (pRBD) statusa
DISCUSSION
In this community-based prospective study of over 12,000 Chinese adults, we observed that participants with pRBD at baseline were approximately 1.5 times more likely to develop stroke, including both ischemic and hemorrhagic types, independent of potential co-determinants, comprising age, sex, obesity, hypertension, and smoking status. Excluding common RBD mimics did not change the significant association between pRBD and stroke risk.
Although RBD preceding stroke has not been studied previously, a cross-sectional relationship between RBD and stroke was reported in previous studies.9–11,26 For example, in a study including 119 stroke Chinese patients, 11% had RBD and presence of RBD was significantly associated with brainstem infarcts and smaller infarct volumes.26 In another multicenter case-control study including 318 idiopathic RBD cases and 318 matched controls (181 sleep disorder controls and 137 healthy volunteers), RBD cases reported more cardiovascular disease (odds ratio [OR] 1.6; 95% CI 1.0–2.5) relative to the controls, and the association became stronger when 181 sleep disorder controls were removed (OR 2.4; 95% CI 1.3–4.6).27 In the same study, a correlation between RBD and a higher odds of having self-reported cerebrovascular disease was also observed (OR 1.3; 95% CI 0.7–2.6), although the association was not significant, which could be due to small number of cerebrovascular disease cases (n = 38) and misclassification due to self-report nature.27
There are several biological mechanisms that may explain the observed association between RBD and stroke. The association could be due to a more profound change, resulting from the disturbed sleep. Indeed, poor or insufficient sleep has been associated with a wide range of autonomic and metabolic consequences, such as diabetes28,29 and cancer.30 Sleep fragmentation may lead to increased sympathetic output, such as changes in heart rate variability and temperature regulation, and impairment in hormonal secretion and immune function.31 Over time, this hyperactivity during sleep might be generalized into an ongoing, daytime sympathetic overactivity, thereby increasing cardiovascular stress.8 Significant association between cardiovascular risk factors and pRBD was observed in 2 previous cross-sectional studies.12,32 However, in the current study, after we controlled for these cardiovascular factors, the significant association between pRBD and stroke persisted, suggesting that pRBD could be a risk factor for stroke beyond these conventional factors. Furthermore, this increased risk may come from RBD itself or possibly from another comorbid sleep disorder, such as OSA.33,34
We observed a strong relation between pRBD and hemorrhagic stroke. This association cannot be explained by the above factors alone. It is possible that hemodynamic abnormalities due to the sleep disruption may play a role. We may also speculate that this is due to the fact that patients with RBD are more likely to have injury during the night.35 Although we excluded individuals with head injury, based on review of medical records, we cannot exclude the possibility of residual confounding due to RBD-related mild injuries. Another potential explanation is that the symptoms we perceived as pRBD may be actually arousals provoked by OSA, as described above. However, the strong association between pRBD and hemorrhagic stroke remained the same after we excluded those participants who snored frequently at baseline (adjusted HR 7.17) or those with intermediate or high risk of OSA during the follow-up (adjusted HR 8.07), suggesting that the effect of OSA on the observed association is probably small to modest. Interestingly, relative to those at low risk of OSA, individuals at intermediate/high risk of OSA were more likely to have incident stroke (1.80% vs 0.63%) and pRBD (5.41% vs 4.79%).
It is also possible that the same factors that increase RBD risk may also increase the risk for cerebral amyloid angiopathy—a common cause of intracerebral hemorrhage in the elderly and that was observed in approximately 25% of individuals with PD.36 In a recent study, a higher sleep fragmentation was associated with a higher number of postmortem identified macroscopic infarcts and more severe atherosclerosis.37 However, it remains unknown whether amyloid angiopathy has a role in RBD pathogenesis. Because of small number of incident hemorrhagic stroke cases (n = 23) during the follow-up period, the observed strong association between pRBD and hemorrhagic stroke could, at least to some extent, be due to chance. Further studies are warranted to replicate our findings and explore the potential underlying biological mechanisms.
Our study has several limitations. RBD status was determined by self-reported questionnaire, without verification by clinical evaluation or polysomnography. For this reason, we used pRBD in this report. To reduce the effects of potential misclassification of RBD status, we controlled other sleep measures, such as daytime sleepiness, insomnia, and snoring, which could mimic RBD symptoms. Further excluding participants with these RBD mimics did not materially change the significant results, suggesting that the effect of these factors on the pRBD–stroke relation could be small to modest. Residual confounding is another concern. We did not collect information on depression or antidepressant use, which were associated with RBD27 and stroke.38 Similarly, we did not collect information on other coronary artery disease (e.g., angina) besides myocardial infarction. The existence of residual confounding could lead to overestimation of the observed association. Further, we did not collect detailed information on OSA until 2014, and only had 78% of participants with known OSA status, which might introduce potential selection bias. However, we used multiple imputation for these missing values and generated similar results after we adjusted the OSA status or excluded those who were considered at intermediate or high risk of OSA. We acknowledge that imputation analysis is likely insufficient to address the bias because the STOP-BANG values might not be missing at random. Further, OSA status was obtained by questionnaire, rather than gold standard testing, which could introduce misclassification. We were not able to examine the sex-specific association because of small sample size for women. In addition, the current study was conducted in Tangshan City, an industrial city in northern China. Our results may lack generalizability to other settings and populations, such as Caucasians. Further, the follow-up period is rather short and we examined only short-term effects of pRBD. We also cannot exclude the possibility that presence of pRBD could merely be the result of any otherwise undiagnosed lacunar strokes within the nigrostriatal system.
The advantages of our study include rigorous data collection for all variables among the participants, consistent follow-up, and within-subject comparisons in this longitudinal design. Overall, in this population-based prospective study, we observed that participants with pRBD had an increased risk of having a stroke, both ischemic and hemorrhagic, during the 3 years of follow-up. Future studies with clinically confirmed RBD, a longer follow-up, and specific measures of hemorrhage risk (such as gradient echo sequence on MRI to evaluate potential comorbid cerebral amyloid angiopathy) would be appropriate to further investigate this association.
GLOSSARY
- AIS
Athens Insomnia Scale
- BMI
body mass index
- CI
confidence interval
- ESS
Epworth Sleepiness Scale
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- ICD-10
International Classification of Diseases–10
- LDL-C
low-density lipoprotein cholesterol
- OR
odds ratio
- OSA
obstructive sleep apnea
- PD
Parkinson disease
- pRBD
probable REM sleep behavior disorder
- RBD
REM sleep behavior disorder
- RBDQ-HK
RBD questionnaire–Hong Kong
- RMB
renminbi
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Chaoran Ma: analysis and interpretation of data, manuscript drafting/revising. Milena Pavlova: critical revision of manuscript for intellectual content. Yesong Liu: collection and interpretation of data, manuscript drafting/revising. Ying Liu: collection and interpretation of data, manuscript drafting/revising. Chunmei Huangfu: collection and interpretation of data, manuscript drafting/revising. Shouling Wu: data collection, study supervision. Xiang Gao: study concept and design, data collection, analysis and interpretation of data, manuscript drafting/revising, study supervision.
STUDY FUNDING
Supported by the National Institute of Neurological Disorders and Stroke at the NIH (NINDS 5R21NS087235-02 and 1R03NS093245-01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
C. Ma reports no disclosures relevant to the manuscript. M. Pavlova served on a committee of the American Academy of Sleep Medicine and received research study funding from Lundbeck Inc. Y. Liu and C. Huangfu report no disclosures relevant to the manuscript. S. Wu received funding from the Chinese Science Foundation. X. Gao has served on committees of the Sleep Research Society, American Academy of Sleep Medicine, and Parkinson Study Group and received funding from the NIH/NINDS. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Postuma RB, Gagnon JF, Montplaisir J. Rapid eye movement sleep behavior disorder as a biomarker for neurodegeneration: the past 10 years. Sleep Med 2013;14:763–767. [DOI] [PubMed] [Google Scholar]
- 2.Howell MJ, Schenck CH. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol 2015;72:707–712. [DOI] [PubMed] [Google Scholar]
- 3.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 2006;67:2236–2238. [DOI] [PubMed] [Google Scholar]
- 5.Ferini-Strambi L, Fantini ML, Zucconi M, et al. REM sleep behaviour disorder. Neurol Sci 2005;26(suppl 3):s186–192. [DOI] [PubMed] [Google Scholar]
- 6.Ferini-Strambi L, Oertel W, Dauvilliers Y, et al. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case-control study. J Neurol 2014;261:1112–1118. [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord 2010;25:2304–2310. [DOI] [PubMed] [Google Scholar]
- 8.Micieli G, Cavallini A. The autonomic nervous system and ischemic stroke: a reciprocal interdependence. Clin Auton Res 2008;18:308–317. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S, Waki R. A discrete pontine ischemic lesion could cause REM sleep behavior disorder. Neurology 2000;55:894–895. [DOI] [PubMed] [Google Scholar]
- 10.Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med 2009;10:143–146. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds TQ, Roy A. Isolated cataplexy and REM sleep behavior disorder after pontine stroke. J Clin Sleep Med 2011;7:211–213. [PMC free article] [PubMed] [Google Scholar]
- 12.Wong JC, Li J, Pavlova M, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology 2016;86:1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lashley T, Holton JL, Gray E, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol 2008;115:417–425. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Jin C, Vaidya A, et al. Longitudinal patterns of blood pressure, incident cardiovascular events, and all-cause mortality in normotensive diabetic people. Hypertension 2016;68:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Zhou Y, Gao X, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke 2013;44:2451–2456. [DOI] [PubMed] [Google Scholar]
- 16.Stroke 1989: recommendations on stroke prevention, diagnosis, and therapy: report of the WHO task force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 17.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med 2010;11:43–48. [DOI] [PubMed] [Google Scholar]
- 18.Shen SS, Shen Y, Xiong KP, et al. Validation study of REM sleep behavior disorder questionnaire–Hong Kong (RBDQ-HK) in east China. Sleep Med 2014;15:952–958. [DOI] [PubMed] [Google Scholar]
- 19.Chung KF, Kan KK, Yeung WF. Assessing insomnia in adolescents: comparison of insomnia severity index, Athens insomnia scale and sleep quality index. Sleep Med 2011;12:463–470. [DOI] [PubMed] [Google Scholar]
- 20.Sun JL, Chiou JF, Lin CC. Validation of the Taiwanese version of the Athens insomnia scale and assessment of insomnia in Taiwanese cancer patients. J Pain Symptom Manage 2011;41:904–914. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 22.Chen NH, Johns MW, Li HY, et al. Validation of a Chinese version of the Epworth Sleepiness Scale. Qual Life Res 2002;11:817–821. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812–821. [DOI] [PubMed] [Google Scholar]
- 24.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One 2015;10:e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 26.Tang WK, Hermann DM, Chen YK, et al. Brainstem infarcts predict REM sleep behavior disorder in acute ischemic stroke. BMC Neurol 2014;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frauscher B, Jennum P, Ju YE, et al. Comorbidity and medication in REM sleep behavior disorder: a multicenter case-control study. Neurology 2014;82:1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 2007;11:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu F, Han J, Laden F, et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med 2015;48:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev 2016;74:321–329. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Qiao Y, Gao X, et al. A community-based study of risk factors for probable rapid eye movement sleep behavior disorder. Sleep Med 2017;30:71–76. [DOI] [PubMed] [Google Scholar]
- 33.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 34.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep 2008;31:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyken ME, Lin-Dyken DC, Seaba P, Yamada T. Violent sleep-related behavior leading to subdural hemorrhage. Arch Neurol 1995;52:318–321. [DOI] [PubMed] [Google Scholar]
- 36.Dugger BN, Adler CH, Shill HA, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord 2014;20:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim AS, Yu L, Schneider JA, Bennett DA, Buchman AS. Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community-dwelling older people. Stroke 2016;47:516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA 2011;306:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]


