Abstract
Objective:
To evaluate whether the axonal protein neurofilament light (NFL) in serum is a sensitive biomarker to detect subtle brain injury or concussion in contact sports athletes.
Methods:
Two prospective cohort studies involving (1) 14 Swedish amateur boxers who underwent fluid biomarker assessments at 7–10 days after bout and after 3 months of rest from boxing and (2) 35 Swedish professional hockey players who underwent blood biomarker assessment at 1, 12, 36, and 144 hours after concussion and when the players returned to play were performed. Fourteen healthy nonathletic controls and 12 athletic controls were also enrolled. Serum NFL was measured using ultrasensitive single molecule array technology.
Results:
Serum NFL concentrations were increased in boxers 7–10 days after bout as compared to the levels after 3 months rest as well as compared with controls (p = 0.0007 and p < 0.0001, respectively). NFL decreased following 3 months of rest, but was still higher than in controls (p < 0.0001). Boxers who received many (>15) hits to the head or were groggy after bout had higher concentrations of serum NFL as compared to those who received fewer hits to the head (p = 0.0023). Serum NFL increased over time in hockey players, and the levels returned to normal at return to play. Importantly, serum NFL could separate players with rapidly resolving postconcussion symptoms (PCS) from those with prolonged PCS.
Conclusions:
The results from these 2 independent cohort studies suggest that serum NFL is a highly sensitive biomarker for concussion.
Mild traumatic brain injury (mTBI) or concussion in participants practicing contact sports, such as American football, boxing, and hockey, is an increasing concern.1 For athletes and nonathletes who have mTBI, postconcussion symptoms (PCS) usually resolve within days; however, in 10%–15%, PCS may persist for months to years.2
At present, there are no valid fluid or imaging biomarkers for diagnosis or prognosis of mTBI.3,4 Axonal injury with cytoskeletal damage and redistribution of neurofilament proteins have been hypothesized to be the key type of damage and the primary determinant of outcome following mTBI.5,6 Neurofilament light (NFL) is CNS-enriched protein, predominantly expressed in the long myelinated subcortical axons.7 Measurement of NFL in CSF of boxers after bout have been shown to correlate with the severity of brain injury.6,8 However, CSF biomarker analysis requires lumbar puncture and is therefore difficult to implement in routine evaluations of athletes. In contrast, blood samples are easily accessible. Thus, blood biomarkers for mTBI would be of great clinical value.
With the above in mind, we recently developed an immunoassay on the single molecule array (Simoa) platform for ultrasensitive quantification of NFL in both plasma and serum.9–11 In the context of traumatic brain injury (TBI), we measured serum NFL with our newly developed assay from patients with severe TBI, where the levels correlated with diffusion tensor imaging (DTI) measures of diffuse axonal injury12 and CSF NFL, suggestive of serum NFL reflecting axonal damage in the brain.9 We therefore hypothesized that serum NFL may serve as a blood biomarker for mTBI.
METHODS
Study population.
For the first longitudinal cohort study, we enrolled 14 amateur boxers who were sampled 7–10 days after boxing and after 3 months of rest from boxing and sparring and 14 neurologically healthy age-matched controls without history of brain trauma or participation in contact sports. For comparison with athletes without head impact, we also analyzed NFL in serum samples (n = 12) of gymnasts.
In addition, we assessed serum NFL in a cohort of concussed professional ice hockey players (n = 35) competing in the Swedish Hockey League (SHL) who underwent repeated blood sampling. The players were diagnosed and managed according to the latest clinical guidelines on sports-related concussion.13 Details on this study have been presented previously.14
Standard protocol approval, registrations, and patient consents.
The regional ethics committee at the University of Gothenburg, Sweden, approved the study. Written informed consent was obtained from all participants.
Severity of brain injury.
The boxers were interviewed at 7–10 days after bout for assessing the number and severity of hits to the head. The severity of the brain injury was assessed by either the number of punches each boxer received to the head or grogginess. No boxer was knocked out.
Severity of injury in hockey players was assessed according to the latest guidelines on the management of sports-related concussion, which is based on the duration of PCS, and a graduated return to play (RTP) protocol, aiming at RTP within 6 days.13,14
Fluid sampling and biochemical procedures.
Blood samples were collected by venipuncture into EDTA tubes for serum and centrifuged within 20–60 minutes. CSF was collected in polypropylene tubes by lumbar puncture (LP) through the L3-4 or L4-5 interspace. All samples were aliquoted and stored at −80°C pending analysis. The participants were examined physically and neurologically prior to sampling, and all were healthy, and had no contradictions to LP. In the cohort of boxers, sampling of serum and CSF was performed at the same occasion both 7–10 days after boxing and following a 3-month resting period without sparring or boxing. The 7- to 10-day time point was considered as the optimal time point for detecting a change in NFL levels as a result of the bout, based on the kinetics of NFL in CSF.6 The hockey players who sustained a concussion underwent blood sampling at 1, 12, 36, and 144 hours after concussion, and players with PCS lasting longer than 6 days were also sampled on the day of RTP.
NFL concentrations in serum were measured using the Simoa platform (Quanterix, Lexington, MA), a magnetic bead-based digital ELISA that allows detection of proteins at subfemtomolar concentrations,15 and an analytical protocol previously described in detail.9 Limit of detection (LOD) for the NFL assay was 0.29 pg/mL and lower limit of quantification (LLOQ) was 2.7 pg/mL when compensated for a 4-fold sample dilution. LOD and LLOQ were determined by mean blank signal +3 SD and +10 SD, respectively. Average intra-assay duplicate coefficient of variation for the samples was 6.5% (SD 8.6%).
CSF NFL concentrations were measured using a commercial ELISA (NF-light ELISA, UmanDiagnostics, Umeå, Sweden) as described previously.16 The CSF results have been reported in a previous publication.6
All samples were analyzed at the same time using the same batch of reagents by board-certified laboratory technicians who were blind to clinical information.
Statistical analysis.
The χ2 test was used to examine differences in categorical variables between the patients with mTBI vs controls. The Spearman rank correlation examined the relationship between changes in NFL levels and age as well as CSF NFL. For the comparisons of serum NFL concentrations vs controls, the Mann-Whitney U test was used. The Wilcoxon signed-rank test was used for comparison between NFL levels at 7–10 days after a bout and the levels at 3 months rest. The area under the receiver operating characteristic curve (AUC) was calculated for assessing the diagnostic accuracy of serum NFL for mTBI. All tests were 2-sided and statistical significance was determined at p < 0.05. All statistical calculations were performed using GraphPad Prism 6.0 (GraphPad Inc., San Diego, CA).
RESULTS
Demographic characteristics of the boxers.
Fourteen boxers (median age 21.5 years, interquartile range [IQR] 20–26 years) and 14 neurologically healthy nonathletic controls (median age 23.5 years, IQR 23–26 years) were enrolled (table e-1 at Neurology.org). One of the boxers declined to undergo a follow-up sampling at 3 months. For further comparison, we also enrolled athletic controls without direct impact: a group of gymnasts (median age 19 years, IQR 18–22 years, all female). However, there was no difference in the levels of serum NFL between nonathletic controls (median 9.0 pg/mL, IQR 7–14 pg/mL) and gymnasts (median 8.5 pg/mL, IQR 7–12; p = 0.90), and the latter group was therefore excluded from further statistical analyses. There was no significant difference in age and sex between the boxers and controls (table e-1). There was no correlation between serum NFL levels and age (r = −0.04, p = 0.90).
Boxing is associated with axonal injury.
The concentrations of NFL in all serum samples from boxers were variable and elevated (median 22.0 pg/mL, IQR 18–34 pg/mL) as compared to the controls (median 9.0 pg/mL, IQR 7–14 pg/mL; p < 0.0001) (table e-1). Serum NFL concentrations were increased in boxers 7–10 days after bout as compared to the levels at 3 months rest and to levels in controls (p = 0.0007 and p < 0.0001, respectively) (figure 1, A and B). Serum levels of NFL decreased at 3 months rest (median 18.0 pg/mL, IQR 16–24 pg/mL), but were still higher than in controls (median 9.0 pg/mL, IQR 7–14 pg/mL; p < 0.0001) (figure 1B). In addition, serum NFL concentration in boxers showed a strong, positive correlation with correspondent CSF NFL concentration (r = 0.86, p = 0.0003) (figure 1C).
Figure 1. Boxing is associated with increased serum neurofilament light (NFL).
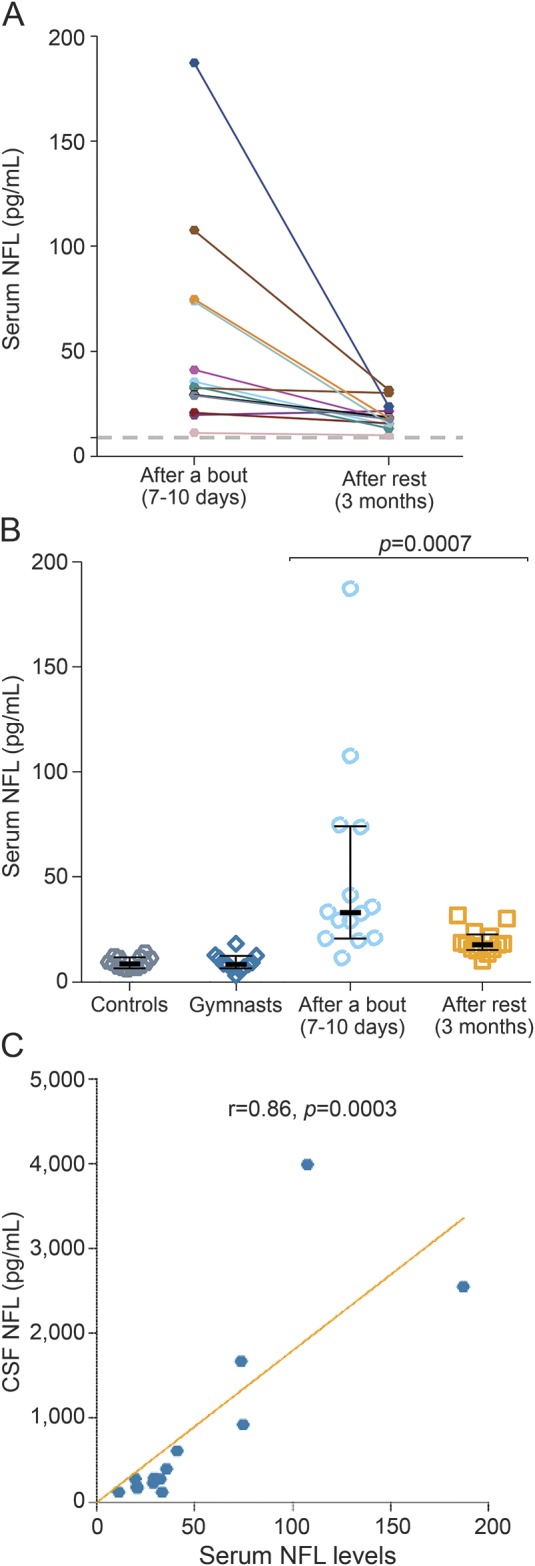
(A) Longitudinal serum NFL changes in boxers. (B) Boxers had elevated levels of serum NFL 7–10 days after a bout compared to the levels at 3 months rest, as well as compared to controls. (C) The levels of serum NFL were related to NFL in CSF from the same individuals. Values are presented as medians; error bars indicate interquartile range. The horizontal dotted line in (A) shows the median of the controls.
Serum NFL correlated with injury severity in boxers.
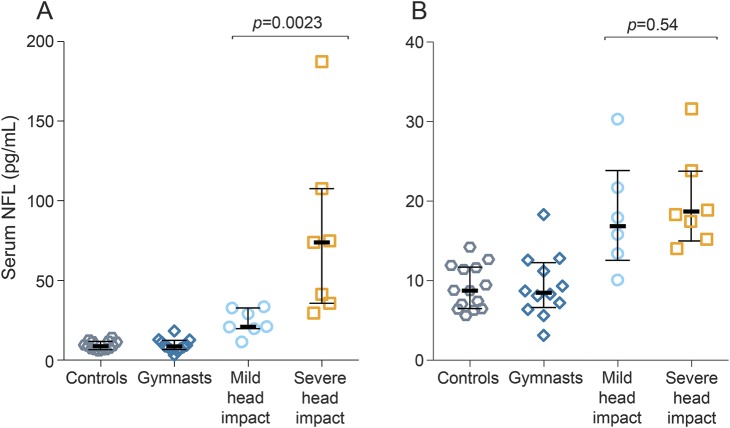
We dichotomized the severity of head impacts in boxers into those who received severe head impact (more than 15 punches or feeling groggy during or after bout) or those who received mild head impact (fewer than 15 punches to the head) (table e-2). Boxers who received severe head impact had higher levels of serum NFL as compared to those who received fewer hits to the head (p = 0.0023) (figure 2A). Also, after 3 months rest, the concentrations of NFL were higher in boxers who had received severe head impacts compared with boxers who had received fewer hits, although the difference was not significant (p = 0.54; figure 2B). However, both the boxers who received severe head impact and those who received milder or fewer hits had increased concentrations of serum NFL at 3 months as compared to controls, with the highest levels observed in the former group (p = 0.0015 and p < 0.0001, respectively) (figure 2B).
Figure 2. Serum neurofilament light (NFL) correlated with injury severity in boxers.
(A) Boxers who received severe head impact (more than 15 hits to the head or experienced grogginess during or after bout) had elevated NFL at 7–10 days after a bout compared to boxers who received mild head impact (fewer than 15 head hits). (B) Also, at 3 months rest, the levels of serum NFL were elevated in boxers who received severe head impact compared to those who received fewer, but not significant. Values are presented as medians; error bars indicate interquartile range.
Diagnostic accuracy of serum NFL in boxers.
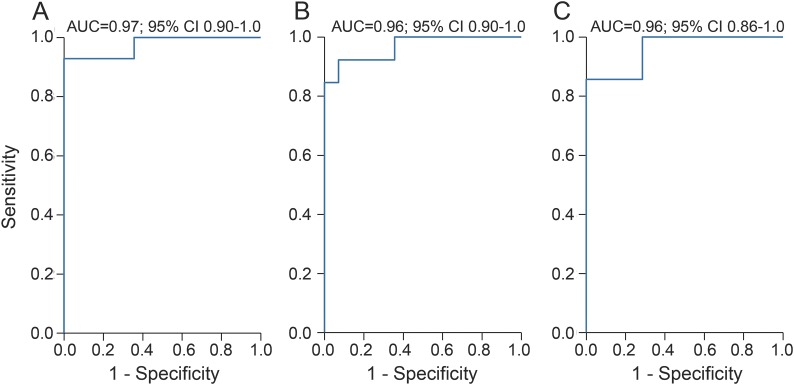
We assessed the diagnostic accuracy of serum NFL vs controls by assessing the AUC. Serum NFL measured at 7–10 days and at 3 months rest vs controls yielded an AUC of 0.97 and 0.96, respectively (figure 3, A and B). Further, serum NFL measured 7–10 days after a bout could separate boxers who received severe head impact from those receiving milder or fewer hits with an AUC of 0.97 (figure 3C).
Figure 3. Diagnostic accuracy of serum neurofilament light (NFL) in boxers.
(A) Serum NFL levels measured 7–10 days after bout vs controls. (B) Serum NFL measured after 3 months rest from boxing vs controls. (C) Serum NFL measured 7–10 days after bout in those who received severe head impact (more than 15 hits to the head or experienced grogginess during or after bout) compared with those who received mild head impact (fewer than 15 head hits). AUC = area under the receiver operating characteristic curve; CI = confidence interval.
Elevated serum NFL concentrations in concussed hockey players.
To validate the findings in the cohort of boxers, we further assessed the diagnostic and prognostic utility of serum NFL in concussed professional hockey players. Of 35 players who sustained a concussion during the first half of the 2012–2013 season in the SHL, 28 consented to undergo repeated blood sampling. Thirteen of the concussed players became symptom-free within a few days of their injury, but in 15 players, symptoms persisted for 6 days or longer. Players with prolonged RTP exhibited persistent symptoms that included cognitive impairment, headache, insomnia, irritability, and mood swings. Two of the players also sustained loss of consciousness, which lasted for less than a minute, and as long as 17 minutes, respectively. One of the players was forced to retire from the game due to persistent PCS for more than 90 days. The median age of the hockey players was 27 years (IQR 23–31 years) as compared to the controls (median 23.5 years, IQR 23–26 years; p = 0.10).
Overall, serum NFL levels were higher in all postconcussion samples (1–144 hours) from hockey players as compared with controls (p = 0.035; figure 4A). Serum concentrations of NFL increased rapidly and in a prolonged fashion in concussed professional hockey players, with the highest levels measured at 144 hours after concussion (figure 4B). The levels of serum NFL normalized (to the same levels as controls) at RTP in all the players except for one, who also had loss of consciousness for 17 minutes and who returned to play after 3 months.
Figure 4. Elevated serum neurofilament light (NFL) in concussed professional hockey players.
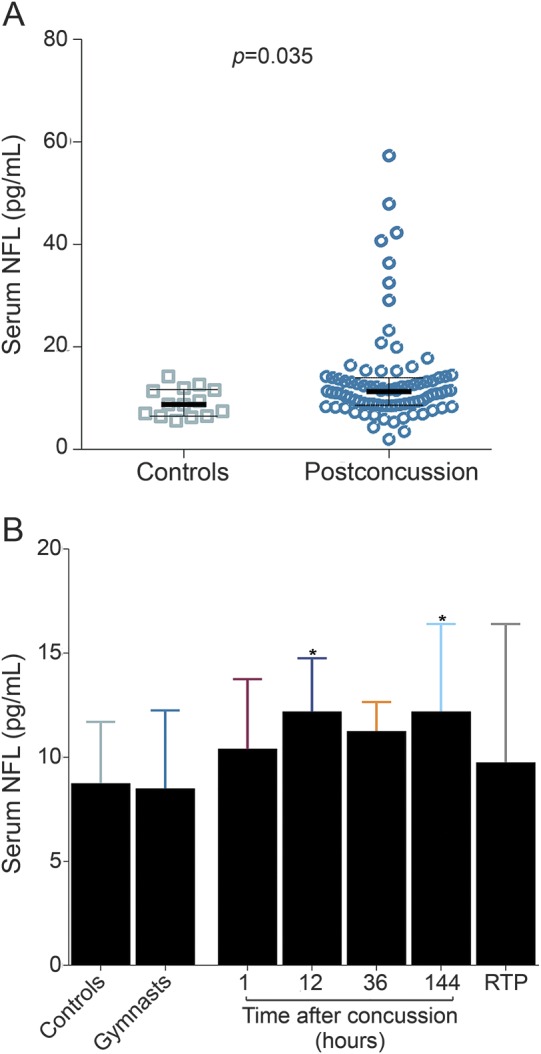
(A) Serum NFL concentrations in postconcussion samples (1–144 hours) and controls. (B) Serum NFL was higher at 12 and 144 hours after concussion as compared to controls (p = 0.036 and p = 0.045, respectively). Values are presented as medians; error bars indicate interquartile range. RTP = return to play.
Serum NFL and severity of concussion in hockey players.
To assess the relationship between longitudinal measures of serum NFL and the persistence of PCS, we compared biomarker levels between concussed players with RTP within 6 days vs players with RTP greater than 6 days (table e-3). Serum NFL measured between 1 and 144 hours after concussion was essentially unchanged in players with rapidly resolving PCS (≤6 days) as compared to controls. In contrast, in players with prolonged PCS (>6 days), serum NFL levels remained elevated from 1 to 144 hours as compared to controls (p = 0.010–0.030). In addition, serum NFL measured at 1 and 36 hours after concussion could differentiate players with rapidly resolving PCS from those with prolonged PCS (p = 0.006 and p = 0.02, respectively; table e-3).
Diagnostic accuracy of serum NFL for concussion in hockey players.
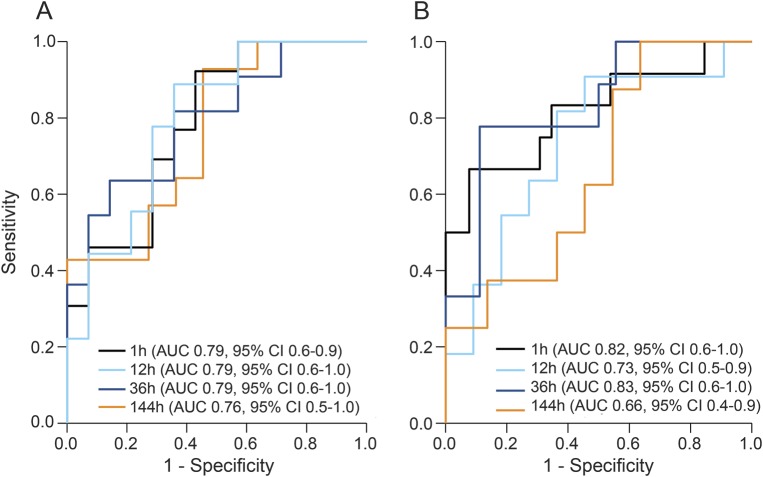
To assess the diagnostic accuracy of serum NFL, the AUC was analyzed comparing different levels of serum NFL at different time points vs controls. Overall, serum NFL between 36 and 144 hours after concussion vs controls yielded an AUC of 0.67–0.71. Serum NFL showed greater diagnostic accuracy for players with persistent PCS for more than 6 days vs controls at 1 hour to 144 hours (AUC 0.79–0.74; figure 5A). Also, serum NFL measured at both 1 and 36 hours after concussion could separate players with rapidly resolving PCS from players with prolonged PCS (AUC 0.82 and 0.83, respectively) (figure 5B).
Figure 5. Diagnostic accuracy of serum neurofilament light (NFL) for concussion in hockey players.
(A) Area under the receiver operating characteristic curve (AUC) for serum NFL measured at different time points postconcussion in players with postconcussion symptoms (PCS) >6 days vs controls. (B) AUC for serum NFL measured at different time points postconcussion in players with persistent PCS ≤6 days vs PCS >6 days. CI = confidence interval.
DISCUSSION
In the present study, amateur boxers had a marked increase in serum NFL 7–10 days after bout. Levels were clearly higher than in controls as well as in nonconcussed gymnasts. Importantly, serum NFL dropped towards normalization after a 3-month period of rest from boxing in all boxers, and correlated with severity of head impacts during the bout. Furthermore, as shown before in other brain disorders, serum levels of NFL correlated strongly with CSF levels in paired samples from the same individuals.17 Serum NFL could separate boxers from controls as well as boxers with severe head impact or many hits to the head vs mild head impact with AUC of 0.97, respectively. In the cohort of professional hockey players, serum NFL concentrations were increased in postconcussion samples as compared to controls. Serum NFL increased rapidly after concussion, with the highest levels measured 144 hours after concussion, and the levels normalized when the athletes returned to play. Importantly, NFL could separate players with rapidly resolving PCS from those with prolonged PCS.
The finding that NFL increases in serum of boxers after bout is in agreement with 2 previous studies on NFL measured in CSF.6,8 Further, serum NFL measured in boxers correlated strongly with the corresponding CSF values, indicating that serum NFL may reflect injury to the large-caliber subcortical axons, which are especially vulnerable to the rotational acceleration forces.18,19 In addition, we measured serum NFL in elite gymnasts whose brains are exposed to rotational acceleration, and found no difference in the levels of serum NFL between the gymnasts and nonathletic controls, suggesting that NFL may mainly be released as a result of direct trauma to the head/axons. Importantly, boxers who had received severe head impact during bouts had higher levels of serum NFL compared with boxers who received milder head impact. The levels of NFL remained higher than controls after 3 months rest from boxing in boxers who had received many hits. Taken together, these results suggest that serum NFL may be used to identify and monitor the course of injury in boxers.
A previous study has shown that serum NFL measured using a standard commercial immunoassay increased in patients with severe TBI; however, the lower limit of detection for the commercial ELSIA method quantifying NFL in serum has been reported to be 3120 to 78 pg/mL,10 which precludes accurate quantification of NFL in samples from mTBI cases as well as healthy controls. In addition, in a recent study comparing 3 different analytical techniques, we showed that ultrasensitive analytical techniques, such as the Simoa platform, are warranted to measure the relatively mild increase in blood NFL in patients with mTBI and to differentiate from control levels.10 Further, other potential blood biomarkers, including the established TBI biomarker S100 calcium binding protein B, may aid in the diagnostic procedure in severe TBI cases, but do not signal in patients with mTBI.7
At present, there is no valid imaging or fluid biomarker for mTBI; diagnosis is largely based on self-reported symptoms after blunt head injury. As such, a blood biomarker that can reliably identify and grade severity of brain damage, predict prognosis and guide clinical management, monitor therapeutic interventions, and, in sports-related TBI, to guide RTP decisions would be highly warranted. An optimal biomarker for mTBI should reflect the key pathophysiology of brain injury. Given that axonal injury, especially injury to long myelinated subcortical axons, which are enriched in neurofilament proteins, is regarded as central in TBI pathophysiology,21 NFL could serve as a marker for this type of injury. To test this hypothesis and validate the findings from the boxing part of this study, we measured NFL in an independent cohort consisting of concussed professional hockey players. We found that serum NFL was elevated in hockey players immediately after concussion and the highest levels of NFL were measured at 144 hours, and the levels normalized when the players returned to play. Importantly, serum NFL relates to the severity of PCS, as assessed by the latest guidelines for sports-related concussion.13 The course of serum NFL from 1 to 144 hours after concussion was essentially unchanged in players with rapidly resolving PCS as compared to controls, while players with prolonged PCS had elevated serum NFL over the same time course as compared to controls. Serum NFL could also separate players with rapidly resolving PCS from those with prolonged PCS. Furthermore, serum NFL has accuracy of AUC 0.74–0.79 measured 1–144 hours after concussion in a subset of cases with prolonged PCS. Taken together, these results suggest that analysis of serum NFL between 1 and 144 hours after injury may be valuable for diagnosis and prognosis of mTBI. In the context of sports-related mTBI, it might aid objective or neurobiologically informed decision on fitness for RTP.
The dynamics of serum NFL seem to differ from those of other axonal injury biomarkers such as tau; while we observed a biphasic release between 1 and 36 hours for plasma tau,14 serum NFL increased between 12 and 144 hours. Having said that, little is known about the half-life or dynamics of NFL. Animal model studies indicate that the half-life for NFL in mice optical axons and retinal ganglion neurons may be in the vicinity of 3 weeks,22 but there are no human studies that have determined the half-life of NFL in either serum or CSF.
There are limitations to this study, including the overall modest sample size, which precludes examining the biomarker level in relation to different form and severity of injury, especially in the hockey cohort. Further, in the cohort of hockey players, the lack of baseline samples for all the players hindered direct comparison of baseline and postconcussion biomarker levels for each individual. In addition, we did not have DTI performed, and therefore, could not compare serum NFL levels to eventual changes on DTI.
The results from these 2 independent prospective cohort studies show that serum NFL is a sensitive and dynamic biomarker for axonal injury in concussive traumatic brain injury. The marker should be useful to detect and monitor CNS injury in concussion.
ACKNOWLEDGMENT
The authors thank the study participants.
GLOSSARY
- AUC
area under the receiver operating characteristic curve
- DTI
diffusion tensor imaging
- IQR
interquartile range
- LLOQ
lower limit of quantification
- LOD
limit of detection
- LP
lumbar puncture
- mTBI
mild traumatic brain injury
- NFL
neurofilament light
- PCS
postconcussion symptoms
- RTP
return to play
- SHL
Swedish Hockey League
- TBI
traumatic brain injury
Footnotes
Supplemental data at Neurology.org
Editorial, page 1780
AUTHOR CONTRIBUTIONS
Drs. Shahim, Zetterberg, and Blennow had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Zetterberg and Blennow. Acquisition of data: Drs. Shahim, Zetterberg, Tegner, and Blennow. Statistical analysis: Drs. Shahim and Zetterberg. Drafting of the manuscript: Drs. Shahim, Zetterberg, and Blennow. Analysis and interpretation of data: Drs. Shahim, Zetterberg, Tegner, and Blennow. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: Drs. Zetterberg and Blennow. Study supervision: Drs. Zetterberg, Tegner, and Blennow.
STUDY FUNDING
The study was supported by grants from the Swedish Research Council, the European Research Council, Centrum för Idrottsforskning, the Torsten Söderberg Foundation, the Knut and Alice Wallenberg Foundation, and Frimurarestiftelsen. The funding source had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
DISCLOSURE
P. Shahim reports no disclosures relevant to the manuscript. H. Zetterberg is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Holding-based platform company at the University of Gothenburg. Y. Tegner reports no disclosures relevant to the manuscript. K. Blennow has served as a consultant or at advisory boards for Alzheon, Eli Lilly, Fujirebio Europe, IBL International, Novartis, and Roche Diagnostics, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Holding-based platform company at the University of Gothenburg. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ellenbogen RG, Berger MS, Batjer HH. The National Football League and concussion: leading a culture change in contact sports. World Neurosurg 2010;74:560–565. [DOI] [PubMed] [Google Scholar]
- 2.Williams WH, Potter S, Ryland H. Mild traumatic brain injury and postconcussion syndrome: a neuropsychological perspective. J Neurol Neurosurg Psychiatry 2010;81:1116–1122. [DOI] [PubMed] [Google Scholar]
- 3.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973;3:270–303. [DOI] [PubMed] [Google Scholar]
- 4.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav 2012;6:244–254. [DOI] [PubMed] [Google Scholar]
- 5.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011;134:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol 2006;63:1277–1280. [DOI] [PubMed] [Google Scholar]
- 7.Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 2013;9:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One 2012;7:e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 11.Gisslen M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2015;3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljungqvist JC, Zetterberg H, Mitsis M, Blennow K, Skoglund TS. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma 2017;34:1124–1127. [DOI] [PubMed] [Google Scholar]
- 13.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47:250–258. [DOI] [PubMed] [Google Scholar]
- 14.Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 2014;71:684–692. [DOI] [PubMed] [Google Scholar]
- 15.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010;28:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res 2003;987:25–31. [DOI] [PubMed] [Google Scholar]
- 17.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmarou CR, Walker SA, Davis CL, Povlishock JT. Quantitative analysis of the relationship between intra-axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma 2005;22:1066–1080. [DOI] [PubMed] [Google Scholar]
- 19.Shitaka Y, Tran HT, Bennett RE, et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 2011;70:551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Nimer F, Thelin E, Nyström H, et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS One 2015;10:e0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013;246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry DM, Millecamps S, Julien JP, Garcia ML. New movements in neurofilament transport, turnover and disease. Exp Cell Res 2007;313:2110–2120. [DOI] [PubMed] [Google Scholar]