Introduction
KEY TEACHING POINTS
|
Mutations in the human calmodulin genes (CALM1, CALM2, and CALM3) are associated with life-threatening conditions in childhood, such as idiopathic ventricular fibrillation (VF) and long QT syndrome (LQTS).1, 2, 3 Furthermore, CALM1 mutations were described in a catecholaminergic polymorphic ventricular tachycardia (CPVT)-like phenotype.4 Sudden unexplained death in the young can be the first clinical manifestation of an underlying arrhythmogenic disorder such as idiopathic VF.5, 6 After an aborted cardiac arrest, determining the diagnosis begins with a systematic clinical evaluation. Subsequently, targeted genetic testing may be considered for genetic confirmation and family screening,7, 8 although in the absence of a significant family history or electrocardiographic abnormalities, identification of the disease etiology should be limited to previously recognized conditions.9
Here we report a novel de novo missense mutation in CALM1 associated with a phenotype compatible with LQTS in a child who experienced an aborted first-episode cardiac arrest.
Case report
The patient was a 6-year-old, otherwise healthy boy (the proband) born to seemingly healthy parents. He had a mild pervasive developmental disorder, with hyperactivity and mild bronchial asthma. His 5 siblings were healthy, and there was no family history of syncope or sudden death.
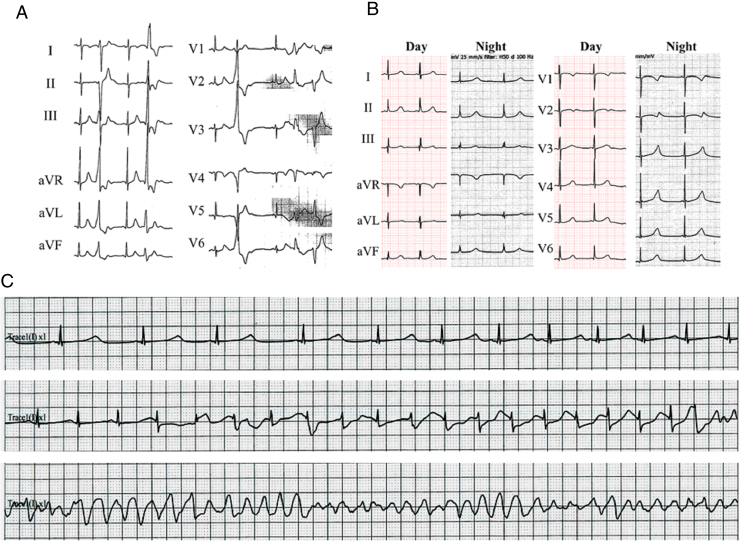
After a breakfast at his home, the boy suddenly lost consciousness, collapsed, and was unresponsive. His mother called for an ambulance. On arrival, the emergency medical staff confirmed cardiopulmonary arrest and performed cardiopulmonary resuscitation. The automated external defibrillator showed VF, and the patient was successfully cardioverted. He was intubated and taken to the local community hospital. Arterial blood gas analysis showed combined respiratory and metabolic acidosis thought to be related to the cardiopulmonary resuscitation. An infusion of dopamine induced premature ventricular contractions, singly and in bidirectional couplets, which responded to xylocaine (Figure 1A). He was then transferred to our hospital for further evaluation and treatment. VF recurred on arrival at our hospital’s emergency room. A direct current shock and 150 mg of amiodarone were administered, and he was transferred to the pediatric intensive care unit. Blood samples taken at the time of admission showed normal biochemistry, including normal troponin T and creatinine kinases. Transthoracic echocardiography was normal, with good left ventricular systolic function. A resting baseline 12-lead electrocardiogram (ECG) did not indicate any abnormality such as LQTS or Brugada syndrome. At midnight, he developed polymorphic ventricular tachycardia, which degenerated to VF with remarkable QT prolongation (Figure 1B). When the patient awoke the next day, torsades de pointes recurred with an increasing heart rate, despite the continuous infusion of xylocaine (1 mg/kg/h) (Figure 1C). An additional continuous infusion of propranolol (0.01 mg/kg/h) and mexiletine (0.5 mg/kg/h) was commenced. The triple regimen of xylocaine, propranolol, and mexiletine was markedly effective. The patient was successfully extubated and was administered oral medication of propranolol and mexiletine. He experienced no further episodes of syncope or seizure on the regimen of beta-blockers and mexiletine. His exercise test on the regimen did not reach a definitive result owing to his intolerance to the test. Invasive electrophysiological testing was not performed. An implantable cardioverter-defibrillator (ICD) was not recommended because of the risk of inappropriate shocks, which could provoke more severe anxiety and possible ICD storms.
Figure 1.
A: Initial 12-lead electrocardiogram shows bidirectional premature ventricular beats. B: QT prolongation during sinus bradycardia at night. C: Torsades de pointes with increasing heart rate.
Genetic testing
Using the candidate gene approach, we initially screened the authentic long QT syndrome genes KCNQ1, KCNH2, SCN5A, and KCNE1 but found no mutation in them. When screening for other candidate genes, we identified a novel missense variation c.A314>C in exon 5 of CALM1. This nucleotide change predicts the substitution of a conserved glutamic acid residue with alanine (p.E105A) within the third EF-hand calcium-binding motif in the C-terminal domain of the encoded calmodulin protein (Figure 2). This variation is not registered in any public DNA variation databases, including the NHLBI GO Exome Sequencing Project, 1000 Human Genome Project, Exome Aggregation Consortium, and Japanese exome database HGVD (http://www.genome.med.kyoto-u.ac.jp/SnpDB/. Accessed June 2016). In silico variant effect prediction programs Polyphen2 and SIFT gave the results “deleterious” (score 0) and “probably damaging” (score 0.997), respectively.
Figure 2.
A novel CALM1 mutation E105A. Upper panel indicates the sequence pherogram of the novel CALM1 missense mutation E105A. Lower panel shows schematic representations of the Ca2+ binding loops in the N-terminal (I and II) and C-terminal (III and IV) and the locations of mutations. Red circle represents the CALM1-E105A identified in our present study and green symbols represents CALM1 and CALM2 mutations previously reported.
Clinical evaluation and genetic testing of family members
The patient had 4 siblings: 3 sisters and 1 brother, aged 8–15 years. None had any known cardiac diseases or symptoms. The parents and siblings had normal ECG indices, including normal QTc intervals. Both parents turned out to be non-carriers of CAML1-E105A, demonstrating that this is a de novo mutation; therefore, we did not further investigate the siblings.
Discussion
Here we described a case of aborted cardiac arrest in a seemingly healthy preschool boy who exhibited profound QT prolongation with an increasing heart rate before the recurrence of polymorphic ventricular tachycardia at a pediatric intensive care unit.
Therapy for a cardiac arrest survivor largely depends on the underlying diagnosis, typically combining condition-specific medication with an ICD. The management of the patient’s family is driven by the outcome of a thorough evaluation of the index cardiac arrest survivor. Therefore, every effort should be made to determine the underlying pathophysiology in order to clarify the prognosis and establish the appropriate therapy. Genetic arrhythmia disorders require further investigation of family members who may be at risk.
As is characteristic of calmodulin gene mutations, the patient had a negative family history of heart disease, and his parents’ ECGs were normal. A cascade gene study was conducted on the index case and his parents, and the results were filtered to include a novel sporadic variant in the proband. Similar to other calmodulin mutations reported so far, E105A is located at the acidic residues located at the EF hands. It is speculated that E105A results in a substantial reduction of Ca2+ affinity, thereby disrupting the ability to transduce intracellular Ca2+ signals and leading to lethal arrhythmias, including severe catecholaminergic QT prolongation and torsades de pointes. It is difficult to identify individuals susceptible to lethal arrhythmias during neonatal ECG screening.
The patient also exhibited a developmental delay and mild bronchial asthma as an extracardiac manifestation. Although channelopathies in the brain are similar to the cardiac channelopathies in some inherited arrhythmia disorders,10 the cause of the patient’s cognitive delay is unknown.
Although the follow-up thus far has been short term (18 months), this patient has successfully responded to beta-blockade, the primary first-line treatment for this disease. Mexiletine therapy was added for the arrhythmia control in the present case as well as in the previous cases published by Crotti et al.11 However, CALM-related calcium channel dysfunction was proposed for the pathophysiological mechanism in calmodulionopathy.12, 13, 14 Therefore calcium antagonist may be a more beneficial treatment of the choice. If the patient continues to have syncope or arrhythmias on medication, he should be strongly considered for ICD implantation or cardiac sympathetic denervation to prevent life-threatening arrhythmias because of his history of bronchial asthma.
Conclusions
We report a novel de novo CALM1 missense mutation associated with a phenotype compatible with LQTS in a child who experienced an aborted first-episode cardiac arrest. Genetic testing for CALM mutations should be performed in children with QT prolongation, especially in those who have experienced an aborted cardiac arrest and who have a negative family history.
References
- 1.Makita N., Yagihara N., Crotti L. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ Cardiovasc Genet. 2014;7:466–474. doi: 10.1161/CIRCGENETICS.113.000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsman R.F., Barc J., Beekman L., Alders M., Dooijes D., van den Wijngaard A., Ratbi I., Sefiani A., Bhuiyan Z.A., Wilde A.A., Bezzina C.R. A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J Am Coll Cardiol. 2014;63:259–266. doi: 10.1016/j.jacc.2013.07.091. [DOI] [PubMed] [Google Scholar]
- 3.Boczek N.J., Gomez-Hurtado N., Ye D. Spectrum and prevalence of CALM1-, CALM2-, and CALM3-encoded calmodulin variants in long QT syndrome and functional characterization of a novel long QT syndrome-associated calmodulin missense variant, E141G. Circ Cardiovasc Genet. 2016;9:136–146. doi: 10.1161/CIRCGENETICS.115.001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyegaard M., Overgaard M.T., Søndergaard M.T. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong L.C., Behr E.R. Sudden unexplained death in infants and children: the role of undiagnosed inherited cardiac conditions. Europace. 2014;16:1706–1713. doi: 10.1093/europace/euu037. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman M., Atkins D.L., Triedman J.K. Sudden cardiac death in the young. Circulation. 2016;133:1006–1026. doi: 10.1161/CIRCULATIONAHA.115.020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerman M.J., Priori S.G., Willems S. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Europace. 2011;13:1077–1109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz P.J., Ackerman M.J., George A.L., Jr, Wilde A.A. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semsarian C., Ingles J., Wilde A.A. Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J. 2015;36:1290–1296. doi: 10.1093/eurheartj/ehv063. [DOI] [PubMed] [Google Scholar]
- 10.Takano K., Liu D., Tarpey P., Gallant E., Lam A., Witham S., Alexov E., Chaubey A., Stevenson R.E., Schwartz C.E., Board P.G., Dulhunty A.F. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum Mol Genet. 2012;21:4497–4507. doi: 10.1093/hmg/dds292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotti L., Johnson C.N., Graf E. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin G., Hassan F., Haroun A.R. Arrhythmogenic calmodulin mutations disrupt intracellular cardiomyocyte Ca2+ regulation by distinct mechanisms. J Am Heart Assoc. 2014;3:e000996. doi: 10.1161/JAHA.114.000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaix M.A., Koopmann T.T., Goyette P. Novel CALM3 mutations in pediatric long QT syndrome patients support a CALM3-specific calmodulinopathy. HeartRhythm Case Rep. 2016;2:250–254. doi: 10.1016/j.hrcr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Hurtado N., Boczek N.J., Kryshtal D.O. Novel CPVT-associated calmodulin mutation in CALM3 (CALM3-A103V) activates arrhythmogenic Ca waves and sparks. Circ Arrhythm Electrophysiol. 2016:9. doi: 10.1161/CIRCEP.116.004161. pii: e004161. [DOI] [PMC free article] [PubMed] [Google Scholar]