Introduction
KEY TEACHING POINTS
|
Catheter ablation therapy for atrial fibrillation (AF) is a safe and effective treatment for patients with both paroxysmal and persistent symptomatic AF. However, given the uncertainty about recurrence after the ablation procedure and continued risk of thromboembolism, it is still recommended to treat with anticoagulation for the long-term prevention of stroke. Left atrial appendage (LAA) occlusion with the WATCHMAN device (Boston Scientific Corp., Natick, MA) has demonstrated equivalent reduction in stroke compared to warfarin as well as a mortality benefit in patients with AF and CHADS2VASC2 score >1,1, 2 and it is increasingly being used as an alternative to warfarin for long-term prevention of stroke. A short-term requirement for antithrombotic therapy remains for 6 months while the device develops an endothelial layer.
Recently, some centers have begun to perform concomitant catheter ablation and WATCHMAN LAA occlusion.3, 4, 5, 6 This strategy has the benefit of providing treatment for symptomatic AF as well as stroke prevention without the need for long-term anticoagulation. However, there are currently no established guidelines for the type and duration of short-term antithrombotic therapy necessary to prevent device-related thrombosis (DRT) and stroke. Current antithrombotic strategies in studies involving concomitant AF ablation and WATCHMAN LAA occlusion are based on a combination of the HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of AF7 and the PROTECT AF trial protocol.1 These studies use differing antithrombotic strategies, however.
Given the numerous strategies used for anticoagulation in this emerging procedure, it is possible, or even expected, that health care providers, especially nonelectrophysiologists, may be confused about the best strategy to use, potentially leading to deleterious consequences in this patient population. We present a case of combined AF catheter ablation and WATCHMAN LAA closure that had early termination of anticoagulation, leading to DRT.
Case report
A 75-year-old woman with a history of aortic valve stenosis with subsequent bioprosthetic valve replacement, history of AF with prior pulmonary vein (PV) isolation via radiofrequency catheter ablation, and pacemaker implantation for an indication of tachycardia-bradycardia syndrome was referred to our practice for recurrent, symptomatic, drug-refractory AF. The patient had been trialed on multiple antiarrhythmic medications including flecainide, dronedarone, and amiodarone without success. In addition, the patient developed significant epistaxis with multiple visits to the emergency department despite low therapeutic international normalized ratios (INRs) and thus had discontinued warfarin. A novel oral anticoagulant (NOAC) drug was not considered because of the treating physicians concern for a lack of a reversal agent at the time. Her CHADS2VASC2 score was 3. After consultation with the patient, it was decided to pursue concomitant AF ablation and WATCHMAN LAA occlusion. The patient was resumption on warfarin and referred for the procedure 2 months later.
Preprocedure assessment
A transthoracic echocardiogram (TEE) revealed normal left ventricular function with an ejection fraction of 70% and mild aortic regurgitation. Computed tomography of the heart was performed to assess LAA features and to assist with appropriate device selection. The study showed a severely enlarged left atrium with a volume of 161 mL. The LAA was cactus shaped, with an ostium measuring 2.2 × 1.5 cm. Two lobes were noted, with depth to the more anterior lobe being 2.7 cm and the more posterior lobe being 2.3 cm. The patient was instructed to continue warfarin uninterrupted. The INR was 2.1 on the morning of the procedure.
Procedure details
The procedure was performed under general anesthesia with TEE guidance for both transseptal puncture and deployment of the LAA occlusion device. A ThermoCool SmartTouch (Biosense Webster Inc., Diamond Bar, CA) radiofrequency catheter was used to perform wide circumference ablation around the antrum of the PVs. Bidirectional block was confirmed within all 4 veins. In addition, the posterior left atrium was isolated with roof and posterior left atrial floor lines with confirmation of isolation.
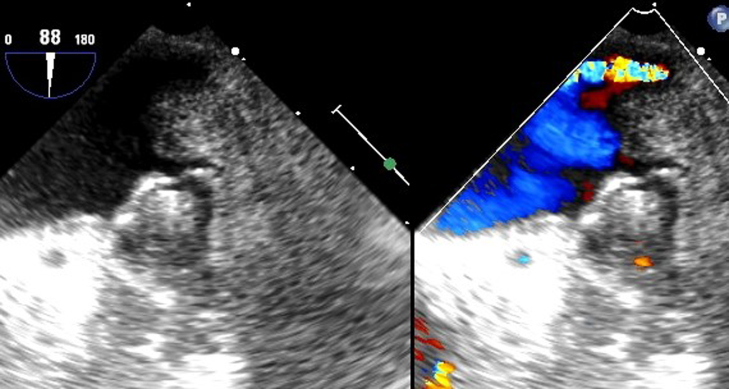
Immediately after the completion of AF ablation, the WATCHMAN LAA occlusion procedure was performed by the same operator. The sheath used for ablation was exchanged over a stiff wire (Amplatz, Boston Scientific Corp., Natick, MA) for the WATCHMAN deployment dual curve sheath. After confirmation of LAA ostium size with both angiography and TEE assessment, a 24-mm device was deployed and released. There was no leak visualized acutely after deployment (Figure 1).
Figure 1.
WATCHMAN initial deployment.
Postprocedural anticoagulation
The patient was continued on warfarin postprocedure. She received enoxaparin 0.5 mg/kg 6 hours after the procedure. She was discharged with a planned regimen of 3 months of warfarin and aspirin followed by 3 months of aspirin and clopidogrel. The INR was 2.2 upon discharge.
Postprocedure course
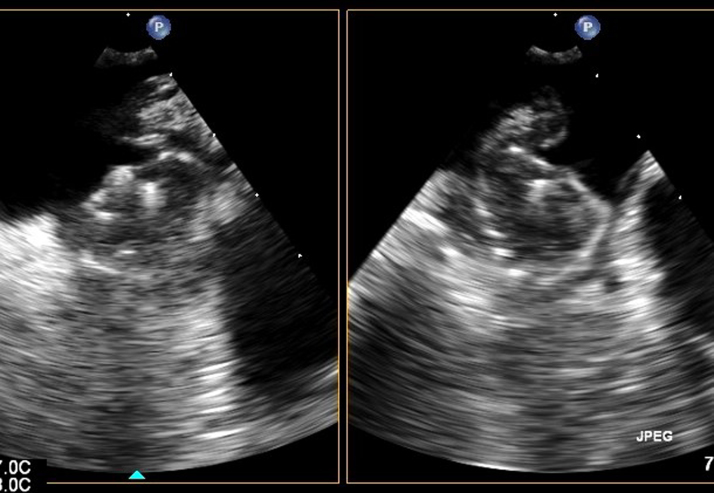
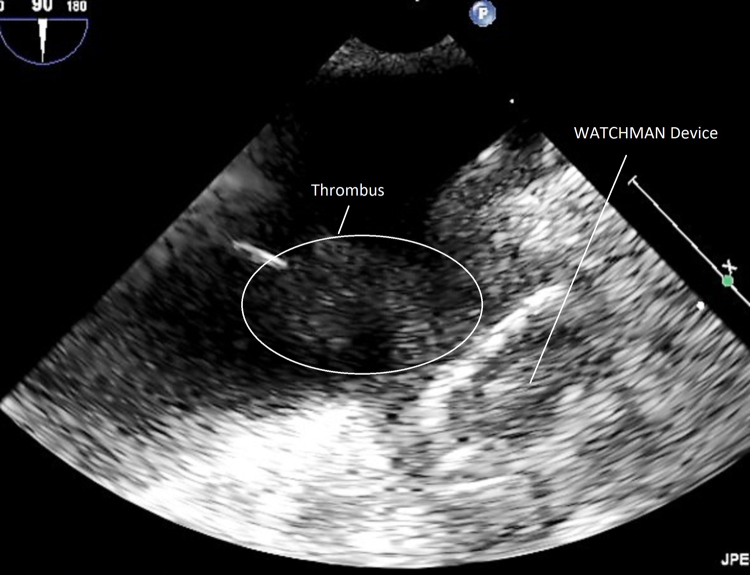
Three weeks postprocedure, the patient returned with heart failure symptoms and AF. A TEE revealed evidence of worsening bioprosthetic aortic valve regurgitation, and thus TEE was performed approximately 25 days postprocedure. This revealed a well-seated WATCHMAN device with no peridevice leak. There was no evidence of left atrial thrombus (Figure 2). However, the patient was noted to have moderate to severe aortic regurgitation. She recovered clinically and was managed as an outpatient. She returned again approximately 10 weeks after the procedure to the outside hospital with recurrent heart failure symptoms and paroxysmal AF. Noted in the admission History and Physical from that hospital was that she was no longer taking warfarin. Notes state that warfarin was discontinued because she was out from her procedure for 45 days. At this point, the outside hospital contacted our facility for her transfer for consideration of transcutaneous aortic valve replacement (TAVR). The patient was transferred to our facility, and TAVR was performed approximately 11.5 weeks after her initial procedure. The patient underwent successful TAVR with a 20-mm Sapien XT valve (Edwards Lifesciences, Irvine, CA). TEE performed after deployment revealed a large layered thrombus across the entirety of the WATCHMAN device along with a large mobile portion extending into the left atrium (Figure 3). The patient was given heparin drip along with warfarin. On the morning of postoperative day 2, the patient was noted to have right-sided weakness with subsequent unresponsiveness. Computed tomography of the head revealed a large left parietal hemorrhagic stroke. Imaging and clinical findings were suggestive of primary ischemic stroke with hemorrhagic conversion. Per patient and family wishes, no aggressive measures were performed. Care was withdrawn, and the patient subsequently died.
Figure 2.
WATCHMAN at 25 days.
Figure 3.
WATCHMAN at 11.5 weeks, showing layered and mobile thrombus.
Discussion
This case highlights 2 important issues: the need to define the optimal regimen and course of antithrombotic agents in combined AF catheter ablation and LAA occlusion cases as well as proper education of the health care team involved in the care of these patients. In this instance, anticoagulation was discontinued at 45 days, with only clopidogrel and aspirin being used subsequently. This may have been partly responsible for the development of DRT and subsequent stroke.
The PROTECT AF and PREVAIL trials used a regimen of anticoagulation for only 45 days before transition to aspirin and clopidogrel in those patients undergoing LAA occlusion with no increase in the incidence of stroke compared with patients receiving warfarin alone. Why then should patients undergoing this procedure concomitantly with AF catheter ablation require longer duration of anticoagulation therapy?
Multiple studies have shown the proinflammatory effects of ablation within the left atrium. One study8 showed a peak in CRP levels at 3 days postablation but a d-dimer level that remained elevated for 30 days postablation. Another study9 showed CRP levels persistently elevated for a median of 49 days after AF ablation. In addition, wide circumference ablation of the PVs typically includes a large amount of ablation along the ridge between the LAA and the left superior PV. This increased amount of local inflammation around the ostium of the LAA may further contribute to increased risk of thrombus formation after combined procedures. These observations have contributed to the HRS/EHRA/ECAS consensus statement,7 recommending at least 2 months of oral anticoagulation after AF ablation.
In patients receiving WATCHMAN LAA occlusion alone, there appears to be a higher incidence of DRT after anticoagulation is terminated. In the PROTECT AF trial, 27 of 485 patients (5.6%) developed DRT. Of these 27 patients, 7 (26%) developed while on warfarin and 19 (70%) were on aspirin and clopidogrel alone when presented at the 6-month follow-up. However, it should be noted that most of these resolved with resumption of anticoagulation therapy and did not result in stroke.1
Given the above information, it is reasonable to conclude that concomitant AF catheter ablation and WATCHMAN procedures confer a higher risk of DRT in the first few months postprocedure and thus a more aggressive antithrombotic strategy is necessary. There are several studies that can give us insight into the potential best strategy. There are 4 recent studies involving combined procedures for AF catheter ablation and WATCHMAN LAA occlusion. One study3 required 2 months of oral anticoagulation, while the other 3 studies4, 5, 6 required 3 months of oral anticoagulation before transition to dual antiplatelet therapy. There was only 1 ischemic event in all these studies. The event occurred 2 years postprocedure and was not associated with DRT. The vast majority of these cases used warfarin as the anticoagulant drug, with 1 study using NOAC therapy in a subset of patients. There was no increase in ischemic events in the NOAC group, leading the authors to conclude that the use of NOAC therapy in a combined procedure for AF catheter ablation and WATCHMAN prosthesis implant is safe.5
The use of NOAC drugs has been increasingly explored as a treatment option for the WATCHMAN procedure. A small study10 showed no DRT at 45 days after the WATCHMAN procedure in patients treated with a NOAC. A more recent and larger registry study11 examining 214 patients who received one of the NOAC drugs after the WATCHMAN procedure showed no difference in DRT or ischemic events during follow-up as compared to a matched warfarin group.
Based on the above observations, it seems the best antithrombotic strategy for combined procedures at this time will include at least 2 months of oral anticoagulation but preferably 3 if patients can tolerate it, followed by continuation of dual antiplatelet therapy to complete a total course of 6 months after lifelong aspirin therapy alone. Either warfarin or NOAC can be used for anticoagulation.
Our practice is to anticoagulate for 3 months with warfarin or NOAC in addition to aspirin, with TEE performed at 3 months instead of 45 days. We then transition to a combination of aspirin and clopidogrel if no significant leak or DRT is noted on the 3-month TEE.
Conclusion
A consensus statement that will allow for both clear recommendations and standardization of postprocedural therapy in this rapidly evolving field is needed. In this case, a misconception that the placement of a WATCHMAN LAA occlusion device required only 45 days of anticoagulation regardless of concomitant procedures may have led to this serious complication.
Acknowledgement
Open access publication of this article is supported by an unrestricted grant from Janssen Pharmaceuticals, a Johnson and Johnson Company. Janssen Pharmaceuticals was not involved in the selection, writing, or peer review of this article.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.hrcr.2016.11.008.
Appendix. Supplementary data
Video 1: Mobile thrombus attached to Watchman Device
Supplementary material
References
- 1.Reddy V.Y., Holmes D., Doshi S.K., Neuzil P., Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 2.Holmes D.R., Kar S., Price M.J., Whisenat B., Sievert H., Doshi S.K., Huber K., Reddy V.Y. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Calvo N., Salterain N., Arguedes H., Macias A., Esteban A., Yebenes M.G., Gavira J., Garcia-Bolao I. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace. 2015;17:1533–1540. doi: 10.1093/europace/euv070. [DOI] [PubMed] [Google Scholar]
- 4.Fassini G., Conti S., Moltrasio M. Concomitant cryoballoon ablation and percutaneous closure of left atrial appendage in patients with atrial fibrillation. Europace. 2016;18:1705–1710. doi: 10.1093/europace/euw007. [DOI] [PubMed] [Google Scholar]
- 5.Phillips K.P., Walker D.T., Humpries J.A. Combined catheter ablation for atrial fibrillation and Watchman left atrial appendage occlusion procedures: five-year experience. J Arrhythm. 2016;32:119–126. doi: 10.1016/j.joa.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panikker S., Jarman J., Virmani R. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:1–12. doi: 10.1161/CIRCEP.115.003710. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H., Kuck K., Cappato R. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;(14):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 8.Lim S., Schultz C., Dang J. Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:83–89. doi: 10.1161/CIRCEP.113.000876. [DOI] [PubMed] [Google Scholar]
- 9.McCabe J.M., Smith L., Tseng Z., Badhwar N., Lee B.K., Lee R.J., Scheinman M.M., Olgin J.E., Marcus G.M. Protracted CRP elevation after atrial fibrillation. Pacing Clin Electrophysiol. 2008;31:1146–1151. doi: 10.1111/j.1540-8159.2008.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosche L.I., Afshari F., Schone D., Ewers A., Mugge A., Gotzmann M. Initial experience with novel oral anticoagulants during the first 45 days after left atrial appendage closure with the Watchman device. Clin Cardiol. 2015;38:720–724. doi: 10.1002/clc.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enomoto Y., Gadiyaram V.K., Gianni C. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017;14(1):19–24. doi: 10.1016/j.hrthm.2016.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Mobile thrombus attached to Watchman Device
Supplementary material