Abstract
Objective:
To compare nonadherence to statins in older black and white adults following an ischemic stroke.
Methods:
We studied black and white adults ≥66 years of age with Medicare fee-for-service insurance coverage hospitalized for ischemic stroke from 2007 to 2012 who filled a statin prescription within 30 days following discharge. Nonadherence was defined as a proportion of days covered <80% in the 365 days following hospital discharge. In addition, we evaluated factors associated with nonadherence for white and black participants separately.
Results:
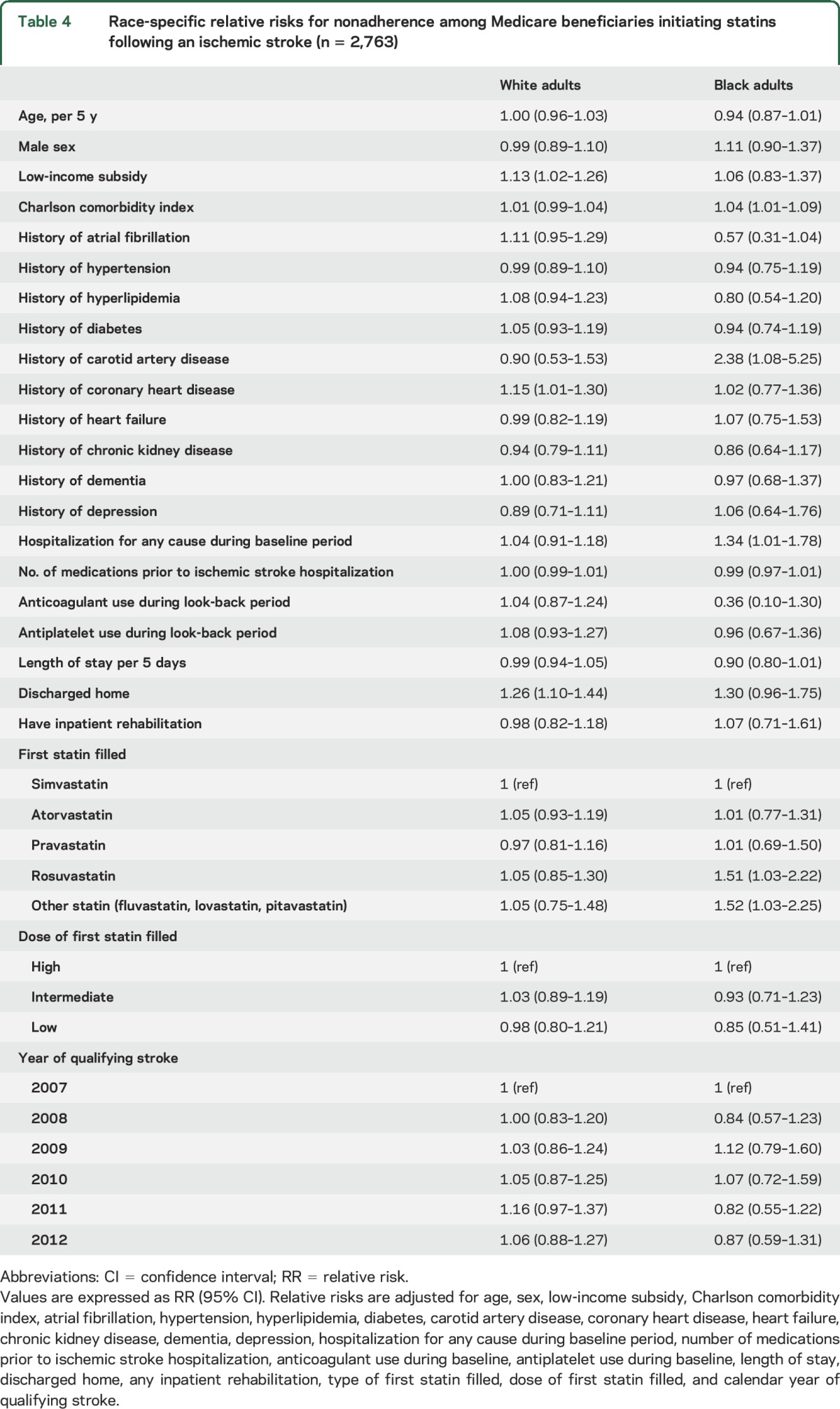
Overall 2,763 beneficiaries met the inclusion criteria (13.5% black). Black adults were more likely than white adults to be nonadherent (49.7% vs 41.5%) even after adjustment for demographics, receipt of a low-income subsidy, and baseline comorbidities (adjusted relative risk [RR] 1.14, 95% confidence interval [CI] 1.01–1.29). Among white adults, receipt of a low-income subsidy (adjusted RR 1.13, 95% CI 1.02–1.26), history of coronary heart disease (adjusted RR 1.15, 95% CI 1.01–1.30), and discharge directly home following stroke hospitalization (adjusted RR 1.26, 95% CI 1.10–1.44) were associated with a higher risk of nonadherence. Among black adults, a 1-unit increase in the Charlson comorbidity index (adjusted RR 1.04, 95% CI 1.01–1.09), history of carotid artery disease (adjusted RR 2.38, 95% CI 1.08–5.25), and hospitalization during the 365 days prior to the index stroke (adjusted RR 1.34, 95% CI 1.01–1.78) were associated with nonadherence.
Conclusions:
Compared with white adults, black adults were more likely to be nonadherent to statins following hospitalization for ischemic stroke.
Each year, 795,000 people in the United States experience a new or recurrent stroke.1 Stroke is a leading cause of long-term adult disability and has estimated total direct medical costs of $71.6 billion.2 The majority of strokes in the United States are ischemic, with 7%–21% of those surviving an initial stroke having a recurrent event within 1 year.3 Studies show that recurrent stroke is even more disabling and costly than the first event.4 Currently there are an estimated 7 million adult stroke survivors in the United States, underscoring the importance of secondary stroke prevention.1
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial demonstrated that statin treatment reduces the risk for recurrent stroke in patients with a history of stroke or TIA.5 Furthermore, a meta-analysis of placebo-controlled and active-comparator trials has shown that statins reduce the risk of stroke in patients with coronary heart disease (CHD).6 Despite the efficacy of statins for stroke prevention, analyses of registry data suggest that many patients discontinue statin therapy within 1 year of stroke hospital discharge.7 While previous studies have shown that black adults are less adherent to statins than white adults following myocardial infarction,8 there are few data on racial differences in statin adherence following ischemic stroke. Determining the factors associated with nonadherence in community-dwelling white and black stroke survivors can guide interventions to improve adherence. Therefore, we conducted a retrospective cohort study to compare statin nonadherence in black and white adults following hospitalization for ischemic stroke. In addition, we determined whether factors associated with statin nonadherence differed for black and white adults. We hypothesized that a higher percentage of black than white adults would be nonadherent and that black and white adults would have different factors associated with statin nonadherence.
METHODS
Data source.
Using the 5% sample from the Centers for Medicare and Medicaid Services (CMS), we conducted a retrospective cohort study of white and black Medicare beneficiaries hospitalized for an ischemic stroke between January 1, 2007, and December 31, 2012. Data were obtained from Medicare enrollment, inpatient (Medicare part A), outpatient (Medicare part B), and prescription drug (Medicare part D) files.
Standard protocol approvals, registrations, and patient consents.
The CMS and the institutional review board at the University of Alabama at Birmingham approved the study.
Inclusion/exclusion criteria.
The current analysis included Medicare beneficiaries with an ischemic stroke (index event), defined by having ICD-9-CM codes 433.x1, 434.x1, or 436 in any discharge diagnosis position in an inpatient file claim. Beneficiaries were required to have continuous full fee-for-service coverage, defined as Medicare parts A, B, and D during the 365-day look-back period prior to hospital admission for the index stroke event, during the stroke hospitalization, and for the 365 days after discharge. Beneficiaries enrolled in Medicare Advantage plans (Medicare part C coverage) were excluded as complete claims were not available for this population.
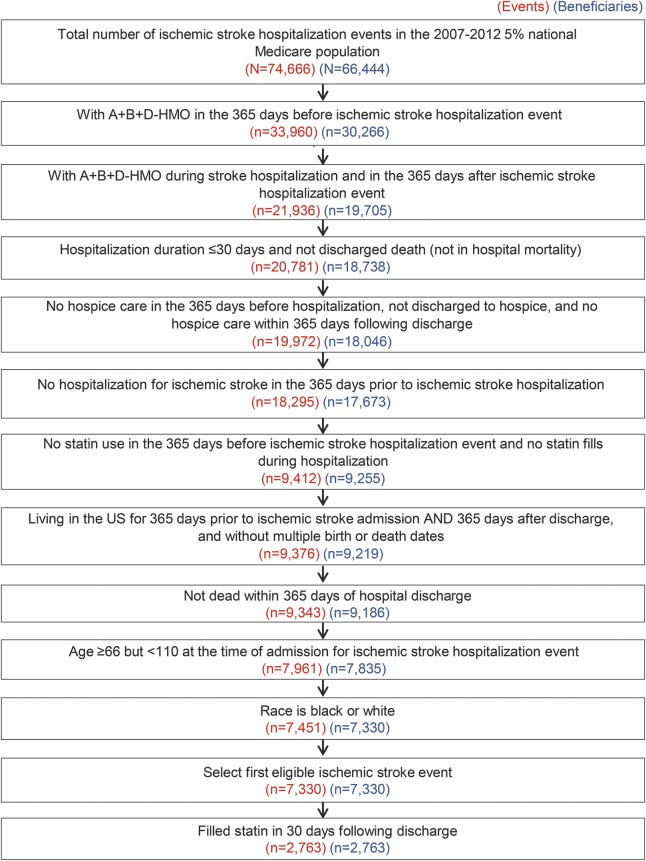
Additional eligibility for the current analysis included the following: (1) an acute care hospital length of stay for the index stroke of less than or equal to 30 days, (2) no claims for hospice care in the look-back period or in the 365 days after discharge, (3) no hospitalization for ischemic stroke during the 365-day look-back period prior to hospital admission for the index stroke event,9 (4) no statin use in the 365 days before the ischemic stroke hospitalization event and no statin fills during hospitalization, (5) living in the United States during the entire look-back period through 365 days after hospital discharge and having valid birth and death dates, (6) alive for 365 days following hospital discharge for the index stroke event, (7) age at least 66 years but less than 110 years at the time of hospital admission for the index stroke event, and (8) self-reported race as black or white. If a Medicare beneficiary had more than one hospitalization for ischemic stroke meeting these inclusion criteria, only the earliest eligible hospitalization was included. We restricted the analysis to Medicare beneficiaries initiating statins following their index stroke event as adherence differs between prevalent and new statin users.10,11 Among Medicare beneficiaries meeting these criteria (n = 7,330), we excluded 4,567 beneficiaries who did not have a Medicare Part D claim for a statin within 30 days of hospital discharge following their stroke, resulting in a sample of 2,763 Medicare beneficiaries for the analyses (figure).
Figure. Flow diagram for the analysis: Medicare beneficiaries in the statin-naive stroke cohort.
Exposure/covariates.
Age, self-reported race, and sex were obtained from the Medicare beneficiary enrollment file. Receipt of a low-income cost subsidy (LIS), a marker of low socioeconomic status, was obtained from the Part D summary file. Diabetes, heart failure, dementia, depression, and Charlson comorbidity index were defined using claims during the look-back period and previously published algorithms.12,13 The algorithm used to define each covariate is shown in table e-1 at Neurology.org.12–16 Hospitalizations during the look-back period were identified using Part A claims. The type and dose of the first statin filled were determined using Medicare Part D claims. Covariates assessed following hospital discharge for the index stroke event included any inpatient rehabilitation (IPR) and skilled nursing facility (SNF) stays with an admission to IPR or SNF within 7 days of hospital discharge.
Outcome: Statin nonadherence.
Statin fills were identified using Medicare Part D claims. Nonadherence was determined by calculating the proportion of days covered (PDC), a metric recommended by the Pharmacy Quality Alliance.17 The PDC was calculated as the number of days for which a day of statin supply was available from the date of the first fill through 365 days following hospital discharge for the index stroke divided by the number of days in this time period. Time spent in inpatient rehabilitation, SNF, or in the hospital was excluded from the PDC calculations. Nonadherence was defined as a PDC <80%.17
Statistical analysis.
Characteristics of the cohort were calculated overall and by race. The median PDC was calculated for black and white adults. The difference in median PDC between black and white adults was calculated using quantile regression with 3 levels of adjustment. The crude difference in the median PDC was calculated, followed by a second model that included adjustment for age and sex, and a third model that also included adjustment for age, sex, LIS, Charlson comorbidity index, atrial fibrillation, hypertension, hyperlipidemia, diabetes, carotid artery disease, coronary heart disease, heart failure, chronic kidney disease, dementia, depression, hospitalization for any cause during baseline period, number of medications prior to ischemic stroke hospitalization, anticoagulant use during baseline, antiplatelet use during baseline, length of stay, discharged home, any inpatient rehabilitation, type of first statin filled, dose of first statin filled, and calendar year of qualifying stroke. The prevalence of statin nonadherence was calculated for black and white adults, separately, and compared using a χ2 test. Relative risks (RR) for nonadherence comparing black to white adults were calculated using generalized linear models with a Poisson distribution, a log link, and a robust variance estimator.18 Models included 3 levels of adjustment, as described above. In a secondary analysis, RRs for statin nonadherence associated with factors from the look-back period, first statin filled, dose of first statin filled, and year of stroke were calculated for white and black adults, separately. All analyses were conducted using SAS 9.4 (SAS Institute, Research Triangle Park, NC).
RESULTS
Cohort characteristics.
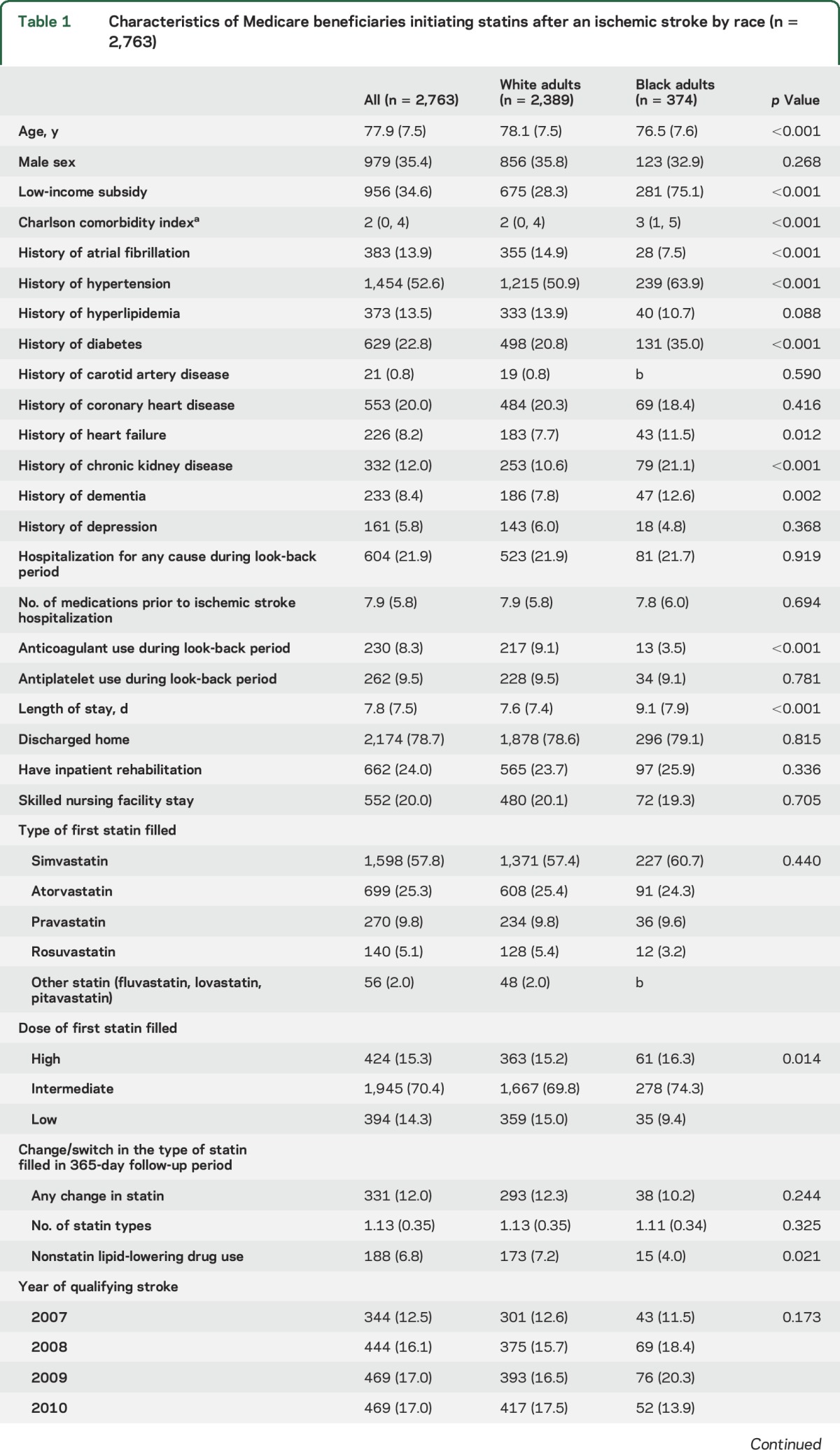
Among Medicare beneficiaries included in the current analysis (n = 2,763), black adults were younger, more frequently received a LIS, had a higher median Charlson comorbidity index, had a lower prevalence of atrial fibrillation, and had a higher prevalence of hypertension, diabetes, heart failure, chronic kidney disease, and dementia when compared to white adults (table 1). Black adults were less likely to be taking anticoagulants during the look-back period and had a longer length of stay during the index stroke than white adults. There were no statistically significant differences in the proportion of black and white adults who were discharged directly home, to inpatient rehabilitation, or to a SNF following hospital discharge. The first statin filled was a low-dose intensity for 15.0% of white adults and 9.4% of black adults. Nonstatin lipid-lowering agents were filled during the follow-up period by 7.2% of white adults and 4.0% of black adults.
Table 1.
Characteristics of Medicare beneficiaries initiating statins after an ischemic stroke by race (n = 2,763)
Factors associated with nonadherence in white and black participants.
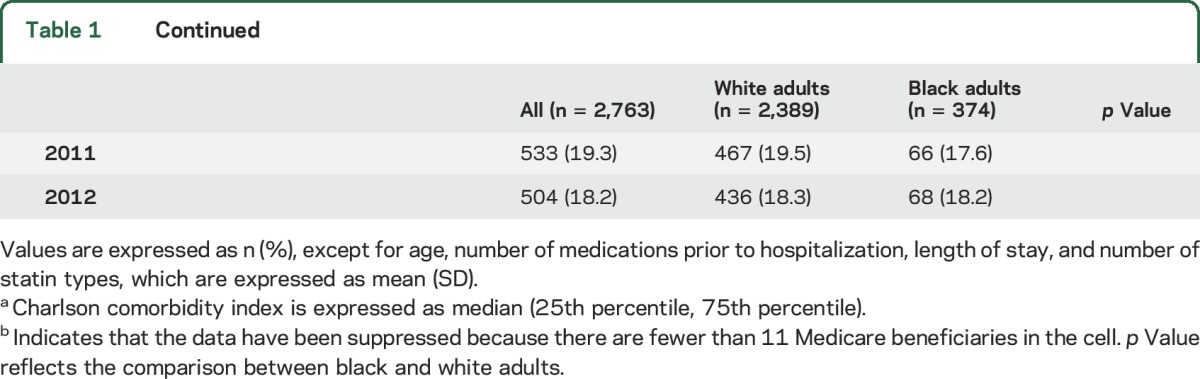
The median PDC was 87.5 for white adults and 80.5 for black adults (table e-2; p < 0.001). After multivariable adjustment, the median PDC was −6.66% (95% confidence interval [CI] −12.2% to −1.12%) lower in black adults compared with white adults. Forty-three percent of ischemic stroke survivors were nonadherent to statins in the year following hospital discharge. When compared with white adults, a higher percentage of black adults were nonadherent to their statin (49.7% vs 41.5%, p = 0.003; table 2). This association remained present after multivariable adjustment (black:white RR 1.14, 95% CI 1.00–1.29).
Table 2.
Statin nonadherence in white and black Medicare beneficiaries following an ischemic stroke
Characteristics of white and black adults who were adherent and nonadherent to statins are shown in table 3. After multivariable adjustment, the factors associated with statin nonadherence among white adults included receipt of a LIS (RR 1.13, 95% CI 1.02–1.26), a history of CHD (RR 1.15, 95% CI 1.01–1.30), and being discharged directly home following ischemic stroke hospitalization (RR 1.26, 95% CI 1.10–1.44, table 4). Among black adults, each one point higher Charlson comorbidity index (RR 1.04, 95% CI 1.01–1.09), history of carotid artery disease (RR 2.38, 95% CI 1.08–5.25), and hospitalization during the look-back period (RR 1.34, 95% CI 1.01–1.78) were associated with statin nonadherence after multivariable adjustment.
Table 3.
Characteristics of Medicare beneficiaries initiating statins following an ischemic stroke by adherence and race (n = 2,763)
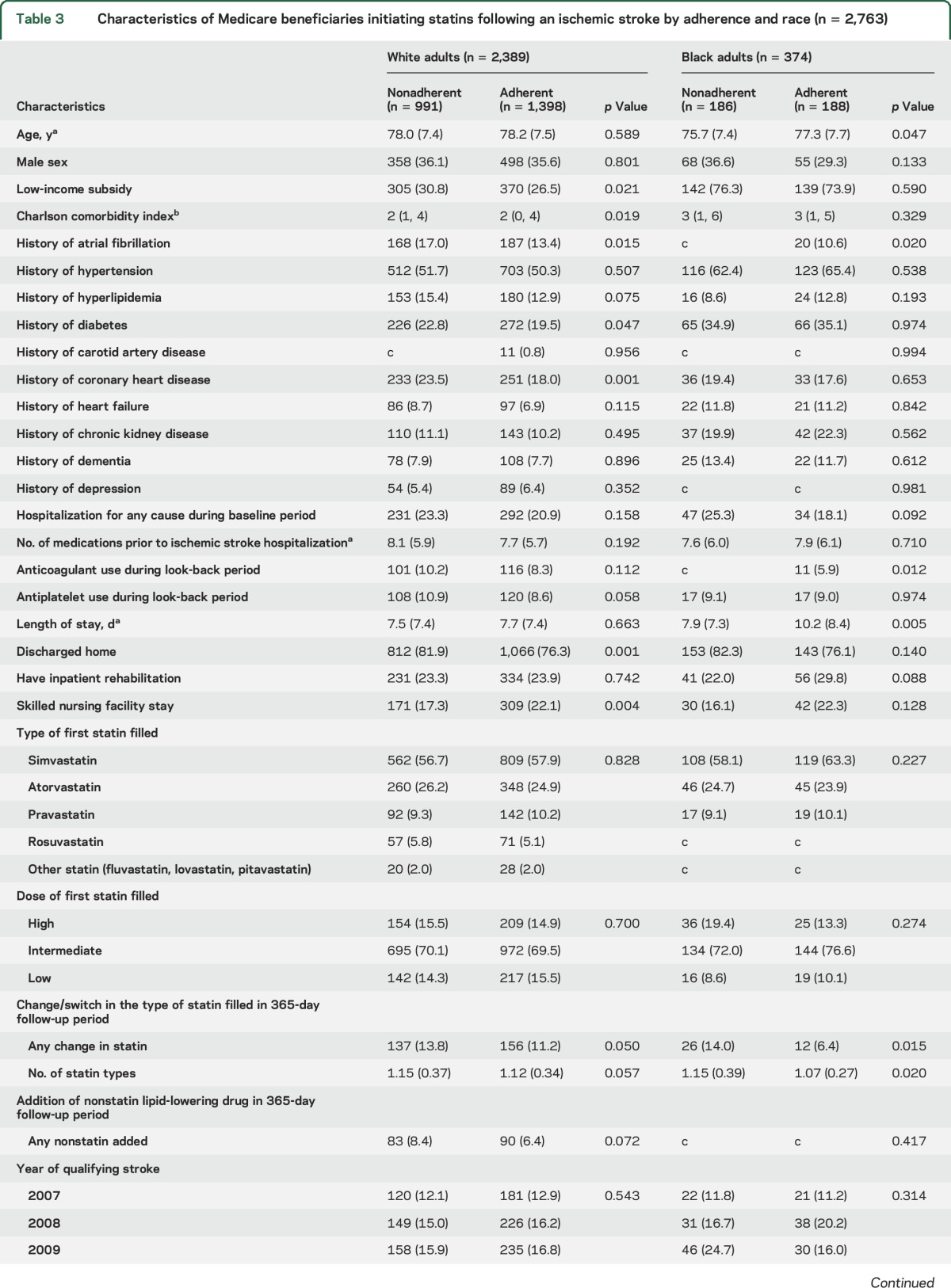
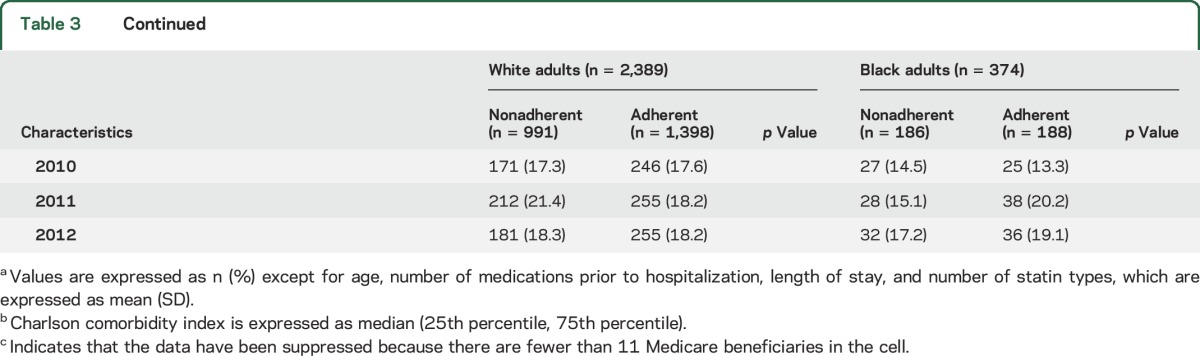
Table 4.
Race-specific relative risks for nonadherence among Medicare beneficiaries initiating statins following an ischemic stroke (n = 2,763)
DISCUSSION
There are several important findings from this study. First, nonadherence to statins was common in the year following hospital discharge for an ischemic stroke and did not improve between 2007 and 2012. Second, black adults were more likely to be nonadherent to statins compared with white adults. This finding remained after adjustment for beneficiary demographics, comorbidities, and proxies of stroke severity. Third, factors associated with statin nonadherence differed for white and black adults. Among white adults, LIS, history of coronary heart disease, and discharge home were associated with statin nonadherence. In black adults, a higher Charlson comorbidity index, hospitalization in the look-back period, and history of carotid artery disease were associated with statin nonadherence. This suggests that white and black adults may have different barriers to medication adherence and that efforts aimed at improving medication adherence may require race-specific interventions.
Prior estimates of statin nonadherence among people in the year following ischemic stroke and TIA have varied widely.7,19 This variation may be due in part to the reliance on self-report for assessing adherence,19 the inclusion of patients who have no lasting disability from their cerebral ischemia (TIA),19 younger patients,19 a small proportion of black adults,7 or being performed in countries where the health care system is inherently different from the US system.7 In the current study, 43% of ischemic stroke survivors were nonadherent to statins in the year following hospital discharge. These data underscore that statin nonadherence is a major challenge for patients following ischemic stroke.
Prior research has shown that statin nonadherence in the general population is associated with an increased risk for cardiovascular disease–related hospitalizations and higher health care costs.20 Given the association of nonadherence with increased morbidity and mortality,21 health care providers should be adequately trained to screen for and recognize medication nonadherence. Screening for medication nonadherence is not routinely performed by physicians.22 Even when screening is performed, physicians often have a difficult time recognizing nonadherence.23 Prior studies suggest that physicians' ability to recognize medication nonadherence can be improved by providing them with access to pharmacy refill data.24 Cost-related nonadherence to medication in adult stroke survivors occurs in 1 in 6 black adults and 1 in 10 white adults.25 Elimination of prescription copayment has been shown to improve adherence for patients following myocardial infarction.26 Two additional strategies that can be employed to improve adherence are the use of mail-order pharmacies and 90-day prescriptions. Observational studies show that these approaches are associated with higher medication adherence.27,28 The high incidence of statin nonadherence among stroke survivors in the current study highlights the need for health care providers to screen for nonadherence and assist patients in addressing barriers to statin nonadherence.
The higher incidence of statin nonadherence among black adults compared with white adults is consistent with the findings of a recent meta-analysis that reported that nonwhite adults were at higher risk of nonadherence compared with white adults.29 Prior studies have reported that older individuals,30 women,31 and individuals with low income,30 depression,30 polypharmacy,30 and less morbidity30 are less likely to be adherent; the association we observed between black race and statin nonadherence did not change appreciably after adjustment for these variables. Reasons for racial differences in adherence likely extend beyond demographics, socioeconomic status, and comorbidities.32 Physician communication behaviors have been shown to affect patient trust, particularly in race discordance between patients and physicians.33 A recent meta-analysis suggested that the risk of nonadherence is higher in patients who have a physician who communicates poorly.34 Studies have shown that training physicians in patient-centered communication skills has the potential to improve patient adherence to treatment.32,34
In the current study, risk factors for statin nonadherence differed by race. For example, having LIS was associated with statin nonadherence in white adults, but not in black adults. Reasons for differential statin nonadherence between black and white stroke survivors are likely multifactorial and may include, but are not limited to, reduced access to physicians,35 lower quality of medical care,36 and lack of trust in providers and the health care system.35
Qualitative interviews of black patients with hypertension revealed that patient-specific barriers such as beliefs and attitudes about the disease and medications, as well as forgetfulness, were the most commonly cited barriers to medication adherence.37 Additional research in black adults with hypertension has shown that perceived discrimination is associated with medication nonadherence and that stress and depression may mediate this association.38 Understanding the barriers to medication adherence has important policy and practice implications that warrant further exploration and testing. To improve outcomes, contain health care costs, and limit preventable recurrent events, it is essential to identify populations at high risk for statin nonadherence and to develop interventions that can improve adherence.
The current study has several strengths. The majority of US adults ≥65 years of age have health insurance through Medicare, providing high generalizability to older adults in the United States. Utilizing a 365-day look-back period allowed us to identify comorbidities present prior to the index stroke. Pharmacy fill data provided an objective adherence measure that is not subject to recall bias.17 The current study also has limitations. Medicare beneficiaries may have limited generalizability to younger adults; however, most US adults having stroke are 65 years of age or older. Lack of evidence of a statin claim in the look-back period cannot guarantee first ever statin use.39 Although we required a 1-year period with no statin fills, beneficiaries could have taken statins in the more distant past. Those with a history of CHD in the current study may have been on a statin previously and discontinued due to side effects. These beneficiaries would be more likely to be nonadherent to their statin following a stroke event. It is also possible that beneficiaries filled a statin outside of Medicare. Previous studies have suggested that up to 20% of beneficiaries may fill a statin without a claim being filed.40 Information on several factors that can influence adherence, including adverse reactions to statins, creatine kinase levels, and glucose, are not available in Medicare claims.
In the current study, black adults were more likely than white adults to be nonadherent to statins in the year following stroke hospitalization. Factors associated with statin nonadherence differed for black and white adults. Identifying medication nonadherence and barriers to medication adherence has the potential to reduce the risk for recurrent events among the 7 million stroke survivors in the United States.
GLOSSARY
- CHD
coronary heart disease
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- ICD-9-CM
International Classification of Diseases–9–clinical modification
- IPR
inpatient rehabilitation
- LIS
low-income cost subsidy
- PDC
proportion of days covered
- RR
relative risk
- SNF
skilled nursing facility
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Karen Albright participated in the design or conceptualization of the study, interpretation of data, and drafted the manuscript. Hong Zhao performed the analysis and participated in revision of the manuscript. Justin Blackburn participated in the design or conceptualization of the study, interpretation of data, and revision of the manuscript. Nita Limdi participated in the design or conceptualization of the study and revision of the manuscript. T. Mark Beasley participated in the design or conceptualization of the study and revision of the manuscript. George Howard participated in the design or conceptualization of the study, interpretation of data, and revision of the manuscript. Vera Bittner participated in the design or conceptualization of the study and revision of the manuscript. Virginia Howard participated in the design or conceptualization of the study, interpretation of data, and revision of the manuscript. Paul Muntner participated in the design or conceptualization of the study, interpretation of data, and revision of the manuscript.
STUDY FUNDING
Supported by the American Heart Association (AHA 14CRP20380256). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHA.
DISCLOSURE
K. Albright, H. Zhao, J. Blackburn, N. Limdi, T. Beasley, G. Howard, V. Bittner, and V. Howard report no disclosures relevant to the manuscript. P. Muntner receives research support from Amgen Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke Statistics: 2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 3.Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–1494. [DOI] [PubMed] [Google Scholar]
- 4.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999;30:338–349. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Bogousslavsky J, Callahan A III, et al. High-dose atorvastatin after stroke or transient ischemic attack. New Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 6.Naci H, Brugts JJ, Fleurence R, Ades AE. Comparative effects of statins on major cerebrovascular events: a multiple-treatments meta-analysis of placebo-controlled and active-comparator trials. QJM 2013;106:299–306. [DOI] [PubMed] [Google Scholar]
- 7.Glader EL, Sjolander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke 2010;41:397–401. [DOI] [PubMed] [Google Scholar]
- 8.Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G. Racial/ethnic, gender gaps in the use and adherence of evidence-based preventive therapies among elderly Medicare part D beneficiaries after acute myocardial infarction. Circulation 2014;129:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen NB, Holford TR, Bracken MB, et al. Trends in one-year recurrent ischemic stroke among the elderly in the USA: 1994–2002. Cerebrovasc Dis 2010;30:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 11.Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 2013;22:1–6. [DOI] [PubMed] [Google Scholar]
- 12.Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 13.Noyes K, Liu H, Lyness JM, Friedman B. Medicare beneficiaries with depression: comparing diagnoses in claims data with the results of screening. Psychiatr Serv 2011;62:1159–1166. [DOI] [PubMed] [Google Scholar]
- 14.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. Identifying atrial fibrillation from electronic medical data: a systematic review. Pharmacoepidemiol Drug Saf 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan H, Li B, Duncan Saunders L, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43:1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with CKD from Medicare claims data: a validation study. Am J kidney Dis 2005;46:225–232. [DOI] [PubMed] [Google Scholar]
- 17.Nau DP. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence [online]. Available at: pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf. Accessed May 30, 2013. [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 19.Bushnell CD, Olson DM, Zhao X, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology 2011;77:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittman DG, Chen W, Bowlin SJ, Foody JM. Adherence to statins, subsequent healthcare costs, and cardiovascular hospitalizations. Am J Cardiol 2011;107:1662–1666. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med 2012;157:785–795. [DOI] [PubMed] [Google Scholar]
- 22.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 23.Meddings J, Kerr EA, Heisler M, Hofer TP. Physician assessments of medication adherence and decisions to intensify medications for patients with uncontrolled blood pressure: still no better than a coin toss. BMC Health Serv Res 2012;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieszk N, Patel R, Heaberlin A, Wlasuk K, Zarowitz B. Detection of medication nonadherence through review of pharmacy claims data. Am J Health Syst Pharm 2003;60:360–366. [DOI] [PubMed] [Google Scholar]
- 25.Levine DA, Morgenstern LB, Langa KM, Piette JD, Rogers MAM, Karve SJ. Recent trends in cost-related medication nonadherence among stroke survivors in the United States. Ann Neurol 2013;73:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–2097. [DOI] [PubMed] [Google Scholar]
- 27.Schmittdiel JA, Nichols GA, Dyer W, Steiner JF, Karter AJ, Raebel MA. Health care system-level factors associated with performance on Medicare STAR adherence metrics in a large, integrated delivery system. Med Care 2015;53:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taitel M, Fensterheim L, Kirkham H, Sekula R, Duncan I.. Medication days' supply, adherence, wastage, and cost among chronic patients in Medicaid. Medicare Medicaid Res Rev 2012;2:mmrr.002.003.a004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J 2013;165:665–678, 678 e661. [DOI] [PubMed] [Google Scholar]
- 30.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–461. [DOI] [PubMed] [Google Scholar]
- 31.Puskas CM, Forrest JI, Parashar S, et al. Women and vulnerability to HAART non-adherence: a literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep 2011;8:277–287. [DOI] [PubMed] [Google Scholar]
- 32.Cooper LA, Roter DL. Patient-provider communication: the effect of race and ethnicity on process and outcomes of healthcare. [online]. Available at: ncbi.nlm.nih.gov/books/NBK220354/. Accessed July 1, 2017. [Google Scholar]
- 33.Martin KD, Roter DL, Beach MC, Carson KA, Cooper LA. Physician communication behaviors and trust among black and white patients with hypertension. Med Care 2013;51:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haskard Zolnierek KB, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care 2009;47:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musa D, Schulz R, Harris R, Silverman M, Thomas SB. Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health 2009;99:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA 2011;305:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogedegbe G, Harrison M, Robbins L, Mancuso CA, Allegrante JP. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis 2004;14:3–12. [PubMed] [Google Scholar]
- 38.Forsyth J, Schoenthaler A, Chaplin WF, Ogedegbe G, Ravenell J. Perceived discrimination and medication adherence in black hypertensive patients: the role of stress and depression. Psychosomatic Med 2014;76:229–236. [DOI] [PubMed] [Google Scholar]
- 39.Young JC, Stürmer T, Lund JL, Funk MJ. Predictors of prevalent statin use among older adults identified as statin initiators based on Medicare claims data. Pharmacoepidemiol Drug Saf 2016;25:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colantonio LD, Kent ST, Kilgore ML, et al. Agreement between Medicare pharmacy claims, self-report, and medication inventory for assessing lipid-lowering medication use. Pharmacoepidemiol Drug Saf 2016;25:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]