Introduction
KEY TEACHING POINTS
|
We present a case of ventricular arrhythmia induced by a premature ventricular beat with a short coupling interval in a young patient with Brugada syndrome, vasodepressive response to tilt test, and a structurally normal heart. A clear vagal trigger appeared in all ventricular arrhythmia events. The patient was implanted with an implantable cardioverter-defibrillator (ICD).
Case report
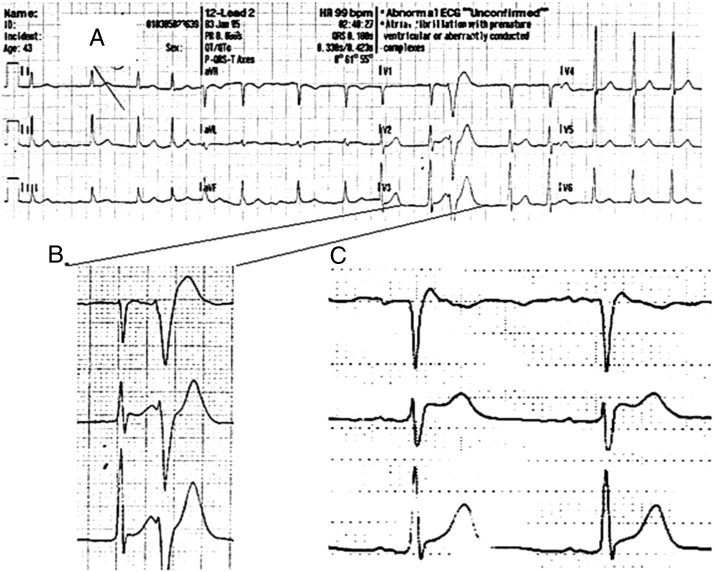
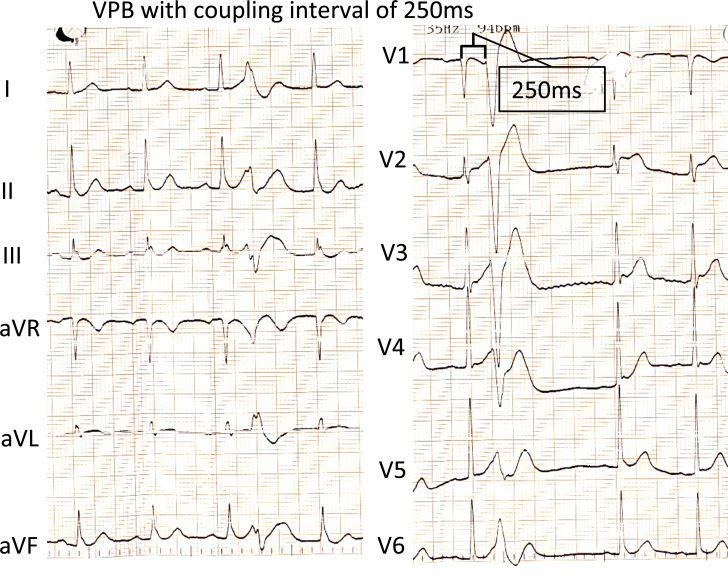
A 44-year-old man was admitted to the hospital after 2 episodes of syncope. Upon waking up at midnight, the patient proceeded to urinate, went back to his bed, and passed out. His wife noticed that he was not responding and started cardiopulmonary resuscitation. After about 2 minutes, the patient started to respond. An emergency medical services team was called, but before their arrival the patient experienced a shorter similar episode. When emergency medical services arrived he was alert, but felt fatigue. His blood pressure was normal. An electrocardiogram (ECG) at home showed slow atrial fibrillation (AF) with short–coupling interval premature ventricular contractions (280 ms) and ST elevation in V2-V3 and weak “coved-type” ST elevation in V1, compatible with Brugada syndrome (Figure 1). The AF converted spontaneously to sinus rhythm over the next few hours. The first ECG in sinus rhythm was recorded in the morning with right bundle branch block pattern and ST elevation compatible with Brugada syndrome, together with short-coupled ventricular premature beats (VPBs) (Figure 2). Later ECGs did not indicate a Brugada pattern.
Figure 1.
Electrocardiogram recorded by emergency medical services team demonstrating slow atrial fibrillation with short–coupling interval ventricular premature beats (280 ms) and ST elevation in V2-V3 and weak “coved-type” ST elevation in V1 compatible with Brugada syndrome.
Figure 2.
Electrocardiogram in sinus rhythm demonstrates ST elevations in V1-V3 consistent with Brugada syndrome together with short-coupled (250 msec) ventricular premature beats.
A 24-hour Holter monitor demonstrated sinus rhythm (average of 80 beats per minute [bpm]) and 320 VPBs with very short coupling intervals, less than 280 ms. An echocardiogram demonstrated normal left ventricular systolic function and normal structure and function of the heart chambers and valves. The exercise test was normal. Because of the normalized ECG, we decided to perform the flecainide test, which was positive.
A tilt table test was performed because of the clinical picture of the neurocardiogenic syncope. At baseline the heart rate was 80 bpm and the blood pressure was 139/90 mm Hg. During the orthostatic challenge the heart rate gradually increased and the blood pressure decreased. After 5 minutes, the patient complained of dizziness; his heart rate increased to 131 bpm, and his blood pressure decreased to 110/70 mm Hg. At minute 9 the patient had frank syncope, with a heart rate of 142 bpm and nonmeasurable blood pressure (the last measured blood pressure was 75/50 mm Hg). The patient was returned to the supine position and regained consciousness. A diagnosis of vasodepressive syncope was made, and exaggerated orthostatic tachycardia was noted. Electrophysiological study (EPS) revealed a relatively long HV interval of 60 ms. A programmed electrical stimulation from both the right ventricular apex and outflow tract was conducted with stimulation up to 350/160/160/150/150 ms with 1-to-1 conduction without induction of any ventricular arrhythmia. We have not performed genetic testing in this patient.
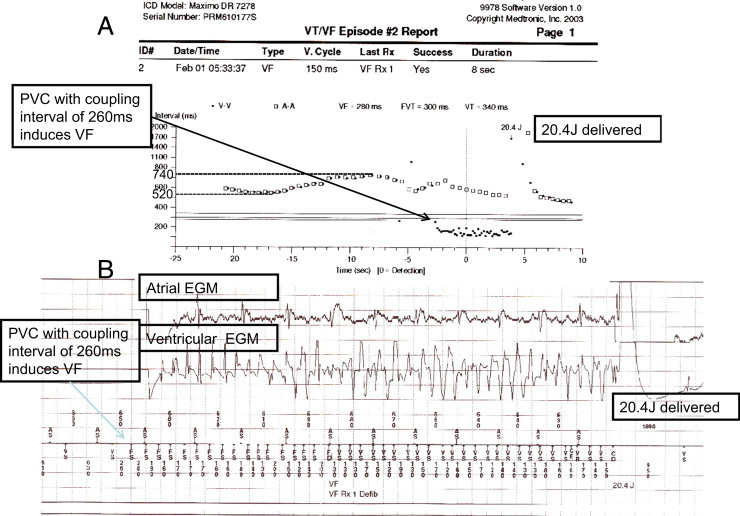
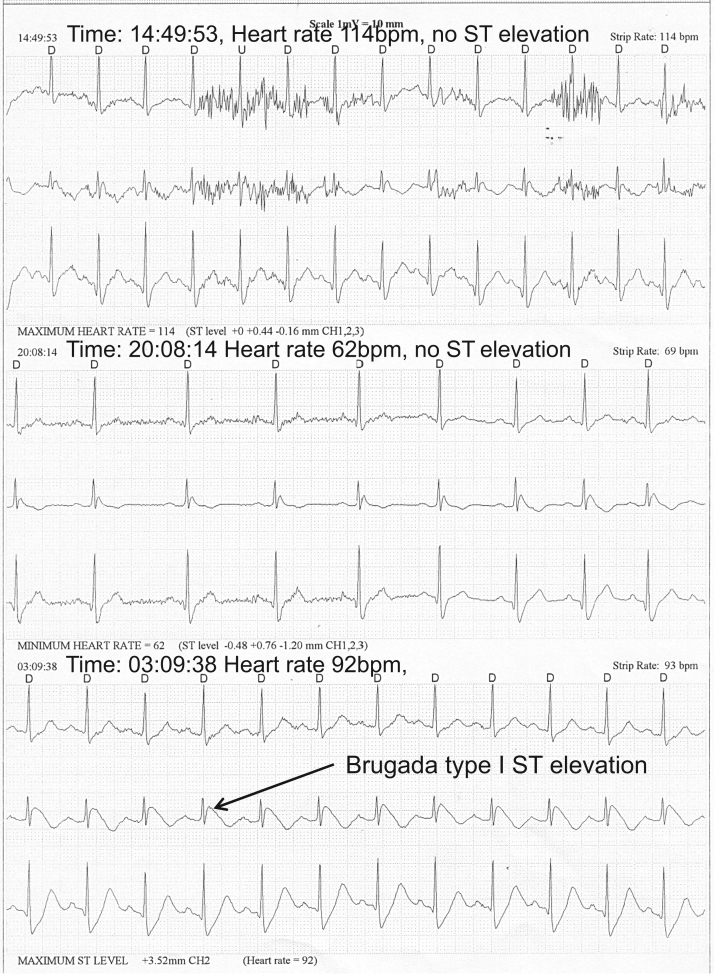
An ICD was implanted because of the spontaneous Brugada at presentation and very short–coupling interval VPBs, despite the noninducible EPS and the demonstration of the vasodepressive (vagal) syncope. The patient was discharged with beta blocker therapy. Three weeks later, the patient experienced an electrical shock delivered by the ICD at 6:33 AM. The patient urinated and washed his face with cool water and felt the electrical shock without any other symptoms or syncope. An ICD interrogation (Figure 3) demonstrated that a VPB with a short coupling interval (260 ms) induced a ventricular fibrillation (VF), which converted into sinus rhythm with 20.4 J. Of note, slowing of the sinus rhythm (from 115 to 81 bpm) preceded the VPB. On the same day at 1 PM, the patient experienced another electrical shock. This was during urination. Again an ICD interrogation showed a VPB with a short coupling interval (280 ms) that induced VF. The patient was hospitalized and 1500 mg of quinidine daily was started. The patient tolerated the quinidine well, with a blood drug level in the expected range without prolongation of the QTc interval. The only ventricular arrhythmia events that were recorded by the ICD over the 11-year follow-up were episodes of nonsustained ventricular tachycardia (VT) during several days when the patient discontinued quinidine owing to a shortage at the local pharmacy. A 24-hour Holter monitor performed at that time (2010) demonstrated ST elevations that were present at night (Figure 4). These ST elevations were not rate dependent because they were absent from the daytime recordings during both slow and fast heart rate, so they were presumably vagally mediated.
Figure 3.
An implantable cardioverter-defibrillator interrogation demonstrates a ventricular premature beat (VPB) with a short coupling interval (260 ms), which induced a ventricular fibrillation, which converted into sinus rhythm with 20.4 J. Slowing of the heart rate (from 520 to 740 msec) precedes a short-coupled VPB.
Figure 4.
A 24-hour Holter monitor performed at 8:10 PM demonstrates that ST elevations are much more prominent at night (Brugada type I pattern). The changes in the magnitude of the ST elevations are not rate dependent (ST elevations are much less prominent in the daytime recordings during both slow and fast heart rates).
Discussion
Brugada syndrome is a distinct clinical and electrocardiographic syndrome associated with syncopal episodes and sudden cardiac death in patients with an ECG that demonstrates ST elevation in right precordial leads.1 It has an autosomal dominant pattern of inheritance2; more than 250 mutations in 13 genes have been described, leading to an imbalance between depolarizing Na and Ca currents and repolarizing the K (Ito) current. The resultant right ventricular transmural inhomogeneity causes short-coupled VPBs and then polymorphic VT or VT through the phase 2 reentry mechanism.3 Sodium channel blockers are used to unmask a Brugada ECG pattern in suspected individuals.4 History of cardiac arrest or syncope, male sex, and spontaneous AF have all been demonstrated to have prognostic significance.5, 6 Management includes ICD implantation,7 quinidine to prevent arrhythmias and recurrent ICD shocks,8 and catheter ablation in selected patients.9
Our patient is a prime example that a clear parasympathetic activation combined with very early VPB induces VF. The transient and intermittent Brugada pattern on ECG can be related to vagal activity changes. In our case all syncopal events and documented ventricular arrhythmia were associated with increased vagal activity such as urination, washing with cool water, and postural changes. Moreover, a slowed heart rate preceded VPB-induced ventricular arrhythmia on the ICD recording. In addition, our patient demonstrated a vasodepressive response during the head-up tilt table test and intermittent ST elevation (type I Brugada during sleep) on the Holter recording (Figure 4).
Increased vagal tone may lead to more pronounced ST-segment elevation and an increased likelihood of arrhythmias in Brugada patients owing to decreasing ICa.10 Acetylcholine was demonstrated to augment ST-segment elevation in patients with Brugada syndrome. Higher vagal tone also explains the higher incidence of arrhythmias at night in these patients.11, 12 An early study demonstrated that in 6 patients with transient rSR′ pattern with ST elevation in right precordial leads and episodes of VF, agents that block sympathetic activation and increase parasympathetic activation caused more pronounced ST elevation and easier VT/VF inducibility on EPS, whereas sympathomimetic and vagolytic agents had the opposite effect.13 Moreover, in some of these patients, Holter ECG monitoring demonstrated an increased high-frequency component (marking increased vagal tone) just before VF (as in our case; Figure 3, Figure 4). One study demonstrated that patients with Brugada syndrome who had increased ST elevation in V1-V2 during the recovery stage of the exercise test also had increased heart rate recovery (as a sign of increased vagal activity). These patients had significantly more ventricular arrhythmias and sudden cardiac death (44% vs 17%) than patients with Brugada syndrome without these findings on the exercise test.14 Another study demonstrated much higher frequency of ST-segment augmentation in right precordial leads (69% vs 7%) in patients with a positive response to the tilt test.15 Ikeda et al 16 proposed a full stomach test to identify patients at risk for Brugada syndrome.
Our patient had short-coupled VPBs (260–280 ms), which initiated episodes of VF. While some reports demonstrated rather long-coupled VPBs initiating ventricular arrhythmias in these patients,13, 17 other studies demonstrated short-coupled VPBs causing ventricular arrhythmias in both experimental models and clinical studies in Brugada syndrome.10, 18, 19 Short-coupled VPB is consistent with the phase 2 reentry hypothesis, which is itself a result of transmural inhomogeneity.10
Isoproterenol’s effectiveness in the prevention of recurrent VF in patients with Brugada syndrome is consistent with a vagal tone proarrhythmic effect in these patients.20, 21 Quinidine is effective in restoring the epicardial action potential dome and normalizing the ST-segment elevation and preventing phase 2 reentry.8, 22 Our patient had 2 syncopal episodes and 2 documented VF events in a period of 3 weeks. Daily treatment with quinidine prevented any syncopal or ventricular arrhythmia event for more than 11 years.
Our patient presented with AF after syncope. AF is the most common atrial arrhythmia in patients with Brugada syndrome and may be its first manifestation23, 24; it is associated with an unfavorable prognosis.6 Brugada syndrome should be excluded in young patients with AF and normal heart and ECG, because the administration of class IC antiarrhythmic agents may cause ventricular arrhythmias and cardiac arrest.
In conclusion, our case shows many faces of Brugada syndrome, including syncopal events, AF at presentation, and an intermittent Brugada pattern on the ECG. The uniqueness of the current case is a clear demonstration of vagal-mediated Brugada’s ST elevation, short–coupling interval VPB, induction of VF, and response to quinidine. The efficacy of the quinidine in this patient may be due not only to the inhibition of the Ito current, but also to its known vagolytic effect.25, 26
Increased vagal activity increased the endocardial-epicardial heterogeneity and predisposed the patient to VF. The combination of ICD implantation to terminate VF episodes and quinidine therapy to prevent the recurrence of VF has been proven to be effective in our patient.
Because ECG signs of Brugada syndrome may be intermittent (as it was in our case), the pharmacologic challenge to exclude Brugada syndrome should be strongly considered in patients presenting with “vagal” syncope, especially a recurrent one. Presence of AF makes this testing even more compulsory.
References
- 1.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q., Kirsch G.E., Zhang D. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morita H., Zipes D.P., Wu J. Brugada syndrome: insights of ST elevation, arrhythmogenicity, and risk stratification from experimental observations. Heart Rhythm. 2009;6:S34–S43. doi: 10.1016/j.hrthm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Gehi A.K., Duong T.D., Metz L.D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–583. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 6.Kusano K.F., Taniyama M., Nakamura K. Atrial fibrillation in patients with Brugada syndrome: relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51:1169–1175. doi: 10.1016/j.jacc.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 7.Sarkozy A., Boussy T., Kourgiannides G. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334–344. doi: 10.1093/eurheartj/ehl450. [DOI] [PubMed] [Google Scholar]
- 8.Belhassen B., Glick A., Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 9.Nademanee K., Veerakul G., Chandanamattha P. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 10.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K., Kurita T., Inagaki M. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J. 1999;20:465–470. doi: 10.1053/euhj.1998.1332. [DOI] [PubMed] [Google Scholar]
- 12.Mizumaki K., Fujiki A., Tsuneda T., Sakabe M., Nishida K., Sugao M., Inoue H. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:667–673. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 13.Kasanuki H., Ohnishi S., Ohtuka M., Matsuda N., Nirei T., Isogai R., Shoda M., Toyoshima Y., Hosoda S. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997;95:2277–2285. doi: 10.1161/01.cir.95.9.2277. [DOI] [PubMed] [Google Scholar]
- 14.Makimoto H., Nakagawa E., Takaki H., Yamada Y., Okamura H., Noda T., Satomi K., Suyama K., Aihara N., Kurita T., Kamakura S., Shimizu W. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol. 2010;56:1576–1584. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Yokokawa M., Okamura H., Noda T., Satomi K., Suyama K., Kurita T., Aihara N., Kamakura S., Shimizu W. Neurally mediated syncope as a cause of syncope in patients with Brugada electrocardiogram. J Cardiovasc Electrophysiol. 2010;21:186–192. doi: 10.1111/j.1540-8167.2009.01599.x. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T., Abe A., Yusu S., Nakamura K., Ishiguro H., Mera H., Yotsukura M., Yoshino H. The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:602–607. doi: 10.1111/j.1540-8167.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 17.Kakishita M., Kurita T., Matsuo K., Taguchi A., Suyama K., Shimizu W., Aihara N., Kamakura S., Yamamoto F., Kobayashi J., Kosakai Y., Ohe T. Mode of onset of ventricular fibrillation in patients with Brugada syndrome detected by implantable cardioverter defibrillator therapy. J Am Coll Cardiol. 2000;36:1646–1653. doi: 10.1016/s0735-1097(00)00932-3. [DOI] [PubMed] [Google Scholar]
- 18.Kyriazis K., Bahlmann E., van der Schalk H., Kuck K.H. Electrical storm in Brugada syndrome successfully treated with orciprenaline; effect of low-dose quinidine on the electrocardiogram. Europace. 2009;11:665–666. doi: 10.1093/europace/eup070. [DOI] [PubMed] [Google Scholar]
- 19.Gang E.S., Priori S.S., Chen P.S. Short coupled premature ventricular contraction initiating ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:837. doi: 10.1046/j.1540-8167.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H., Kinoshita O., Uchikawa S., Kasai H., Nakamura M., Izawa A., Yokoseki O., Kitabayashi H., Takahashi W., Yazaki Y., Watanabe N., Imamura H., Kubo K. Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1293–1294. doi: 10.1046/j.1460-9592.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- 21.Maury P., Hocini M., Haissaguerre M. Electrical storms in Brugada syndrome: review of pharmacologic and ablative therapeutic options. Indian Pacing Electrophysiol J. 2005;5:25–34. [PMC free article] [PubMed] [Google Scholar]
- 22.Schweizer P.A., Becker R., Katus H.A. Successful acute and long-term management of electrical storm in Brugada syndrome using orciprenaline and quinine/quinidine. Clin Res Cardiol. 2010;99:467–470. doi: 10.1007/s00392-010-0145-7. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T., Watanabe I., Okumura Y. Atrial electrophysiological abnormality in patients with Brugada syndrome assessed by P-wave signal-averaged ECG and programmed atrial stimulation. Circ J. 2006;70:1574–1579. doi: 10.1253/circj.70.1574. [DOI] [PubMed] [Google Scholar]
- 24.Letsas K.P., Sideris A., Efremidis M. Prevalence of paroxysmal atrial fibrillation in Brugada syndrome: a case series and a review of the literature. J Cardiovasc Med (Hagerstown) 2007;8:803–806. doi: 10.2459/JCM.0b013e3280112b21. [DOI] [PubMed] [Google Scholar]
- 25.Alboni P., Paparella N., Pirani R., Cappato R., Cuccí A.M., Ruffilli E., Tomasi A.M. Different electrophysiological modes of action of oral quinidine in man. Eur Heart J. 1985;6:946–953. doi: 10.1093/oxfordjournals.eurheartj.a061792. [DOI] [PubMed] [Google Scholar]
- 26.Cappato R., Alboni P., Codecà L., Guardigli G., Toselli T., Antonioli G.E. Direct and autonomically mediated effects of oral quinidine on RR/QT relation after an abrupt increase in heart rate. J Am Coll Cardiol. 1993;22:99–105. doi: 10.1016/0735-1097(93)90822-i. [DOI] [PubMed] [Google Scholar]