Abstract
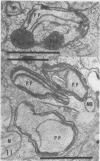
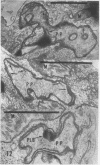
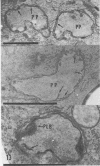
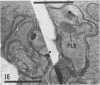
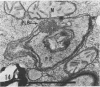
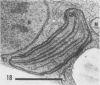
The herbicide SAN 9789 (4-chloro-5-(methylamino)-2-(α,α,α-trifluoro-m-tolyl-3- (2H)pyridazinone) blocks carotenoid synthesis in growing and resting cells of Euglena at concentrations of 20 to 100 μg/ml without affecting cell viability. Although the inhibition is immediate and complete, in resting cells no decrease in already synthesized carotenoids is found indicating a lack of turnover. In cells growing in the dark, carotenoids are diluted out as the cells divide. Cells dividing in the light in the presence of SAN 9789, eventually lose viability, presumably because of photooxidations usually prevented by carotenoids. During 72 hours of light-induced plastid development in dark-grown resting cells, none of the usual carotenoids increase while phytoene accumulates, indicating that SAN 9789 blocks carotenoid synthesis at this point. Chlorophyll synthesis and membrane formation are also blocked by the herbicide, but these inhibitions appear to be secondary to the inhibition of carotenoid synthesis. That carotenoid levels are strongly correlated with and may control the synthesis of chlorophyll and the formation of plastid membranes is suggested by the following data. (a) If dark-grown dividing cells are placed in the presence of the herbicide for various periods, rested and exposed to light in the presence of the drug, different amounts of carotenoids remain in the cells and the amount of chlorophyll finally synthesized is proportional to the amount of carotenoids present. (b) Photodestruction of chlorophyll is excluded, since the same amounts of chlorophyll are formed at intensities of 10 to 100 foot-candles of light. (c) Photoconversion of protochlorophyll(ide) to chlorophyll(ide) in dark-grown cells is not blocked by the herbicide. (d) Initial rates of chlorophyll synthesis are the same in treated and nontreated cells. (e) The extent of membrane formation appears to parallel the amount of carotenoids present as judged by electron microscopy.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett C. J., Walne P. L., Schwarz O. J., Brown D. H. Large Scale Isolation and Purification of Eyespot Granules from Euglena gracilis var. bacillaris. Plant Physiol. 1972 Jun;49(6):881–885. doi: 10.1104/pp.49.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kekwick R. G. Mevalonate kinase in green leaves and etiolated cotyledons of the french bean Phaseolus vulgaris. Biochem J. 1973 Jun;133(2):335–347. doi: 10.1042/bj1330335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRINSKY N. I., GOLDSMITH T. H. The carotenoids of the flagellated alga. Euglena gracilis. Arch Biochem Biophys. 1960 Dec;91:271–279. doi: 10.1016/0003-9861(60)90501-4. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I., Gordon A., Stern A. I. The Appearance of Neoxanthin during the Regreening of Dark-grown Euglena. Plant Physiol. 1964 May;39(3):441–445. doi: 10.1104/pp.39.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYMAN H., EPSTEIN H. T., SCHIFF J. A. Studies of chloroplast development in Euglena. I. Inactivation of green colony formation by u.v. light. Biochim Biophys Acta. 1961 Jun 24;50:301–309. doi: 10.1016/0006-3002(61)90328-6. [DOI] [PubMed] [Google Scholar]
- Liaaen-Jensen S., Andrewes A. G. Microbial carotenoids. Annu Rev Microbiol. 1972;26:225–248. doi: 10.1146/annurev.mi.26.100172.001301. [DOI] [PubMed] [Google Scholar]
- RABOURN W. J., QUACKENBUSH F. W., PORTER J. W. Isolation and properties of phytoene. Arch Biochem Biophys. 1954 Feb;48(2):267–274. doi: 10.1016/0003-9861(54)90341-0. [DOI] [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Seliskar C. J. Separation of phytylated and non-phytylated chlorophylls by thin-layer chromatography. Anal Biochem. 1966 Oct;17(1):174–177. doi: 10.1016/0003-2697(66)90021-2. [DOI] [PubMed] [Google Scholar]
- Stern A. I., Epstein H. T., Schiff J. A. Studies of Chloroplast Development in Euglena. VI. Light Intensity as a Controlling Factor in Development. Plant Physiol. 1964 Mar;39(2):226–231. doi: 10.1104/pp.39.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Mercer E. I., Goodwin T. W. Incorporation of [14C] carbon dioxide and [2-14C] mevalonic acid into terpenoids of higher plants during chloroplast development. Biochem J. 1966 Apr;99(1):239–245. doi: 10.1042/bj0990239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne P. L., Haber A. H., Triplett L. L. Photodestruction of chloroplast ultrastructure by red light: location of chlorophyll. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1501–1504. doi: 10.1073/pnas.67.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin M. H., Schiff J. A. RNA metabolism during light-induced chloroplast development in euglena. Plant Physiol. 1967 Jul;42(7):922–932. doi: 10.1104/pp.42.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]