Abstract
Objective:
To assess if visual discrimination training improves performance on visual perimetry tests in chronic stroke patients with visual cortex involvement.
Methods:
24-2 and 10-2 Humphrey visual fields were analyzed for 17 chronic cortically blind stroke patients prior to and following visual discrimination training, as well as in 5 untrained, cortically blind controls. Trained patients practiced direction discrimination, orientation discrimination, or both, at nonoverlapping, blind field locations. All pretraining and posttraining discrimination performance and Humphrey fields were collected with online eye tracking, ensuring gaze-contingent stimulus presentation.
Results:
Trained patients recovered ∼108 degrees2 of vision on average, while untrained patients spontaneously improved over an area of ∼16 degrees2. Improvement was not affected by patient age, time since lesion, size of initial deficit, or training type, but was proportional to the amount of training performed. Untrained patients counterbalanced their improvements with worsening of sensitivity over ∼9 degrees2 of their visual field. Worsening was minimal in trained patients. Finally, although discrimination performance improved at all trained locations, changes in Humphrey sensitivity occurred both within trained regions and beyond, extending over a larger area along the blind field border.
Conclusions:
In adults with chronic cortical visual impairment, the blind field border appears to have enhanced plastic potential, which can be recruited by gaze-controlled visual discrimination training to expand the visible field. Our findings underscore a critical need for future studies to measure the effects of vision restoration approaches on perimetry in larger cohorts of patients.
Stroke damage to the primary visual cortex (V1) is a major cause of vision loss in humans. Clinically, cortically induced blindness (CB) is assessed with Goldmann, Humphrey, and other forms of visual perimetry, presenting as homonymous defects contralateral to the damaged brain hemisphere.
While substantial spontaneous recovery can occur in the first few weeks to months postdamage, CB defects are then thought to become stable and permanent.1 Patients are commonly sent home without visual rehabilitation, and when therapy is recommended, it tends to focus on developing compensatory eye movement strategies2 or on using substitution, such as prism lenses.3 Although able to improve visual functioning and quality of life, neither form of therapy alters the visual defect.4 In fact, there is currently no widely accepted clinical method to restore vision in CB,5 although multiple research studies have shown visual training to recover particular functions within chronic CB fields (see Melnick et al.6 for review). However, whether restitution therapies improve perimetry has been relatively little explored, and results vary widely. In addition, some prior studies were confounded by poor standards and controls,7,8 while others yielded promising results.9–16 As such, significant controversy remains about the ability of restitution therapy to improve cortically blind visual fields.
The goal of the present study was to quantify the effect of visual discrimination training on Humphrey automated perimetry in chronic CB. We performed a retrospective analysis of Humphrey visual fields (HVFs) from patients with chronic CB trained using the Huxlin discrimination paradigm, with performance measured using online fixation control.16–18 Changes in pretraining/posttraining HVFs were also compared with those from HVFs collected at 2 time points prior to the onset of training—i.e., in untrained CB (UCB) controls. Our data suggest significant benefits of visual training for perimetry in chronic CB, which warrant further exploration in controlled clinical trials.
METHODS
Participants.
HVFs were analyzed retrospectively from 24 patients with CB (table e-1 at Neurology.org), recruited for visual retraining.16–18 Inclusion criteria were adults at least 6 months after stroke-induced occipital damage (verified using structural MRIs), with reliable 24-2 and 10-2 HVFs (<20% fixation losses, false-positive and false-negative errors) in both eyes and ability to fixate precisely (error smaller than ±1 degree relative to fixation spot) during psychophysical testing. Exclusion criteria were unreliable HVFs, ocular disease, neglect, neurologic disease unrelated to occipital stroke, use of neuroactive drugs, and inability to fixate precisely (error greater than ±1 degree relative to fixation spot) during psychophysical testing. In 5 patients (UCB1–UCB5), 2 HVFs were collected before training, allowing assessment of HVF stability. UCB1 then completed training and was designated CB1. UCB2–UCB5 failed to complete training or to generate reliable posttraining HVFs (appendix e-1); thus, they were not included in our trained cohort. Three participants, who successfully completed pretraining tests and training, then failed to obtain reliable HVFs posttraining and were excluded from the analysis. As such, the data presented include 5 untrained and 17 trained CB patients.
Standard protocol approvals, registrations, and patient consents.
All patient-related procedures performed in the presented study were approved by the Institutional Review Board of the University of Rochester Medical Center. Testing and training were conducted after obtaining written informed consent from each participant.
Experimental design.
HVFs were collected by a single ophthalmic technician, who was blinded to each participant's training status. Psychophysical testing by laboratory personnel was then used to establish training locations, as described previously.16–18 UCB1–UCB5 repeated HVFs after 1.4–13.3 months before training (table e-1). CB1–CB17 trained for 3–14 months before returning to the laboratory for verification of training performance and to repeat HVFs (table e-1).
Training.
Patients trained on left-right direction discrimination of random dot stimuli (n = 6), vertical-horizontal orientation discrimination of static Gabors (n = 5), or both tasks (n = 6) at nonoverlapping, blind field locations (table e-1), as previously described.16–18 Stimuli and task details are also provided in appendix e-1 and illustrated in figure e-1, A and B. Training locations were chosen as sites where performance first dropped to chance (50% correct) during blind field border mapping. Patients trained at home, performing 300 trials per day, per location, at least 5 days per week. They e-mailed data log files automatically generated by the training software back to the laboratory for analysis weekly. Once performance became comparable to that at equivalent, intact field locations (measured during pretests), training moved 1 degree deeper into the blind field along the X-axis (Cartesian coordinate space). While home training was performed without an eye tracker, patients were instructed to fixate whenever a fixation spot was present. In addition, after 6 months of training, or recovering normal discrimination performance at ≥2 blind field locations, home training was verified in laboratory with fixation control enforced using an Eyelink 1000 eye tracker (SR Research Ltd., Kanata, Canada).
Quantitative analysis of HVFs.
HVFs were collected as detailed in appendix e-1 and several metrics calculated by the Humphrey STATPAC software (Zeiss Humphrey Systems, Atlanta, GA) were analyzed as follows:
Pattern deviation (PD): deviation from the age-corrected population mean for each HVF testing location.
Perimetric mean deviation (PMD): overall difference in sensitivity between the tested and expected hill of vision for an age-corrected, normal population.
Short term fluctuations (STF): test/retest variance during 10-2 HVF test.
Composite, binocular HVFs were generated in MATLAB (MathWorks, Inc., Natick, MA) by first averaging luminance detection thresholds (dB) from monocular HVFs at identical test locations between both eyes (figure e-2A), justified given the homonymous nature of the deficit. These binocular 24-2 and 10-2 HVFs were then combined (figure e-2A), with 5 overlapping locations averaged together (green dots, figure e-2A). Natural-neighbor interpolation was applied between test locations with 0.1 degree2 resolution, creating composite visual fields of 121 tested locations and 161,398 interpolated data points, covering an area 1,616 degrees2 in size. Difference maps were generated (figure e-2B) by subtracting the initial, composite, noninterpolated HVF from the second HVF, then interpolating the difference to create a smooth map of visual sensitivity change (trained patients: figure e-3; untrained patients: figure e-4). From these difference maps, we calculated the following:
Area of HVF-defined visual deficit: impaired region defined by PD < −5 dB.
Area of HVF where sensitivity changed by ≥6 dB: improved regions had luminance sensitivity that increased by ≥6 dB relative to baseline; worsened regions had sensitivity that dropped by ≥6 dB. The 6 dB value was selected as it was roughly double the 24-2 HVF test/retest variability (Humphrey STATPAC, Zeiss Humphrey Systems), and the STFs measured during 10-2 HVFs (figure e-5A).
Primary outcome measures were changes in PMD and the area of the HVF where sensitivity increased or decreased by ≥6 dB. The secondary outcome measure was the change in performance on the training tasks.
Statistical analyses.
Values provided are mean ± SEM and 95% confidence intervals (CI), with 2-tailed t tests or analyses of variance (ANOVAs) used to assess significance using p < 0.05 (VassarStats.net). Post hoc power analyses for relevant t test comparisons were performed for the primary and secondary outcomes using G*Power (version 3.1.9.2), and outcomes are reported in the Results.
RESULTS
Effect of training on the trained tasks.
Except for CB1, CB2, CB5, CB13, and CB17, training results were published previously (Huxlin et al., 200916; Das et al., 201417). Before training, participants could not reliably perform discriminations within the blind field (gray bars, figure e-1, C and D), despite excellent intact field performance (white bars, figure e-1, C and D). Pretraining blind and intact field performances were found to be different (paired t tests, direction discrimination: t11 = 10.74 p < 0.0001; orientation discrimination: t10 = 11.28, p < 0.0001). Posttraining performance (black bars, figure e-1, C and D) reached 81 ± 2% correct for direction discrimination after 62 ± 12 sessions, and 87 ± 2.5% correct for orientation discrimination after 30 ± 11 sessions, an improvement over pretraining values (paired t tests, direction: t11 = 6.83, p < 0.0001, CI95 = ±6.22%; orientation: t10 = 7.93, p < 0.0001, CI95 = ±7.5%). Post hoc analyses revealed 97% power for both these comparisons.
Effect of training vs no training on HVFs.
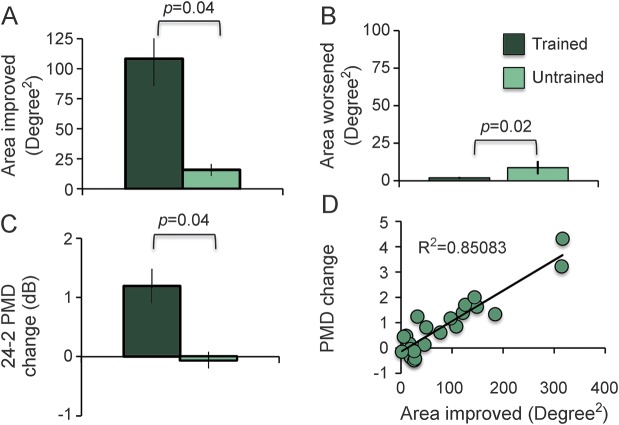
Pretraining, Humphrey-derived metrics revealed no significant baseline differences between trained and untrained groups (figure e-5). After training, luminance detection sensitivity improved ≥6 dB over 108.1 ± 22.8 degrees2 (see figure e-3 for individual maps), greater than the area improved (16 ± 5 degrees2) in untrained controls (figure 1A, figure 2, see figure e-4 for individual maps; independent t test, unequal variance: t17.5 = 17.49, p = 0.001, CI95 = ±49.4 degrees2). Post hoc analysis revealed 83% power for this comparison. In untrained patients, sensitivity improved by up to 14.5 dB (7.3 ± 0.1 dB), less (independent t test: t20 = 3.46, p = 0.003) than trained patients, who improved by up to 28 dB (9.48 ± 0.4 dB).
Figure 1. Effects of discrimination training on Humphrey visual field (HVF)–derived metrics.
(A) Area of the HVF that improved by ≥6 dB in trained and untrained participants. (B) Area of the HVF that worsened by ≥6 dB in trained and untrained participants. (C) Change in perimetric mean deviation (PMD) averaged across the 2 eyes, computed from 24-2 HVF. (D) Positive correlation between the area of the HVF that improved by ≥6 dB (from A) with the change in PMD (from C). Values in A–C are means ± SEM.
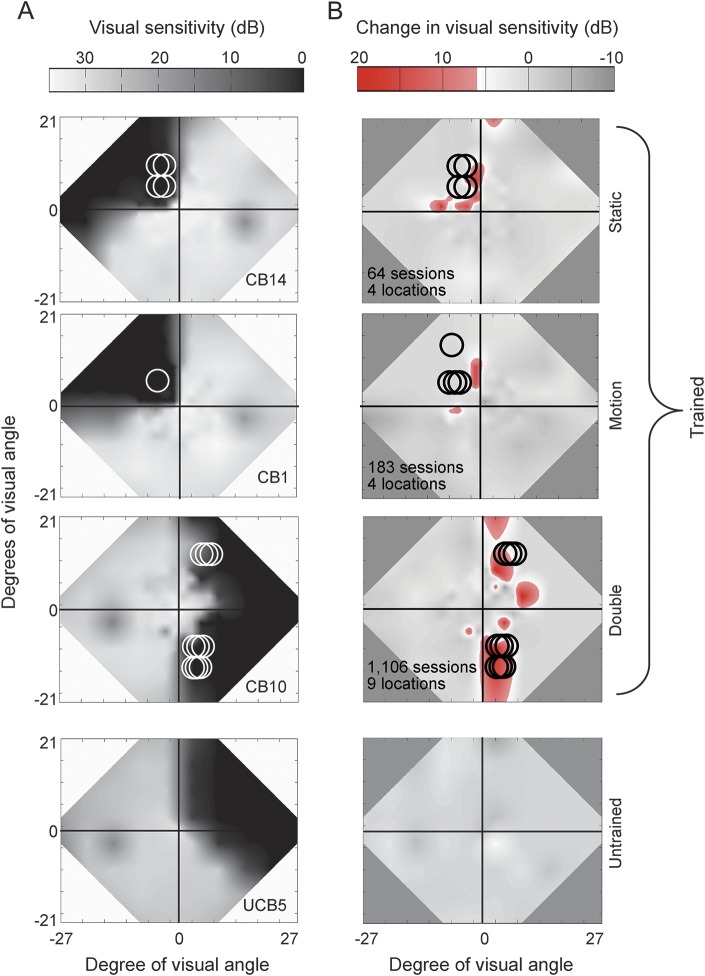
Figure 2. Effect of visual discrimination training on Humphrey visual fields (HVFs).
(A) Composite HVFs with circles indicating the size and location of training stimuli. (B) Difference HVF maps on the same patients as in A with red hues indicating locations of significant improvement in luminance sensitivity (i.e., ≥6 dB of improvement). Number of training sessions and number of locations trained are listed for each trained patient. Details of how the maps were created are provided in appendix e-1.
Both groups also had locations that worsened ≥6 dB. Worsening occurred in 59% of trained patients (n = 10/17) but 80% of untrained patients (n = 4/5). The average area of worsening in trained patients was 1.9 ± 0.7 degrees2, smaller (independent t test: t20 = −2.62, p = 0.016, CI95 = ±5.4 degrees2) than the 8.7 ± 4.5 degrees2 area of decreased sensitivity in untrained patients (figure 1B). The magnitude of worsening was similar in untrained (−7.3 ± 0.2 dB) and trained patients (−7.1 ± 0.2 dB; independent t test: t13 = −0.34, p = 0.74).
The luminance sensitivity improvements in trained patients increased PMD by 1.2 ± 0.29 dB in the 24-2 HVFs (figure 1C). In contrast, PMDs of untrained controls decreased by 0.06 ± 0.14 dB, a substantial difference from trained patients (independent t test, unequal variance: t19.8 = 3.79, p = 0.0012, CI95 = 0.67 dB). Post hoc analysis revealed 86% power for this comparison. Moreover, PMD change correlated tightly with our computed area of improvement (figure 1D, linear correlation, r = 0.9224, independent t test: t20 = 10.68, p < 0.0001).
Effect of type of training.
Double-trained patients (n = 6) exhibited improvements over 114 ± 20 degrees2, while orientation (n = 5) and direction (n = 6) trained patients improved over 101 ± 56 degrees2 and 109 ± 48 degrees2, respectively. A one-way ANOVA revealed no effect of training type on area improved (F2,14 = 0.02, p = 0.98). However, there was an effect of training type (figure 2) on area of worsening (one-way ANOVA: F2,14 = 4.5, p = 0.032), which was driven by a single, direction-trained outlier (CB6, more than 2 SD greater than the mean). Removing this outlier eliminated the effect of training type on area worsened (one-way ANOVA: F2,13 = 2.54, p = 0.1176).
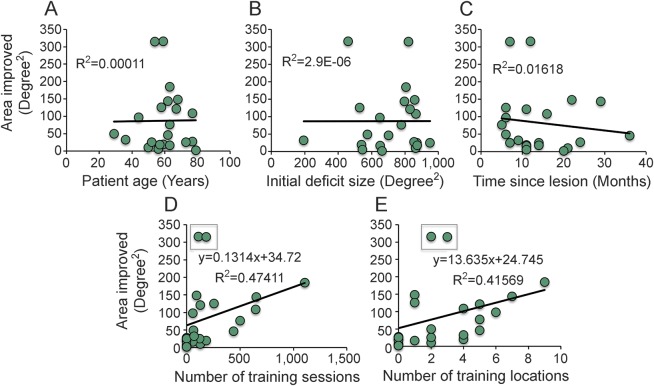
Effect of patient age, lesion age, deficit size, and number of training sessions.
The area of the HVF improved ≥6 dB was not correlated with patient age (figure 3A, r = 0.0105, t20 = 0.05, p = 0.48), time between initial lesion and start of training (figure 3B, r = −0.1272, t20 = −0.56, p = 0.29), or the original (Humphrey-defined) deficit size (figure 3C, r = 0.0016, t20 = 0.01, p = 0.50). However, there were near-significant correlations between the HVF area that improved and the number of training sessions performed (figure 3D, r = 0.3378, t20 = 1.6, p = 0.06), as well as between area improved and the number of locations trained (figure 3E, r = 0.3408, t20 = 0.162, p = 0.06). Removing 2 outliers (CB6 and CB13) from these datasets resulted in both correlations becoming significant (area improved/number of training sessions: r = 0.6886, t18 = 4.03, p = 0.0003; area improved/number of training locations: r = 0.6448, t18 = 3.58, p = 0.001). No other correlation became significant with the removal of these 2 outliers.
Figure 3. Effect of patient demographics on Humphrey visual field (HVF) improvements.
Both trained and untrained patients were included in these analyses. (A) The age of the patient at the time of recruitment did not correlate with the area of HVF improvement (≥6 dB). (B) The size of the HVF-defined deficit at the time of enrollment did not correlate with the area of improvement. (C) The time between stroke and enrollment also failed to correlate with improvement. CB10 was removed as an outlier from this analysis (time since lesion was 226 months, area of improvement = 184.79 degrees2). (D) The number of training sessions correlated strongly with the area of improvement measured on HVFs, as did (E) the number of locations trained. R2s and equations are presented after removing 2 outliers. Including these outliers generates R2 values of 0.114 for D and 0.116 for E.
Location of HVF changes.
As seen in figure 2, HVF change always included, but also extended beyond, trained locations. Training locations accounted for ∼5% of Humphrey improvement ≥6 dB (or 4.9 ± 2.3 degrees2, figure 4A), which extended up to 29.5 degrees away (gray data points, figure 4C). Likewise, most worsening occurred outside trained locations (figure 4B).
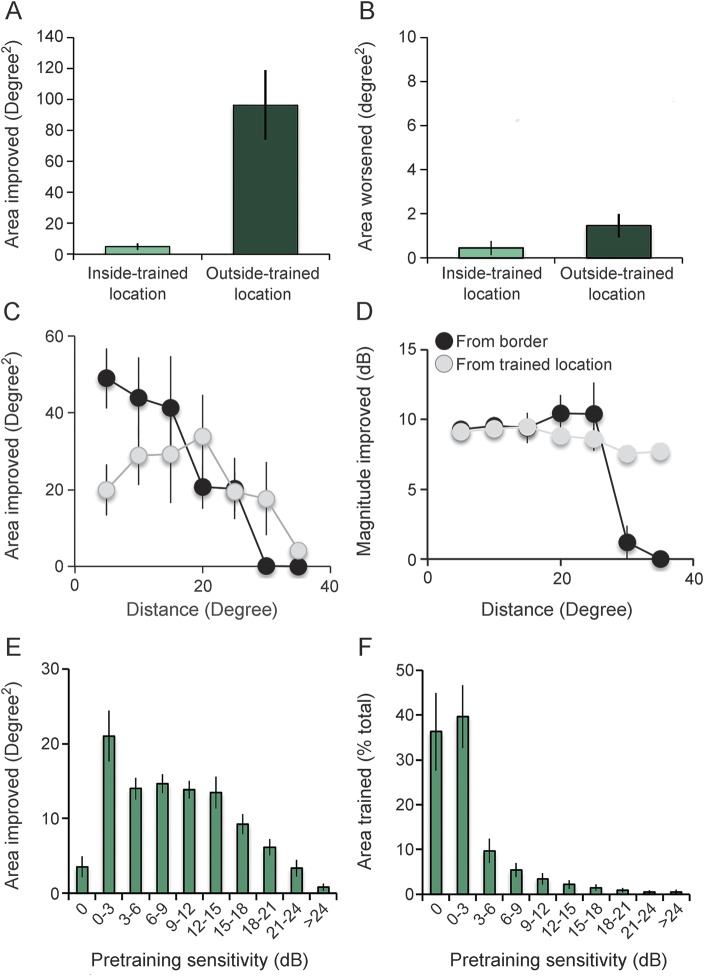
Figure 4. Humphrey visual field (HVF) changes inside and outside trained locations.
(A) Area of the visual field that improved ≥6 dB inside and outside of the trained blind field locations in CB1–CB16. (B) Area of the visual field that worsened by ≥6 dB inside and outside of the trained blind field locations in CB1–CB16. (C) Area improved as a function of distance from training locations (light data points) and the pretraining blind field border (black data points). Distance was binned in 5-degree increments. (D) Plot of the average magnitude of improvement in each 5-degree distance bin. (E) Pretraining visual sensitivity in areas of the Humphrey field that improved by ≥6 dB posttraining in CB1–CB16. Area improved in each bin is expressed as a percentage of the total area improved. (F) Pretraining visual sensitivities in areas of the blind field covered by training stimuli (expressed as % of total area trained) in CB1–CB16. Bins are greater than the lower number and ≤ greater number. Zero bin encompasses only locations with 0 dB sensitivity. Values are means ± SEM.
Most HVF improvement occurred within 5.2 ± 0.7 degrees of the original blind field border (76% occurred within 10 degrees, black data points in figure 4C), and its average magnitude hovered between 9.6 and 10.4 dB up to ∼25 degrees from this border (figure 4D).
However, ∼86% of HVF improvement occurred where pretraining sensitivity was between 3 and 18 dB. Improvements dropped almost linearly above 15 dB, with only ∼10% occurring where pretraining sensitivity was >18 dB. Similarly, only 25% of improvements occurred at locations with 0–3 dB of sensitivity, typically located deeper in the blind field (figure 4E). Critically, locations with 0–3 dB of pretraining sensitivity were where 75% of training areas were located (figure 4F), explaining the disconnect between regions of HVF improvement and training.
DISCUSSION
While cortical visual impairment is thought to be irreversible, here we show that visual discrimination training reduces the size of HVF defects in chronic CB, generating large swaths of visual improvement along the blind field border, and potentially reversing progressive vision loss. Our findings are exciting, as what little rehabilitation is currently available to patients tends to focus on eye movements (compensation therapy)2,19–21 or using prisms (substitution therapy).3,22 While these approaches improve visual functioning in daily life,2,19 neither is designed to restore vision.4 This is the purview of visual training inside CB fields (restitution therapy).9–13,15,16,23,24 However, prior to our study, there was little systematic information about how restitution training affects visual perimetry, the primary method for assessing CB fields. In addition, a significant, long-standing controversy about the efficacy of restitution therapies emerged within the field.7,8 The present work does not claim to resolve this controversy, but instead, offers new methodology to quantify changes in automated perimetry, with broad applicability to CB, as well as other conditions affecting central vision, such as glaucoma or macular degeneration. While interpretation of our results is tempered by small sample sizes, partial blinding, and lack of randomization, bias was partially reduced because all participants were recruited with the intent to train. They were thus treated identically in terms of testing, except that some had 2 baseline HVFs, allowing us to consider native stability of HVFs. Coupled with the previously reported lack of improvement in untrained CB patients,1 our findings both motivate and inform future clinical trials designed to critically examine the effects of vision restoration on perimetry in larger cohorts of patients.
The present results expand our previous work demonstrating substantial transfer of learning across untrained visual functions.16–18 We now show that training chronic CB patients to discriminate global motion, static orientation, or both also shrinks perimetrically measured field defects. This shrinkage was associated with significant improvements in PMD (∼1 dB), the small magnitude of which is likely due to the fact that PMD is computed across the entire HVF. Here, significant improvements occurred over ∼108 degrees2 or 6.6% of the total HVF. Nonetheless, prior work showed changes ≥0.6 dB to be meaningful in glaucoma patients with similarly sized visual loss as our patients.25 A PMD change of 0.7 dB over placebo was also considered significant in patients with idiopathic intracranial hypertension and mild visual loss (NORDIC Committee26).
Critically, HVF improvements were not influenced by patient age, time since lesion, or initial deficit size (suggesting that lesion size may not affect recovery). Thus, any patient with chronic CB may recover some lost vision following rigorous training. However, the number of training sessions and locations correlated with the area of HVF improvement. From figure 3D, one can estimate that substantial improvements in visual sensitivity over an area 80 degrees2 in size can be attained with ∼150 consecutive training sessions (of 300 trials each) at 2–3 blind field locations. Assuming 2 sessions/d, such training should take ∼3 months to complete. However, our data also suggest that continued training may generate continued improvement. Thus, patients should train as long as improvement is observed.
HVFs repeated without intervening training revealed decreases in both local and overall (PMD) sensitivity. Whether this represents a form of visual disuse atrophy, a consequence of retrograde degeneration of neurons in the dorsal lateral geniculate nucleus (dLGN) and retina,27–31 or whether patients learned to ignore weak, unreliable vision near their blind field remains to be determined. That worsening was not systematically reported previously may be due to HVF analyses in prior studies lacking sensitivity to such changes. Nonetheless, our results suggest that visual discrimination training, even when started >6 months poststroke, can reverse potential declines in sensitivity.
That HVF improvements occurred within trained blind field locations is not surprising. Training to detect or discriminate stimuli in the blind field improves contrast sensitivity17,24 and HVFs are, in essence, a luminance contrast detection task with broadband stimuli.32,33 However, improvements in trained tasks are typically restricted to trained locations in CB patients,16,24 while here, 80% of HVF improvements occurred within 10 degrees of the original blind field border, suggesting enhanced plasticity in this region. In addition, close to 86% of improvements occurred where pretraining sensitivities ranged from 3 to 18 dB, while 75% of the trained areas had baseline sensitivity <3 dB, a consequence of our procedure for selecting training locations. These findings highlight an interesting difference in visual functions assessed by clinical perimetry vs laboratory psychophysics: namely regions with <3 dB sensitivity on Humphrey perimetry appear to possess measurable, residual visual abilities, which can be retrained back to normal.
While speculative, a possible substrate of training-induced visual improvements in CB is engagement of extrageniculostriate pathways. Projections from the dLGN that bypass V1 provide direct input to V2/V3,34–36 V4,29 and MT/MST.37 These pathways may mediate blindsight38—residual visual processing present in some CB fields.39,40 After V1 damage, extrageniculostriate pathways are thought to rely primarily on koniocellular (K-cell), as opposed to parvocellular (P-cell) and magnocellular (M-cell), dLGN neurons, partly because K-cells appear to be more resistant to retrograde degeneration.30,41 K-cells also possess contrast sensitivity and spatial frequency preferences that match responses seen in blindsight42 and our patients posttraining.17 Finally, K-cell pathways may switch from a modulatory to a driving role following damage to V1.35 Repeated, directed activation of these pathways through visual discrimination training could strengthen their driving role. This in turn may allow the residual visual system to better utilize information bypassing V1, measurably improving conscious vision both perimetrically and in visual discrimination tasks.
We used a fine-grained, quantitative analysis of HVFs to show that visual discrimination training at discrete blind field locations can generate large swaths of visual improvement and may prevent progressive vision loss in chronic CB patients. Together with the observed benefit of visual discrimination training on perimetry, the lack of effect of time since lesion on recovery suggests that a controlled, randomized, blinded, crossover clinical trial would be the optimal design to further elucidate this phenomenon in a larger patient population. Despite the limitations inherent in this pilot study, our findings remain exciting for several reasons. First, they illustrate yet another form of learning transfer in CB: a recovery of luminance sensitivity following visual discrimination training in which neither luminance nor contrast was varied. Second, this boost in sensitivity was reliably reported by CB patients during perimetry and can presumably be used in their day-to-day lives. Third, the amount of perimetry improvement attained did not depend on major demographic parameters, but was proportional to the amount of training performed. Finally, training-induced sensitivity improvements occupied previously impaired regions along the blind field border. Together, these data provide compelling evidence that contrary to established thought, cortical visual impairment is reversible in part. Specifically, visual discrimination training in chronic CB fields improves fixation-controlled visual performance on both the trained tasks and Humphrey perimetry.
ACKNOWLEDGMENT
The authors thank Terrance Schaefer for performing Humphrey visual field tests on all the patients and Drs. David Heeger, Elisha Merriam, and Duje Tadin for their constructive comments on the manuscript.
GLOSSARY
- ANOVA
analysis of variance
- CB
cortically induced blindness
- CI
confidence interval
- dLGN
dorsal lateral geniculate nucleus
- HVF
Humphrey visual field
- PD
pattern deviation
- PMD
perimetric mean deviation
- STF
short term fluctuations
- UCB
untrained cortically induced blindness
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Matthew Cavanaugh: study concept and design, data acquisition, analysis, and interpretation. Krystel Huxlin: study concept and design, data interpretation, study supervision.
STUDY FUNDING
This work was supported by grants from the NIH (EY021209 to K.R.H., Core Center Grant P30 EY001319 to the Center for Visual Science [CVS], training grant T32 EY007125 to CVS) and by an unrestricted grant from the Research to Prevent Blindness (RPB) Foundation to the Flaum Eye Institute.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Zhang X, Kedar S, Lynn M, Newman N, Biousse V. Natural history of homonymous hemianopia. Neurology 2006;66:901–905. [DOI] [PubMed] [Google Scholar]
- 2.Spitzyna GA, Wise RJ, McDonald SA, et al. Optokinetic therapy improves test reading in patients with hemianopic alexia: a controlled trial. Neurology 2007;68:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peli E. Field expansion for homonymous hemianopia by optically induced peripheral exotropia. Optom Vis Sci 2000;77:453–464. [DOI] [PubMed] [Google Scholar]
- 4.Lane AR, Smith DT, Ellison A, Schenk T. Visual exploration training is no better than attention training for treating hemianopia. Brain 2010;133:1717–1728. [DOI] [PubMed] [Google Scholar]
- 5.Pollock A, Hazelton C, Henderson CA, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev 2011;10:CD008388. [DOI] [PubMed] [Google Scholar]
- 6.Melnick MD, Tadin D, Huxlin KR. Relearning to see in cortical blindness. Neuroscientist 2016;22:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFadzean RM. NovaVision: vision restoration therapy. Curr Opin Ophthalmol 2006;17:498–503. [DOI] [PubMed] [Google Scholar]
- 8.Horton JC. Disappointing results from Nova Vision's visual restortation therapy. Br J Ophthalmol 2005;89:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chokron S, Perez C, Obadia M, Gaudry I, Laloum L, Gout O. From blindsight to sight: cognitive rehabiliation of visual field defects. Restorative Neurol Neurosci 2008;26:305–320. [PubMed] [Google Scholar]
- 10.Bergsma DP, Elshout JA, van der Wildt GJ, van den Berg AV. Transfer effects of training-induced visual field recovery in patients with chronic stroke. Top Stroke Rehabil 2012;19:212–225. [DOI] [PubMed] [Google Scholar]
- 11.Bergsma DP, van der Wildt GJ. Visual training of cerebral blindness patients gradually enlarges the visual field. Br J Ophthalmol 2009;94:88–96. [DOI] [PubMed] [Google Scholar]
- 12.Raemaekers M, Bergsma D, van Wezel R, can der Wildt G, van den Berg A. Effects of vision restoration training on early visual cortex in patients with cerebral blindness investigated with functional magnetic resonance imaging. J Neurophysiol 2011;105:872–882. [DOI] [PubMed] [Google Scholar]
- 13.Raninen A, Vanni S, Hyvarinen L, Nasanen R. Temporal sensitivity in a hemianopic visual field can be improved by long-term training using flicker stimulation. J Neurol Neurosurg Psychiatry 2007;78:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahraie A, Trevethan CT, MacLeod MJ. Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia 2008;46:879–885. [DOI] [PubMed] [Google Scholar]
- 15.Vaina LM, Soloviev S, Calabro FJ, Buonanno F, Passingham R, Cowey A. Reorganization of retinotopic maps after occipital lobe infarction. J Cogn Neurosci 2014;26:1266–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxlin K, Martin T, Kelly K, et al. Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci 2009;29:3981–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Tadin D, Huxlin KR. Beyond blindsight: properties of visual relearning in cortucally blind fields. J Neurosci 2014;34:11652–11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavanaugh M, Zhang R, Melnick M, et al. Visual recovery in cortical blindness is limited by high internal noise. J Vis 2015;15:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg J, Diller L, Gordo WA, et al. Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch Phys Med Rehabil 1977;58. [PubMed] [Google Scholar]
- 20.Kerkhoff G. Restorative and compensatory therapy approaches in cerebral blindness: a review. Restorative Neurol Neurosci 1999;15:255–271. [PubMed] [Google Scholar]
- 21.Kerkhoff G. Neurovisual rehabiliation: recent developments and future directions. J Neurol Neurosurg Psychiatry 2000;68:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi PW, Khefyets S, Reding MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology 1990;40:1597–1599. [DOI] [PubMed] [Google Scholar]
- 23.Sahraie A, Trevethan CT, MacLeod MJ, Murray AD, Olson JA, Weiskrantz L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proc Natl Acad Sci USA 2006;103:14971–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahraie A, Trevethan CT, Weiskrantz L, et al. Spatial channels of visual processing in cortical blindness. Eur J Neurosci 2003;18:1189–1196. [DOI] [PubMed] [Google Scholar]
- 25.Tattersall CL, Vernon SA, Menon GJ. Mean fluctuation in eyes with stable Humphrey 24-2 visual fields. Eye 2007;21:362–366. [DOI] [PubMed] [Google Scholar]
- 26.NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee, Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;11:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cellss and optic tract in hemianopic monkeys and humans. Brain 2011;134:2149–2157. [DOI] [PubMed] [Google Scholar]
- 28.Jindahra P, Petrie A, Plant G. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132:628–634. [DOI] [PubMed] [Google Scholar]
- 29.Cowey A, Stoerig P. Projection patterns of surviving neurons in the dorsal lateral geniculate nucleus following discrete lesions of striate cortex: implications for residual vision. Exp Brain Res 1989;75:631–638. [DOI] [PubMed] [Google Scholar]
- 30.Cowey A, Stoerig P, Perry VH. Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of P beta cells. Neuroscience 1989;29:65–80. [DOI] [PubMed] [Google Scholar]
- 31.Millington RS, Yasuda CL, Jindahra P, et al. Quantifying the pattern of optic tract degeneration in human hemianopia. J Neurol Neurosurg Psychiatry 2014;85:379–386. [DOI] [PubMed] [Google Scholar]
- 32.Kraft A, Roehmel J, Olma MC, Schmidt S, Irlbacher K, Brandt SA. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp Brain Res 2010;207:283–290. [DOI] [PubMed] [Google Scholar]
- 33.Carl Zeiss Meditec Inc. Humphrey Field Analyzer User Manual. Dublin, CA: Carl Zeiss Meditec Inc.; 2010. [Google Scholar]
- 34.Bullier J, Kennedy H. Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Exp Brain Res 1983;53:168–172. [DOI] [PubMed] [Google Scholar]
- 35.Schmid M, Panagiotaropoulos T, Augath M, Logothetis N, Smirnakis S. Visually driven activation in macaque areas V2 and V3 without input from the primary visual cortex. PLoS One 2009;4:e5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci 2000;23:127–153. [DOI] [PubMed] [Google Scholar]
- 37.Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 2004;7:1123–1128. [DOI] [PubMed] [Google Scholar]
- 38.Schmid MC, Mrowka SW, Turchi J, et al. Blindsight depends on the lateral geniculate nucleus. Nature 2010;466:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following restricted occipital ablation. Brain 1974;97:709–728. [DOI] [PubMed] [Google Scholar]
- 40.Sanders MD, Warrington EK, Marshall J, Weiskrantz L. “Blindsight”: vision in a field defect. Lancet 1974;1:707–708. [DOI] [PubMed] [Google Scholar]
- 41.Dineen J, Hendrickson A, Keating EG. Alterations of retinal inputs following striate cortex removal in adult monkey. Exp Brain Res 1982;47:446–456. [DOI] [PubMed] [Google Scholar]
- 42.Ajina S, Rees G, Kennard C, Bridge H. Abnormal contrast responses in extrastriate cortex of blindsight patients. J Neurosci 2015;35:8201–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]