Abstract
Objective:
Because the d-2-hydroxyglutarate (D2HG) product of mutant isocitrate dehydrogenase 1 (IDH1mut) is released by tumor cells into the microenvironment and is structurally similar to the excitatory neurotransmitter glutamate, we sought to determine whether IDH1mut increases the risk of seizures in patients with glioma, and whether D2HG increases the electrical activity of neurons.
Methods:
Three WHO grade II-IV glioma cohorts from separate institutions (total N = 712) were retrospectively assessed for the presence of preoperative seizures and tumor location, WHO grade, 1p/19q codeletion, and IDH1mut status. Rat cortical neurons were grown on microelectrode arrays, and their electrical activity was measured before and after treatment with exogenous D2HG, in the presence or absence of the selective NMDA antagonist, AP5.
Results:
Preoperative seizures were observed in 18%–34% of IDH1 wild-type (IDH1wt) patients and in 59%–74% of IDH1mut patients (p < 0.001). Multivariable analysis, including WHO grade, 1p/19q codeletion, and temporal lobe location, showed that IDH1mut was an independent correlate with seizures (odds ratio 2.5, 95% confidence interval 1.6–3.9, p < 0.001). Exogenous D2HG increased the firing rate of cultured rat cortical neurons 4- to 6-fold, but was completely blocked by AP5.
Conclusions:
The D2HG product of IDH1mut may increase neuronal activity by mimicking the activity of glutamate on the NMDA receptor, and IDH1mut gliomas are more likely to cause seizures in patients. This has rapid translational implications for the personalized management of tumor-associated epilepsy, as targeted IDH1mut inhibitors may improve antiepileptic therapy in patients with IDH1mut gliomas.
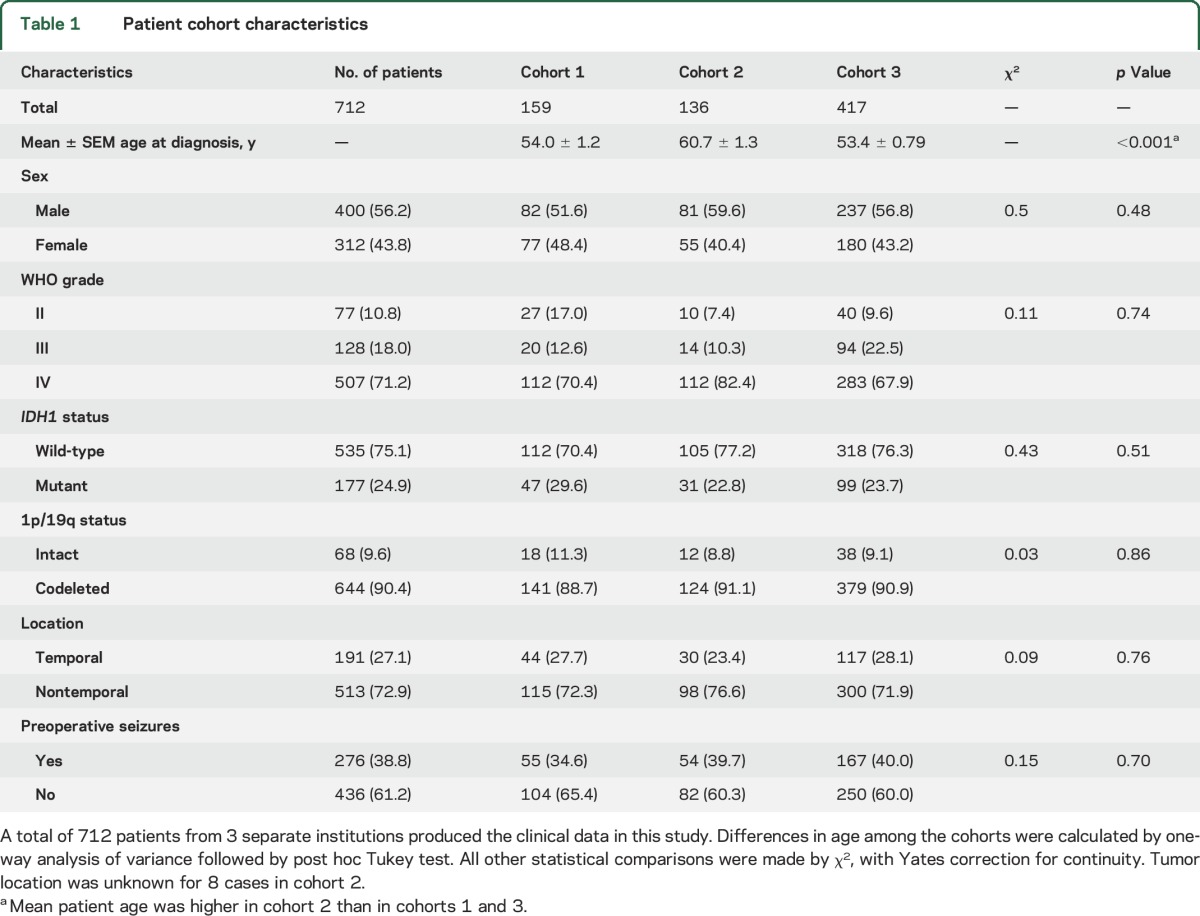
WHO grade II–III gliomas are more likely to induce epilepsy than grade IV glioblastomas (GBMs), though the reason for this is unclear.1 One possible explanation is that the former group of tumors usually have mutations in isocitrate dehydrogenase 1 (IDH1mut), whereas the latter usually do not. IDH1 is a metabolic enzyme located in the cytosol and peroxisomes, catalyzing the oxidative decarboxylation of isocitrate to α-ketoglutarate. In contrast, mutant enzyme reduces α-ketoglutarate to d-2-hydroxyglutarate (D2HG). This is so efficient that the concentration of D2HG can exceed 30 mM in IDH1mut gliomas.2 IDH1mut cells release D2HG into their surroundings in culture, in vivo, and into the CSF of patients with glioma.2,3 Furthermore, infiltrative glioma cells readily migrate along axons and dendrites in the gray and white matter, forming close physical associations with neurons (figure 1, A and B). Thus, IDH1mut glioma cells can expose neurons to D2HG.
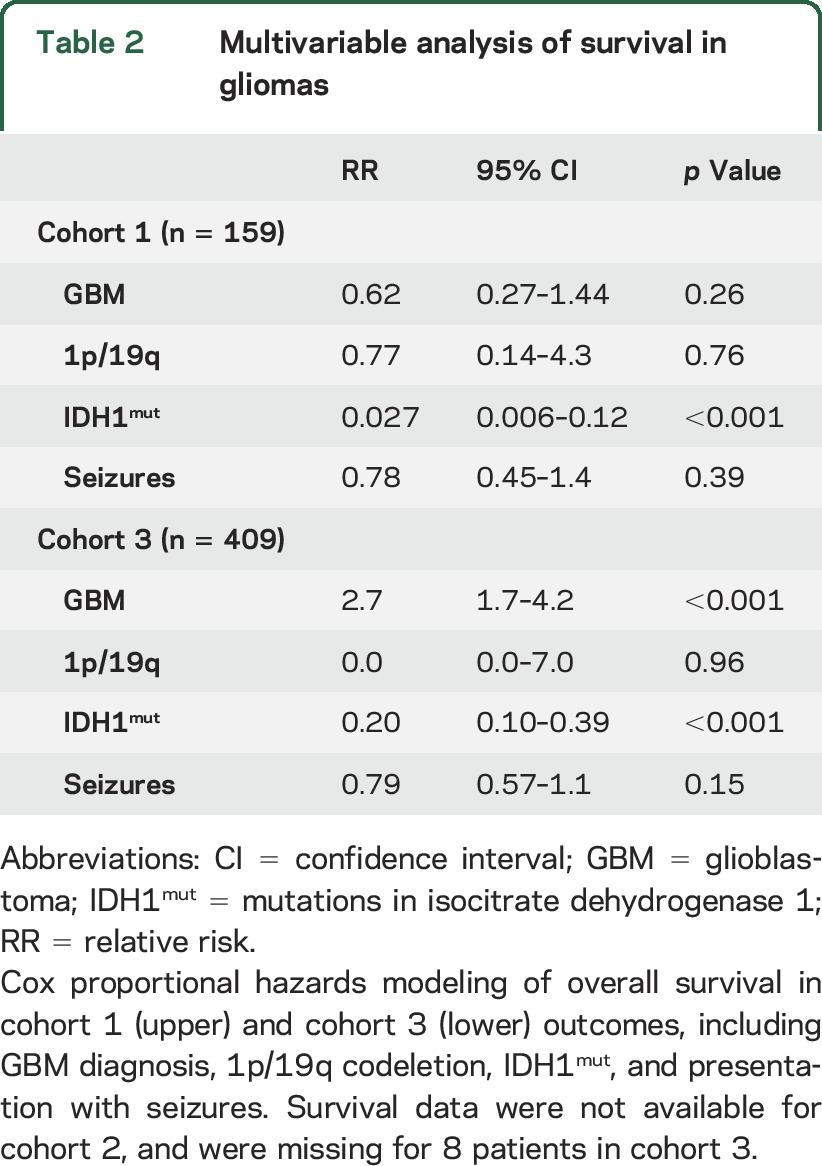
Figure 1. Chemical structure of d-2-hydroxyglutarate (D2HG) and the close relationship of mutations in isocitrate dehydrogenase 1 (IDH1mut) glioma cells to neurons.
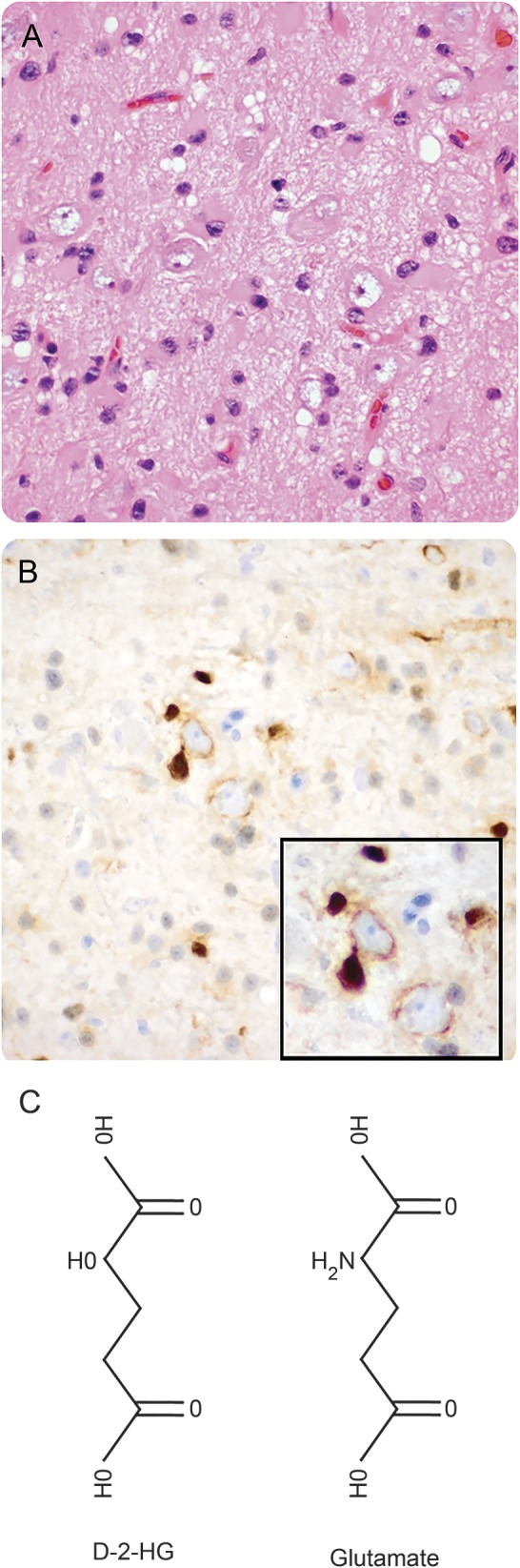
(A) Hematoxylin & eosin section of the left frontal cortex from a 34-year-old man who presented with seizures shows scattered infiltrative oligodendroglioma cells that were highlighted by R132H IDH1 immunohistochemistry (B). Many tumor cells were closely apposed to neurons, even wrapping their processes around the neurons (B, inset). (C) The chemical structure of D2HG, compared with the structure of the excitatory neurotransmitter, glutamate.
The D2HG product of IDH1mut bears a strong structural resemblance to glutamate, the most widespread excitatory neurotransmitter in the CNS (figure 1C). Because glutamate is the primary excitatory neurotransmitter in the brain, its baseline concentration is tightly regulated in the extracellular space around neurons.4 Thus, infiltrative glioma cells releasing a glutamate mimetic could disrupt the balance between inhibition and excitation, and potentially lead to seizures. In this study, we analyzed clinical data from 712 patients representing 3 separate institutions, and examined the effects of D2HG on cortical neuron activity, in order to determine whether there is a link between IDH1mut and seizures in patients with glioma.
METHODS
Patient cohorts.
This was a retrospective cohort study of WHO grade II–IV infiltrative astrocytomas, oligodendrogliomas, and GBM, from patients aged 18 years and older, with known IDH1/2 mutation status (collectively referred to as IDH1mut). Cohort 1 consisted of 159 gliomas that had been tested for IDH1mut as part of routine clinical care at the University of Kentucky (table 1). This cohort included 13 grade II astrocytomas, 14 grade II oligodendrogliomas, 16 grade III astrocytomas, 4 grade III oligodendrogliomas, and 112 GBMs from 2009 to 2014. (Some cases had originally been resected prior to 2009, but for clinical reasons required retrospective testing.) Cohort 2 consisted of 136 gliomas that had been tested for IDH1mut at New York University (NYU) from 2010 to 2014, including 4 grade II astrocytomas, 6 grade II oligodendrogliomas, 10 grade III astrocytomas, 4 grade III oligodendrogliomas, and 112 GBMs. Both of these cohorts have recently been described in a separate study.5 Cohort 3 consisted of 417 gliomas from 2010 to 2016, including 26 grade II astrocytomas, 14 grade II oligodendrogliomas, 70 grade III astrocytomas, 24 grade III oligodendrogliomas, and 283 GBMs with known IDH1mut status from Northwestern Memorial Hospital (NMH). In all 3 cohorts, patient charts were retrospectively reviewed for the presence of any kind of seizures as part of the documented preoperative clinical presentation. A remote history of childhood seizures, or seizures attributed to other causes (e.g., alcohol withdrawal, head trauma), were excluded. Fewer than 1% of all patients with glioma had such a seizure history. To avoid potential therapy-related confounding factors, only seizures in new patients, with no prior surgical or adjuvant therapy, were included. To avoid bias, chart reviewers were blinded to the IDH1mut status of each tumor. Average and maximal follow-up for each patient, from the time of initial surgery, in cohort 1 was 18 and 243 months, respectively. In cohort 3, average and maximal follow-up from the time of initial surgery was 13 and 141 months, respectively. Survival data were obtained in cohort 1 via the Kentucky Cancer Registry, and in cohort 3 by the Northwestern University Tumor Registry, but were not available for cohort 2.
Table 1.
Patient cohort characteristics
Mutation screening was done via R132H IDH1 immunohistochemistry (Dianova, Hamburg, Germany). Immunonegative tumors were tested for less common IDH1/2 mutations via pyrosequencing (University of Kentucky, NMH) or by 450K methylation array profiling (NYU) as described previously.6
Multielectrode array recording in cell culture.
Primary dissociated cortical cultures were prepared from postnatal day 0–1 Sprague-Dawley rat pups (Charles River, Malvern, PA) for multielectrode array (MEA) plates as previously described.7 1.5 × 105 neurons were plated on 12-well MEA plates (Axion Biosystems, Atlanta, GA) precoated with poly-l-lysine (0.5 mg/mL) (Sigma-Aldrich, St. Louis, MO) and laminin (20 μg/mL) (Gibco, Langley, OK). Cultures were maintained in NeuralQ basal medium supplemented with 2% GS21 (MTI-GlobalStem, Gaithersburg, MD) and GlutaMAX (Thermo Fisher Scientific, Waltham, MA) with 1% Penn/Strep (Gibco) at 37°C in 5% CO2. Cytrabine (Sigma-Aldrich) (2.5 μM) was added 4 days after plating.
MEA recordings were performed on DIV 14. Extracellular potentials were recorded at 37°C using Maestro channel amplifier with AxIS software (Axion Biosystems, Atlanta, GA) with 1,200× gain and a 12.5 kHz/channel sampling rate. Plates were placed on the amplifier and equilibrated for 30 minutes before recording. Baseline and treatment measurements were recorded for 10 minutes each. d-α-Hydroxyglutaric acid (Sigma-Aldrich) was prepared in culture media at specified concentrations on the day of recording. dl-2-Amino-5-phosphonopentanoic acid (Tocris, Bristol, UK) (100 μM) was utilized to block NMDA receptors and was added between the baseline and treatment measurements for 10 minutes.
MEA data were analyzed, while blinded to experimental groups, using AxIS software and MATLAB-based Neural Metric Tool (Axion Biosystems). Recordings were passed through a Butterworth bandpass filter (300–5,000 Hz) and spikes were detected using an adaptive threshold crossing (6× SD). Bursts were classified as having a minimum of 5 spikes with a max ISI of 100 milliseconds. Raster plots were generated with Neural Metric Tool.
Developing IDH1mut prediction model.
We used previously described patient cohorts to evaluate whether seizures can improve the accuracy for the prediction of IDH1mut in addition to age, GBM, and history of grade II/III glioma, which were 3 predictors identified in that article.8 For this purpose, we calculated the difference in the area under the receiver operating characteristic curve (ΔAUC) between the logistic regression model with seizures and the later 3 predictors and the logistic regression model only with the later 3 predictors by using the ROCR package of R software version 3.2.3. We also calculated the 95% confidence interval (CI) of ΔAUC by the following bootstrap procedure. We generated 1,000 bootstrap samples of the original data. For each bootstrap sample, we fitted 2 logistic regression models, one with seizures and age, GBM, and history of grade II/III glioma and the other one only with the latter 3 predictors, and calculated the ΔAUC between those 2 models. We then obtained the lower and upper limits of the 95% CI by calculating the 2.5 and 97.5 percentiles of ΔAUC in those 1,000 bootstrap samples.
Statistical evaluation.
Statistical significance of differences between groups was determined via 2-sided t test, one-way analysis of variance with post hoc Tukey test, Fisher exact test, or log-rank test, as appropriate, using GraphPad software (La Jolla, CA). Odds ratios (ORs) were based on exact logistic regression. Multivariable hazard ratios (HRs) were based on Cox proportional hazards models. In all analyses, the significance target was p ≤ 0.05.
Standard protocol approvals, registrations, and patient consents.
Institutional research board approval for the retrospective collection and analysis of deidentified patient data was obtained at University of Kentucky, NYU, and NMH prior to study initiation. Institutional Animal Care and Use Committee approval was obtained from the University of California, Davis, prior to neuronal harvesting and culture.
RESULTS
To determine whether there is an association between IDH1mut and seizures in patients with glioma, we obtained clinical data from 3 separate cohorts of patients, totaling 712 newly diagnosed WHO grade II–IV infiltrative gliomas (table 1). Aside from cohort 2 having a higher mean patient age at presentation, the cohorts were similar in proportions of sex, WHO grades, IDH1mut frequency, 1p/19q codeletion, temporal lobe vs extratemporal tumor location, and patients who presented with seizures. Combining all 3 cohorts, IDH1mut frequency was 80.5% for grade II gliomas, 54.7% for grade III gliomas, and 8.9% for grade IV gliomas.
Current data indicate that testing for IDH1mut and 1p/19q effectively stratifies gliomas into 3 major clinical–pathologic groups: (1) IDH1mut with 1p/19q codeletion (i.e., oligodendroglioma), (2) IDH1mut without 1p/19q codeletion (i.e., astrocytoma), (3) neither IDH1mut nor 1p/19q codeletion (i.e., GBM).9–11 Whole-arm 1p/19q codeletion does not occur in the absence of IDH1mut.12 In all 3 cohorts, IDH1mut gliomas were more likely to present with seizures than gliomas with only wild-type IDH1 (IDH1wt) (figure 2A). This increase in seizure risk was 56.6% in cohort 1 (74.5% vs 17.9%, χ2 44.4, p < 0.001), 32.1% in cohort 2 (64.5% vs 32.4%, χ2 9.0, p = 0.003), and 25.3% in cohort 3 (59.4% vs 34.1%, χ2 17.6, p < 0.001). In all 3 cohorts, there was no difference in seizure frequency between IDH1mut, 1p/19q-codeleted gliomas, and IDH1mut, 1p/19q-intact gliomas (83.3% vs 69.0%, p = 0.32 in cohort 1; 66.7% vs 63.2%, p = 1.0 in cohort 2; 60.5% vs 57.4%, p = 0.84 in cohort 3). On multivariable analysis of other major factors associated with seizure risk, including 1p/19q codeletion, WHO grade, and temporal lobe location, IDH1mut was an independent correlate with seizures (OR 2.5, 95% CI 1.6–3.9, p < 0.001) (table e-1 at Neurology.org).
Figure 2. Seizures and mutations in isocitrate dehydrogenase 1 (IDH1mut) in patients with glioma.
(A) Patients with newly diagnosed infiltrative gliomas from 3 cohorts were assessed according to whether seizures were a part of their presenting symptoms, segregated into the 3 main molecular subgroups of gliomas: wild-type isocitrate dehydrogenase 1 (IDH1wt) (yellow), IDH1mut (white), and IDH1mut with 1p/19q codeletion (blue). (B, C) Overall survival in cohorts 1 (B) and 3 (B), according to IDH1mut and seizures on clinical presentation. p Values were calculated based on log-rank tests between IDH1wt or IDH1mut curves. (Cohort 2 lacked sufficient follow-up data for survival analyses.)
We had previously created a web-based algorithm to help guide the efficient molecular workup of gliomas by providing accurate pretest probability of IDH1mut.8 That model included patient age, history of glioma, and GBM diagnosis, as those were the variables that contributed to the predictive accuracy of the overall model. We found that, in the combined cohorts of that study, incorporating seizures at clinical presentation into the algorithm improved the model's accuracy (△AUC 0.008, 95% CI 0.0004–0.017), further underscoring the relationship between IDH1mut and seizures in patients with glioma.
In cohorts 1 and 3, where survival data were available, there was no difference in median survival among IDH1mut gliomas without and with seizures (undefined vs undefined in cohort 1, HR 2.1, 95% CI 0.29–15.5, p = 0.45; undefined vs undefined in cohort 3, HR 0.64, 95% CI 0.17–2.4, p = 0.51) (figure 2, B and C). Among IDH1wt gliomas, only cohort 3 showed a modest increase in median survival when patients presented with seizures (7.6 vs 12.6 months in cohort 1, HR 1.1, 95% CI 0.66–1.9, p = 0.25; 13.6 vs 18.4 months in cohort 3, HR 1.5, 95% CI 1.1–2.1, p = 0.01). On multivariable analysis, however, only IDH1mut was an independent correlate with overall survival in both cohorts, not seizures (IDH1mut relative risk [RR] 0.027 and 0.20, 95% CI 0.006–0.12 and 0.57–1.1, p < 0.001 and <0.001; seizures RR 0.78 and 0.79, 95% CI 0.45–1.4 and 0.57–1.1, p = 0.39 and 0.15) (table 2).
Table 2.
Multivariable analysis of survival in gliomas
We found that exogenous D2HG markedly increased the in vitro electrical activity of rat cortical neurons (figure 3, A and B, video). D2HG specifically increased the duration of synchronized bursts of firing in the neuronal networks (figure 3C), a characteristic effect of epileptogenic agents.13,14 Along with an increase in network burst duration, D2HG decreased network burst frequency (figure 3D). IDH1mut gliomas contain up to 30 mM D2HG, cells expressing IDH1mut release D2HG into their environment, and elevated D2HG has been detected in the circulating CSF of IDH1mut patients with glioma.2,3 Infiltrative glioma cells readily migrate along axons and dendrites in the gray and white matter, forming close physical associations with neurons (figure 1). Thus, IDH1mut glioma cells are poised to expose neurons to very high local concentrations of D2HG. We found a dose-response relationship between D2HG and neuronal activity, with increased activity detectable at 3 and 10 mM (figure 3E). The selective NMDA receptor antagonist, (2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate (AP5), completely blocked the excitatory effect of D2HG on neurons (figure 3F).
Figure 3. Effects of d-2-hydroxyglutarate (D2HG) on neuronal activity.
(A, B) Raster plots of cultured rat cortical neurons, before (A) and after (B) treatment with 10 mM D2HG. (C, D) Network burst duration (C) and frequency (D), before and after treatment with 10 mM D2HG. (E) Neuronal activity, expressed as mean firing rate relative to baseline, in the presence of increasing concentrations of D2HG. p = 0.003 Via one-way analysis of variance, *p < 0.05 vs 0 and 1 mM via post hoc Tukey test. (F) Mean firing rate of cultured neurons with control vehicle (0 mM D2HG), 10 mM D2HG, or 10 mM D2HG + 100 µM AP5. ***p < 0.001. (C, D, F) Seven wells per condition from 2 biological replicates; E shows 5 wells per condition from 2 biological replicates.
DISCUSSION
Seizures are a common manifestation of infiltrative gliomas. Over 50% of patients with an infiltrative glioma will experience at least one seizure during the course of their disease, and over 30% will develop tumor-associated epilepsy (TAE), defined as multiple seizures.15,16 This is a major cause of tumor-associated morbidity and greatly diminishes quality of life. At least 10% of patients with glioma will develop status epilepticus, a life-threatening condition wherein a seizure lasts longer than 5 minutes, or when multiple seizures occur without regaining consciousness.1 TAE is managed by a combination of antitumor therapies (including surgical resection, chemotherapy, and radiation), as well as antiepileptic drugs (such as lamotrigine, levetiracetam, and valproate). Even multimodal strategies fail to control seizures in up to a third of patients.17 A better understanding of the risk factors and mechanisms associated with TAE are therefore needed in order to improve patient quality of life.
The relationship between IDH1mut and seizures in patients with glioma has been controversial.1,10,18–26 A direct mechanistic link between the D2HG product of IDH1mut and neuronal activity strengthens the hypothesis that IDH1mut gliomas are more likely to induce TAE than IDH1wt gliomas. Glutamate binds to and activates multiple receptors in neurons, including ligand-gated NMDA and AMPA ion channels, allowing influx of Na2+, K2+, and sometimes Ca2+ ions. Depolarization caused by glutamate thus increases the likelihood that a neuron will fire an action potential. In an artificial model of human embryonic kidney cells expressing NMDA or AMPA receptors, D2HG bound and activated ionotropic NMDA receptors, but not AMPA receptors.27 In the current study, we directly tested the effect of D2HG on cultured neurons using MEAs, which are widely used to measure neuronal activity in vitro.28 Unlike intracellular recordings that are limited to measurement of electrophysiologic activity in a single cell for a short duration, MEAs allow for noninvasive, simultaneous, and long-term recordings of local field potentials and extracellular action potentials from a population of neurons within a millisecond time scale.28 MEAs can quantitatively measure properties of electrical activity across neuronal networks that are altered when changes in the inhibitory/excitatory balance lead to seizures.29–31 Our data demonstrate that D2HG may activate neuronal NMDA receptors, thereby promoting the same kind of heightened network synchrony that is characteristic of seizure-inducing agents (figure 3, video).
A direct excitatory role for the D2HG product of IDH1mut helps explain a number of patterns regarding epilepsy in gliomas. First, older studies reported that oligodendrogliomas are more likely to cause seizures than those of astrocytic lineage.32–34 Our data suggest that this is because the molecular profile of true oligodendrogliomas necessarily includes IDH1mut, whereas the molecular profile of astrocytic gliomas (including GBM) is more heterogeneous. Second, seizures are more common in lower-grade gliomas than in GBM, even though the latter are more invasive and destructive.1,35 Our multivariable data show that IDH1mut is a significant correlate of seizures, independent of WHO grade (figure 2A, table e-1). Third, presenting with seizures is commonly thought to correlate with longer overall survival in patients with glioma, possibly because such tumors are detected earlier.16 However, IDH1mut gliomas are much less aggressive than those harboring only IDH1wt,36 raising the possibility that seizures at clinical presentation only predict longer survival to the extent that they are linked with IDH1mut. Our data suggest that the association between seizures at clinical presentation and better prognosis is driven mostly by the intrinsic biological differences between IDH1mut and IDH1wt gliomas, rather than by seizures prompting earlier clinical intervention (figure 2, B and C, and table 2).
Although seizures were more common in IDH1mut gliomas, a sizeable proportion of IDH1wt gliomas also caused pretherapy seizures (figure 2A). This underscores the fact that tumors can cause seizures by a variety of mechanisms, such as abnormal regulation of excitatory and inhibitory receptors, altered expression of ion transporters, increased mTOR activity, and inflammation.37,38 Our data suggest that the production of D2HG, which is a central, defining feature of IDH1mut gliomas, may be another seizure-inducing mechanism.
While these findings suggest a mechanistic link between IDH1mut, its D2HG product, and glioma-induced seizures, there are some limitations to this study. First, although the clinical data consist of a large number of patients from 3 separate institutions, it is a retrospective study and the presence of seizures is based on history-taking and subjective clinical impressions, not EEG data. Second, while MEA studies firing patterns among interconnected neurons over long periods of time, and high-density in vitro neuronal networks show synapse-specific modifications similar to what is seen in vivo,39 single electrode approaches in low-density neuronal culture allow for the detailed study of D2HG-evoked currents. It will therefore be necessary to extend the current findings in future studies, using prospective patient-based analyses, single-electrode recordings, and in vivo models, in order to more fully understand the effects of D2HG on neuronal activity and epilepsy.
To date, the majority of glioma research on D2HG and IDH1mut has focused on their effects within the tumor cell. Our data indicate that IDH1mut may also exert a considerable effect on surrounding non-neoplastic cells, including neurons, through the release of its D2HG oncometabolite into the tumor microenvironment. In a preclinical model of IDH1mut glioma, pharmacologic inhibition of IDH1mut effectively lowered D2HG production and showed promising effects against tumor growth.40 Currently, there are multiple clinical trials aimed at evaluating IDH1mut inhibitors in gliomas, including AG-120 (NCT02073994), AG-881 (NCT02481154), BAY1436032 (NCT02746081), and IDH305 (NCT02381886). To our knowledge, none of these trials include seizure monitoring as a primary or secondary endpoint. Yet, even if none of these drugs effectively controls glioma growth, they might help control TAE in patients with IDH1mut gliomas, either as a monotherapy or in conjunction with other antiepileptic drugs, by directly blocking the production of D2HG (figure 4).
Figure 4. Proposed proseizure effect of isocitrate dehydrogenase 1 (IDH1mut) glioma cells.
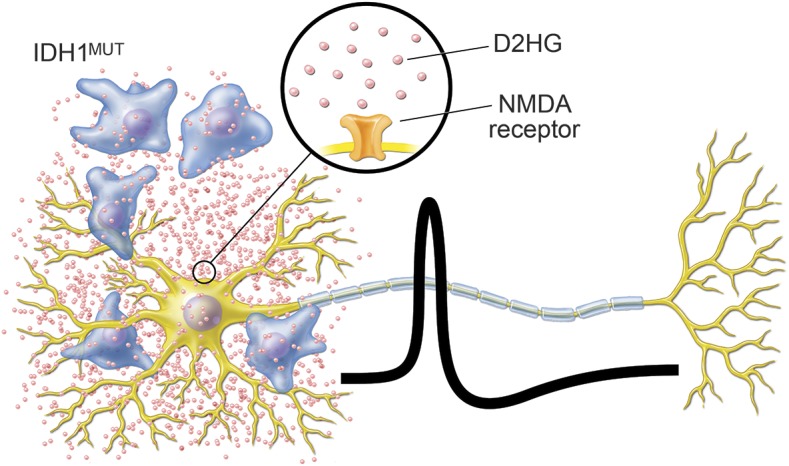
IDH1mut glioma cells infiltrate gray matter and track along neurons. The d-2-hydroxyglutarate (D2HG) product of the mutant IDH1 enzyme is released by tumor cells and activates neuronal NMDA receptors, thereby creating excitatory postsynaptic potentials and increasing the likelihood of action potentials.
ACKNOWLEDGMENT
The authors thank Michael Gallagher for figure 4 and Jayne Jedlicka (Northwestern University Tumor Registry) and Bernice Slone (Kentucky Cancer Registry) for outcome data.
GLOSSARY
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- D2HG
d-2-hydroxyglutarate
- GBM
glioblastoma
- HR
hazard ratio
- IDH1mut
mutations in isocitrate dehydrogenase 1
- IDH1wt
wild-type isocitrate dehydrogenase 1
- MEA
multielectrode array
- NMH
Northwestern Memorial Hospital
- NYU
New York University
- OR
odds ratio
- RR
relative risk
- TAE
tumor-associated epilepsy
Footnotes
Supplemental data at Neurology.org
Editorial, page 1782
AUTHOR CONTRIBUTIONS
Hao Chen performed the in vitro neuronal experiments and assisted with the manuscript. Pamela Lein designed the in vitro experiments. Laith Khoury collected patient data from University of Kentucky. Cheddhi Thomas, Carolina Benjamin, Donato Pacione, John Golfinos, and Matija Snuderl collected patient data from NYU. Jonathon Judkins, Farhad Ghamsari, and Priya Kumthekar collected patient data from NMH. Meijing Wu performed biostatistical analyses on all 3 patient cohorts. Li Chen provided the IDH1mut prediction modeling. Dane Chetkovich assisted with the manuscript. Craig Horbinski conceived the project, designed the study, and wrote the manuscript.
STUDY FUNDING
C.H. was supported by the National Cancer Institute (K08CA155764). The methylation profiling of brain tumors at NYU is supported by The Friedberg Charitable Foundation.
DISCLOSURE
H. Chen, J. Judkins, C. Thomas, M. Wu, L. Khoury, C. Benjamin, D. Pacione, J. Golfinos, P. Kumthekar, F. Ghamsari, L. Chen, P. Lein, D. Chetkovich, and M. Snuderl report no disclosures relevant to the manuscript. C. Horbinski was supported by the National Cancer Institute (K08CA155764). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia 2013;54(suppl 9):12–17. [DOI] [PubMed] [Google Scholar]
- 2.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalinina J, Ahn J, Devi NS, et al. Selective detection of the d-enantiomer of 2-hydroxyglutarate in the CSF of glioma patients with mutated isocitrate dehydrogenase. Clin Cancer Res (in press 2016). [DOI] [PMC free article] [PubMed]
- 4.Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 2011;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012;22:425–437. [DOI] [PubMed] [Google Scholar]
- 7.Cotterill E, Hall D, Wallace K, Mundy WR, Eglen SJ, Shafer TJ. Characterization of early cortical neural network development in multiwell microelectrode array plates. J Biomol Screen 2016;21:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol 2014;16:1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372:2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015;372:2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 2015;129:133–146. [DOI] [PubMed] [Google Scholar]
- 12.Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology 2010;74:1886–1890. [DOI] [PubMed] [Google Scholar]
- 13.Jiruska P, Csicsvari J, Powell AD, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci 2010;30:5690–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truccolo W, Ahmed OJ, Harrison MT, et al. Neuronal ensemble synchrony during human focal seizures. J Neurosci 2014;34:9927–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007;6:421–430. [DOI] [PubMed] [Google Scholar]
- 16.Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer 1998;34:98–102. [DOI] [PubMed] [Google Scholar]
- 17.Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist 2014;19:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Wang Z, Wang Y, You G, Jiang T. IDH1/2 mutation is associated with seizure as an initial symptom in low-grade glioma: a report of 311 Chinese adult glioma patients. Epilepsy Res 2015;109:100–105. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Bai HX, Wang Z, Jiang Y, Yang L. Association of IDH1/2 mutation with preoperative seizure in low-grade gliomas: how strong is the evidence? Epilepsy Res 2015;112:154–155. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Zhang Z, Wang Y, You G, Jiang T. Response to “Association of IDH1/2 mutation with preoperative seizure in low-grade gliomas: how strong is the evidence?” Epilepsy Res 2015;115:145–146. [DOI] [PubMed] [Google Scholar]
- 21.Liubinas SV, D'Abaco GM, Moffat BM, et al. IDH1 mutation is associated with seizures and protoplasmic subtype in patients with low-grade gliomas. Epilepsia 2014;55:1438–1443. [DOI] [PubMed] [Google Scholar]
- 22.Morokoff A, Liubinas S. In response: IDH1 mutation expression is associated with seizures and protoplasmic subtype in patients with low-grade glioma. Epilepsia 2014;55:1679–1680. [DOI] [PubMed] [Google Scholar]
- 23.Gonen T, Grossman R, Sitt R, et al. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J Neurosurg 2014;121:1133–1138. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan L, Ryan E, O'Brien M, et al. Genetic features of oligodendrogliomas and presence of seizures: the relationship of seizures and genetics in LGOs. Clin Neuropathol 2014;33:292–298. [DOI] [PubMed] [Google Scholar]
- 25.Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 2012;21:194–197. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, You G, Zhang W, et al. Correlation of preoperative seizures with clinicopathological factors and prognosis in anaplastic gliomas: a report of 198 patients from China. Seizure 2014;23:844–851. [DOI] [PubMed] [Google Scholar]
- 27.Kolker S, Pawlak V, Ahlemeyer B, et al. NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur J Neurosci 2002;16:21–28. [DOI] [PubMed] [Google Scholar]
- 28.Obien ME, Deligkaris K, Bullmann T, Bakkum DJ, Frey U. Revealing neuronal function through microelectrode array recordings. Front Neurosci 2014;8:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters Ca(2)(+) dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicol Sci 2012;130:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Z, Zou X, Cui Y, et al. Rapid throughput analysis demonstrates that chemicals with distinct seizurogenic mechanisms differentially alter Ca2+ dynamics in networks formed by hippocampal neurons in culture. Mol Pharmacol 2015;87:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinette BL, Harrill JA, Mundy WR, Shafer TJ. In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Front Neuroeng 2011;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg 2008;108:227–235. [DOI] [PubMed] [Google Scholar]
- 33.Whittle IR, Beaumont A. Seizures in patients with supratentorial oligodendroglial tumours: clinicopathological features and management considerations. Acta Neurochir 1995;135:19–24. [DOI] [PubMed] [Google Scholar]
- 34.Lebrun C, Fontaine D, Ramaioli A, et al. Long-term outcome of oligodendrogliomas. Neurology 2004;62:1783–1787. [DOI] [PubMed] [Google Scholar]
- 35.Ruda R, Soffietti R. What is new in the management of epilepsy in gliomas? Curr Treat Options Neurol 2015;17:351. [DOI] [PubMed] [Google Scholar]
- 36.Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol 2013;125:621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med 2011;17:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huberfeld G, Vecht CJ. Seizures and gliomas: towards a single therapeutic approach. Nat Rev Neurol 2016;12:204–216. [DOI] [PubMed] [Google Scholar]
- 39.Jimbo Y, Tateno T, Robinson HP. Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons. Biophysical J 1999;76:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013;340:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]




