Introduction
KEY TEACHING POINTS
|
Implantable cardioverter-defibrillators (ICDs) have become a life-saving adjunct treatment over the past 2 decades for preventing sudden cardiac death (SCD) due to ventricular arrhythmias.1 Despite the proven clinical benefit of ICDs in preventing SCD in various clinical trials, endovascular lead-related complications and inappropriate therapy remain a challenge. The subcutaneous ICD (S-ICD) system (Boston Scientific, Marlborough, MA) offers an effective alternative treatment option in a select cohort of high-risk patients.2
Inappropriate ICD shocks (IASs) contribute to adverse outcomes in patients with ICD, and therefore, device technology has been upgraded over the years to include rate-, duration-, and stability-based algorithms that prevent such events. Although the S-ICD demonstrated a greater specificity in discriminating supraventricular from ventricular tachycardia in the Subcutaneous versus Transvenous Arrhythmia Recognition Testing (START) study, the rate of IASs in various studies ranges between 5% and 25%.3, 4 T-wave oversensing (TWOS), low amplitude sensing, and supraventricular arrhythmia above the discrimination zone were the most commonly reported reasons in various studies including the EFFORTLESS registry and IDE study.2, 5 The incidence of IASs can be reduced by preprocedural screening (particularly in young patients with hypertrophic cardiomyopathy [HCM]), appropriate vector selection (alternate vector being a predictor of oversensing), exercise testing, and dual-zone programming.6, 7, 8
While IASs due to low amplitude R waves with position change in patients programmed to the alternate vector has been described before, ICD discharge due to an increase in R-wave amplitude and a shift in baseline has not previously been reported in the current EMBLEM S-ICD (Boston Scientific, Marlborough, MA).9
Case report
A 23-year-old female patient with a family history of cardiomyopathy and positive genetic testing for TNNT gene mutation received a Boston Scientific A209 EMBLEM S-ICD 4 weeks postpartum. She previously had a transvenous ICD with a Medtronic Sprint Fidelis lead placed at the age of 13. A pediatric cardiologist had recommended device placement after positive genetic screening of the patient and the demise of her father and a sibling due to sudden cardiac arrest. This device had reached elective replacement indicator during the last trimester of her pregnancy and almost concurrently developed high ICD pace-sense lead impedance suggestive of impending lead fracture. Given the patient’s age, prior endovascular lead-related complications, unwillingness to proceed with lead extraction, and reported history of “appropriate” ICD shocks by her mother, she was considered a suitable candidate for S-ICD implantation. Her baseline laboratory data were within normal limits, and an echocardiogram showed normal left ventricular ejection fraction and no hypertrophy. An electrocardiogram (ECG) at baseline showed sinus rhythm and a narrow QRS complex with nonspecific ST-segment depression. Device implantation was performed after the patient passed standard ECG screening algorithm in two vectors in both supine and standing positions. The baseline R-wave amplitude was large but within acceptable limits, and the device sensing vector was set to primary vector by the automatic algorithm. In addition, 2 zones were programmed with a shock zone at 240 beats/min and a conditional zone at 200 beats/min.
The patient presented with multiple ICD shocks 2 weeks later while she was walking. None of the episodes were preceded by anticipated symptoms. She had no evidence of ventricular arrhythmia upon device interrogation. Her QRS amplitude was noted to have increased since the implantation, exceeding the preset device sensing algorithm. This led to a shift in the baseline and predisposed the patient to double counting and oversensing (Figure 1). The device was reprogrammed to the alternate vector with smallest amplitude QRS (Figure 2), and device function was confirmed in different positions as well as with exercise. No further oversensing has been recorded since reprogramming.
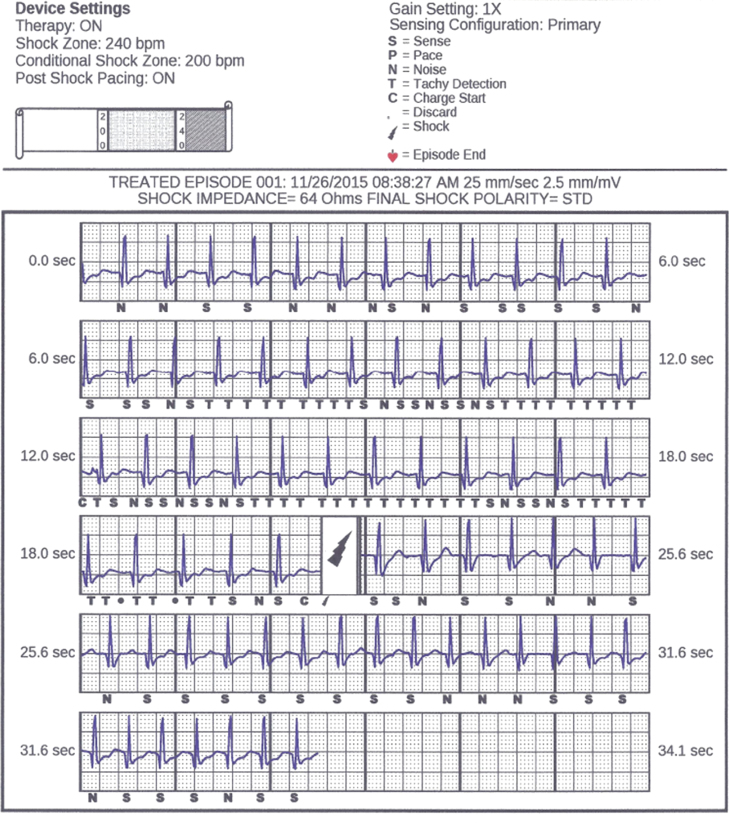
Figure 1.
Subcutaneous implantable cardioverter-defibrillator interrogation strip displaying patient in sinus tachycardia. Overcounting and inappropriate therapy resulted from signal rectification in the presence of tall R waves and shift in baseline.
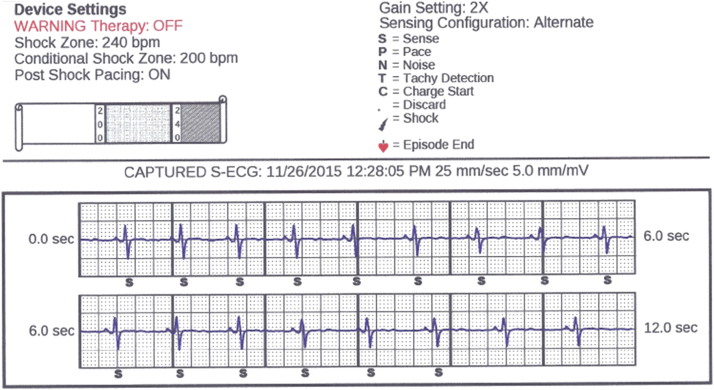
Figure 2.
Alternate vector selection with lowest amplitude R wave.
Discussion
We report a case of inappropriate shocks in a patient with S-ICD due to a rare cause of oversensing and signal classification. R-wave amplitude variation is a known entity, particularly in younger patients, with age and exercise. Prior clinical reports and registry data suggest that IASs with TWOS is observed more commonly with exercise, alternate vector setting, low amplitude R waves, and posture-related R-wave amplitude change.9, 10, 11 To prevent signal and noise oversensing of subcutaneous ECG signals, the S-ICD is equipped with a sensing algorithm threshold that adapts to R-wave amplitude and decays over time. In addition, cardiac signals are run through 4 double-detection algorithms that correlate similarity between stored template at rest and tachycardia beats, current and previous tachycardia beats (beat-to-beat polymorphic variation), QRS width compared to the baseline (beat-to-beat monomorphic relationship), and correlation of the existing complex to previous complexes.3, 12 There are previously reported cases of inappropriate ICD discharge occurring with low amplitude R waves, resulting in rapid decay of sensing threshold and oversensing of non-QRS physiological signals.9
To our knowledge however, there have been no prior reports in the EMBLEM series devices of an increase in R-wave amplitude leading to inappropriate signal classification. To date, a small number of such events (<10 of the 17,000 S-ICD implants) have been reported correlating with a rate of 0.06% on a per device basis (reported based on personal communication with Boston Scientific)13. The potential to introduce errors of this nature during signal classification is more likely when the device attempts to digitalize analog signals of amplitudes larger than the maximum range of the S-ICD system.
The S-ICD system is designed to accommodate R-wave amplitudes that exceed ~3 times the typical peak amplitudes of ~1.0 mV. The system analyzes the analog input signal slew rate (in mV/ms) before its digital conversion. The slew rate limit defined in the S-ICD system is based on cardiac signals whereby amplitude changes between baseline and the maximum allowed amplitude (~4.0 mV) of the S-ICD system change rapidly (eg, within a few milliseconds, ~10 ms). In this case, a baseline offset was introduced as the rapid amplitude change exceeded the slew rate design limit.
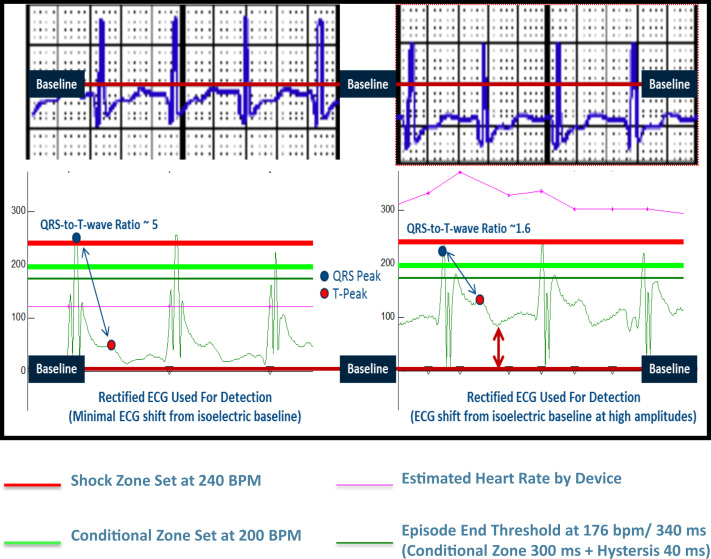
Furthermore, the ECG used for sensing is rectified before detection, thereby converting it into all positive signals above the “zero” baseline. A QRS-T ratio of ≥3.5 is recommended to decrease the likelihood of oversensing. However, a shift in baseline from zero to nonzero value results in the reduction of the QRS-T with subsequent overcounting and IASs (Figure 3). After shock delivery, the threshold is reset to that originally programmed. Using the characteristics of the ECGs (high QRS amplitude, high slew rates, and elevated heart rates) from these episodes, nonzero baseline along with oversensing was successfully reproduced with internal testing. An engineering design limitation to accommodate high slew rates especially at elevated heart rates was identified as the most likely root cause (Figure 4).
Figure 3.
Baseline offset with high QRS amplitude decreased the QRS/T-wave ratio and an increased predisposition to oversensing. ECG = electrocardiogram. © Boston Scientific, published with permission.
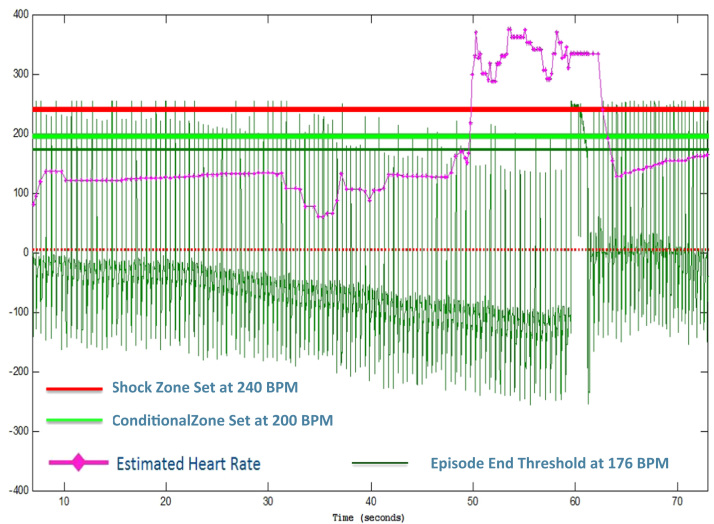
Figure 4.
Recreated oversensing event with nonzero baseline, high QRS amplitude, and slew rates during internal testing. © Boston Scientific, published with permission.
Since this patient had been screened with a standard ECG algorithm and the alternate vector was available for selection, the device was reprogrammed. Device function was tested after reprogramming in multiple positions and with exercise. No further events have been recorded to date.
The current guidelines recommend against the placement of a primary prevention ICD in genotype-positive patients with HCM in the absence of left ventricular hypertrophy or high-risk features based on potential harm of the procedure and long-term risk of device-related complications. Recent registry data have identified patients with HCM as the cohort particularly susceptible to IASs due to TWOS. Given the multiple ECG abnormalities including high R- or T-wave amplitude, presence of T-wave inversion in >2 leads, and ST-segment depression, a higher percentage of these patients maybe ineligible for S-ICD than previously reported.8, 10 It is also important to note that a number of these patients are young, more likely to achieve higher heart rate with activity, exhibit R-wave amplitude variation, and will carry the device for a long period given the overall good prognosis. A subset of these patients will, however, develop progressive left ventricular dysfunction and correlate changes in ECG with the possibility of device failure. This is also the population that is at higher risk of having malignant ventricular arrhythmia. Although our patient did have baseline T-wave abnormalities in more than 2 leads, she had passed the initial screening in 2 vectors and positions before implantation.
Suggested solutions for addressing TWOS in patients with cardiomyopathies particularly HCM is screening with a disease-specific preimplantation algorithm, such as addition of 2.5 mm/mV gain and selection of alternate vector in select cases. Although off-label, some operators also choose to implant the lead on the right side of the sternum instead of left if it allows for a more suitable R-wave amplitude. The new SMARTPASS feature, which is included in the latest EMBLEM S-ICD software update, has been observed with bench testing data to reduce the likelihood of oversensing caused by transient large R-wave amplitude variation. The update is expected to reduce the risk of this unusual clinical problem.
Conclusion
S-ICDs have become a popular alternative therapy to prevent SCD particularly in younger patients with cardiomyopathy who do not require pacing. There are still limitations to the widespread use of this device pertaining to its function when presented with baseline ECG abnormalities, R- and T-wave amplitude variations in particular. Future device technology and software upgrades are expected to counteract these problems.
Acknowledgments
We thank Steven Donnelley, MSS, and the Boston Scientific Engineering Department for their continued support in the preparation of this manuscript.
References
- 1.Priori G.S., Blomström-Lundqvist C., Mazzanti A. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 2.Burke M.C., Gold M.R., Knight B.P. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1616. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Gold M.R., Theuns D.A., Knight B.P., Sturdivant J.L., Sanghera R., Ellenbogen K.A., Wood M.A., Burke M.C. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012;23:359–366. doi: 10.1111/j.1540-8167.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 4.Aziz S., Leon A.R., El-Chami M.F. The subcutaneous defibrillator: a review of the literature. J Am Coll Cardiol. 2014;63:1473–1479. doi: 10.1016/j.jacc.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Olde Nordkamp L., Abkenari L.D., Boersma L.V.A., Maass A.H., de Groot J.R., van Oostrom A.J., Theuns D.A., Jordaens L.J., Wilde A.A., Knops R.E. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939. doi: 10.1016/j.jacc.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Gold M.R., Weiss R., Theuns D.A., Smith W., Leon A., Knight B.P., Carter N., Husby M., Burke M.C. Use of a discriminator algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2014;11:1352–1358. doi: 10.1016/j.hrthm.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Kooiman K.M., Knops E.R., Olde Nordkamp L., Wilde A.A., de Groot J.R. Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T-wave oversensing can be prevented: implications for management. Heart Rhythm. 2014;11:426–434. doi: 10.1016/j.hrthm.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Gersh B.J., Maron B.J., Bonow R.O. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Sharma D., Sharma P.S., Miller M.A., Singh S.M., Kalahasty G., Ellenbogen K.A. Position and sensing vector-related triple counting and inappropriate shocks in the subcutaneous implantable cardioverter-defibrillator system. Heart Rhythm. 2015;12:2458–2460. doi: 10.1016/j.hrthm.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Maurizi N., Olivotto I., Olde Nordkamp L., Baldini K., Fumagalli C., Brouwer T.F., Knops R.E., Cecchi F. Prevalence of subcutaneous implantable cardioverter-defibrillator candidacy based on template ECG screening in patients with hypertrophic cardiomyopathy. Heart Rhythm. 2016;13:457–463. doi: 10.1016/j.hrthm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Olde Nordkamp L., Brouwer T.F., Barr C. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135. [DOI] [PubMed] [Google Scholar]
- 12.Brisben A.J., Burke M.C., Knight B.P., Hahn S.J., Herrmann K.L., Allavatam V., Mahajan D., Sanghera R., Gold M.R. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol. 2015;4:417–423. doi: 10.1111/jce.12612. [DOI] [PubMed] [Google Scholar]
- 13.Steven Donnelly M.S.S. Boston Scientific Technical Services. Verbal/Written Communication on May 6. 2016 [Google Scholar]