Introduction
KEY TEACHING POINTS
|
The mechanism(s) perpetuating atrial fibrillation (AF) is poorly understood, and long-term success with catheter ablation remains suboptimal, especially in persistent AF. Ablation strategies beyond pulmonary vein (PV) isolation (PVI) have not been shown to provide incremental benefit in terms of maintaining sinus rhythm.1 However, there are data suggesting that acute termination of AF during ablation of extra-PV sources could lead to improved freedom from AF recurrence.2 As such, there is intense interest to better characterize and identify such potential “drivers” of AF, which may be amenable to ablation and improve outcomes. We recently described a hierarchical schema using stepwise analysis of bipolar electrogram (EGM) periodicity and unipolar QS morphology to identify focal sources (FS) that may be of significance in maintaining AF.3 In this report, we describe our experience in using this algorithm to identify and ablate extra-PV FS, which led to arrhythmia termination in a patient with persistent AF.
Case report
A 65-year-old woman presented with symptomatic, amiodarone refractory persistent AF for at least 5 months. Transthoracic echocardiography revealed a dilated left atrium (LA; 37 mL/m2) and preserved ventricular function with no significant valvular abnormalities. Institutional review board approval and written informed consent were obtained for PVI and real-time FS mapping/ablation, this being her first AF ablation procedure. Amiodarone was continued, and she was in AF at the commencement of the procedure.
Mapping protocol
A 4-pole catheter in the inferior vena cava was used as an indifferent terminal for unipolar EGM recordings. Double transseptal access was performed that permitted mapping of the LA with a variable curve 20-pole circular catheter (Lasso NAV, 15–25 mm diameter with 2-6-2 mm interelectrode spacing, Biosense Webster Inc., Diamond Bar, CA) and the CARTO-3 system (Biosense Webster Inc.). All regions in the LA and proximal PVs were carefully sampled with the circular catheter to obtain a high-density map containing 684 points. At each catheter location, 10 bipolar (30–500 Hz) and 10 unipolar (0.3–500 Hz) EGMs were simultaneously recorded for 5 seconds using a custom workstation running in parallel with the CARTO-3 system.
Hierarchical schema of EGM analysis
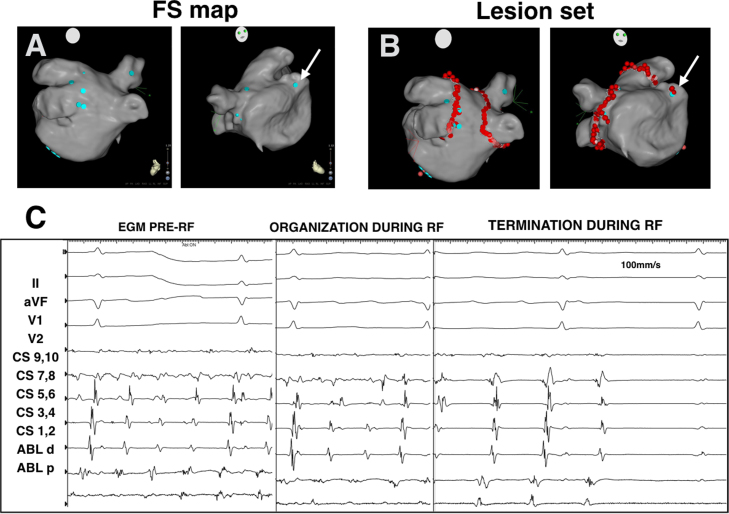
Upon completion of LA mapping, the EGM data were processed to identify FS using our previously described hierarchical analysis schema (Online Supplemental Methods).3 In brief, all sites with dominant periodicity within a cycle length (CL) range of 100–200 ms were identified using fast Fourier transformation of local bipolar EGMs. Once dominant periodicity in a particular location was confirmed, all periodic activations of that periodicity CL were annotated in the local bipolar EGM. These annotations were transposed to the corresponding unipolar EGM in order to define EGM onset and thereby characterize morphology features. FS were identified on the basis of bipolar EGM periodicity and stable unipolar QS morphology, defined as an R/S ratio <0.1 in at least 90% of periodic activations over 5 seconds. FS locations in the LA and PVs were then projected onto the anatomic map provided by the CARTO-3 system. A total of 15 FS were identified. Nine were within the PV antra (8 right, 1 left), and the remaining 6 were outside in the inferolateral LA (n = 5) and anterior base of the LA appendage (LAA) (n = 1) (Figure 1A).
Figure 1.
Distribution of focal sources (FS) and ablation lesions in the left atrium (LA). A: Distribution of FS (blue tags) in the LA and pulmonary veins (PV) shown in posteroanterior (left) and anteroposterior (right) views. B: Circumferential PV ablation (red tags) and FS ablation (red tags) sites. C: Termination of AF during ablation of FS at the base of the left atrial appendage (LAA) (white arrow).
Catheter ablation
Circumferential PV antral ablation was performed using 25–30 W of irrigated radiofrequency (RF) energy and 10–20 g of contact force delivered with a SmartTouch NaviStar Surround Flow catheter (Biosense Webster Inc.) (Figure 1B). Entrance block in all 4 PVs failed to terminate AF. All extra-PV FS outside the antral ablation lines were then ablated during ongoing AF using similar RF parameters, with the end point of reducing local bipolar EGM amplitude by >90%. Ablation of a FS along the base of the LAA terminated AF after a brief period of activation organization (Figure 1C). The total RF time to complete PV antral ablation was 58 minutes and an additional 12 minutes for extra-PV FS ablation.
Characteristics of FS and their relationship with other common EGM properties
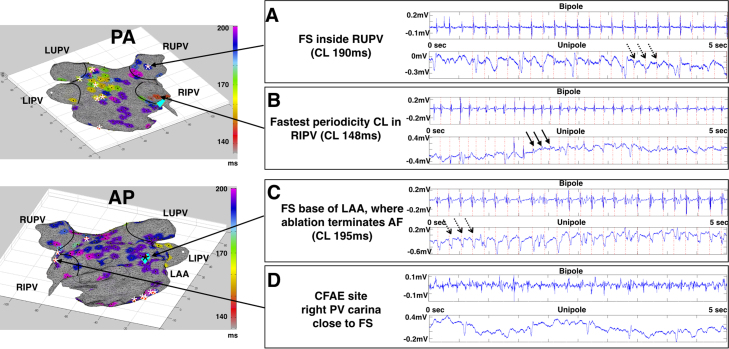
Figure 2 presents the LA periodicity map showing nonhomogeneous clusters of periodicity ranging in CL from 140 to 200 ms. The short periodicity CL (<170 ms) was predominant in the posterior wall near the left PVs, while the shortest periodicity CL of 140 ms was located in the right inferior PV (left top panel). A longer periodicity CL of 200 ms was present diffusely in the anterior wall and LAA (left bottom panel). The bipolar and unipolar EGMs of 2 FS are also shown in Figure 2, one in the right upper PV (panel A) and the other at the base of the LAA where ablation terminated AF (panel C). Both manifest a periodicity CL of ~190 ms and unipolar QS morphology. In contrast, the fastest periodicity CL occurred in the right inferior PV, but a distinct R wave was present in the unipolar EGM (panel B). By comparison, a highly fractionated EGM near the right PV carina did not show any periodicity or unipolar QS morphology (panel D).
Figure 2.
Periodicity map and EGM features of focal sources (FS). The left panel shows spatial distribution of dominant periodicities and their periodicity cycle length (CL) in the left atrium (LA) and pulmonary veins (PVs). White asterisk indicates FS location. AP = anteroposterior view; PA = posteroanterior view. The right panel shows bipolar and unipolar EGMs of selected LA and PV sites. Red dashed vertical lines indicate periodic activations in the bipolar EGM, which are transposed to the corresponding unipolar EGM. A: FS inside the right upper PV with unipolar QS complexes (dashed arrows). B: Fastest periodicity CL in the right inferior PV with a unipolar RS complex (solid arrows). C: FS at the base of the left atrial appendage (LAA) where AF terminated with ablation, showing unipolar QS complexes (dashed arrows). Black asterisk indicates location of this FS. D: CFAE site in the right PV carina close to FS with no periodicity or unipolar QS complex.
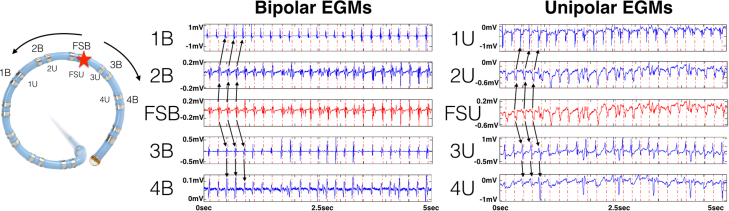
In order to verify wave propagation away from these FS, the bipolar and unipolar EGMs from adjacent recording electrodes of the same circular catheter acquisition were reviewed. Figure 3 demonstrates 4 key features supporting wave propagation away from the FS at the base of the LAA where ablation terminated AF. First, the periodicity CL of bipolar EGMs in adjacent electrodes was identical to that of the FS. Second, local activation times of bipolar EGMs in adjacent electrodes were later than those of the FS. Third, the polarity of the bipolar EGM reversed for electrodes on one side of the FS relative to those on the other side. Fourth, R-wave progression was present in the unipolar EGM of adjacent electrodes relative to the unipolar QS EGM at the FS.
Figure 3.
EGMs of focal source (FS) where AF terminated with ablation. The left panel shows a circular catheter. Red star indicates FS recording site. Arrows indicate wave propagation away from FS to adjacent bipoles (B) and unipoles (U). The right panels indicate bipolar and unipolar EGMs of FS and adjacent electrodes, confirming wave propagation away from FS (black arrows). Dashed vertical lines indicate periodic activations in the focal source - bipolar (FSB) recording channel, which are extended to adjacent bipolar channels and transposed to unipolar channels to provide a timing reference relative to the FSB channel.
Voltage mapping and complex fractionated atrial electrogram map
LA voltage and complex fractionated atrial electrogram (CFAE) maps were created to evaluate their spatial relationship to FS. The low-voltage substrate map (peak-to-peak bipolar EGM amplitude <0.5 mV) identified extensive regions of low bipolar voltage throughout the LA (Online Supplemental Figure S1A). The majority of FS in the LA and PV antra were located on the border zone of these low-voltage regions (13 of 14), including the FS at the base of the LAA where ablation terminated AF. The CFAE map (bipolar EGM interval confidence limit [ICL] >5) defined very few regions with ICL >5. Among the 6 small CFAE regions, only 1 in the left superior PV posterior antrum colocalized with a FS (Online Supplemental Figure S1B). In the remaining CFAE sites, the median distance to the closest FS was 1 cm.
Discussion
The optimal ablation strategy for patients with persistent AF remains unknown. In this population, PV antral ablation alone yielded 1-year AF freedom rates of only 50%–60%.1 As such, there is tremendous interest to identify novel ablation strategies to improve sinus rhythm maintenance post-PVI.4 However, randomized trials have not shown incremental benefit when additional non-PV sites, such as CFAE,5 dominant frequency (DF), or linear lesions, are targeted for ablation.6 FS represent another potential ablation target and have been demonstrated in patients with persistent AF using high-resolution intraoperative activation mapping of epicardial bipolar EGMs along with unipolar EGMs showing a QS pattern.7 Phase mapping of endocardial unipolar EGMs recorded from 64-electrode basket catheters in patients undergoing AF catheter ablation have also provided evidence for FS.8 Recently, Lin et al5 identified FS in patients with persistent AF on the basis of bipolar EGMs showing high similarity index and a vector field with low curl and high divergence.
In contrast, our hierarchical analysis schema identifies FS by determining the presence of local bipolar EGM periodicity and unipolar QS morphology among the periodic activations.3 When this algorithm was applied off-line to a series of 41 patients, 60% of all bipolar EGMs exhibited dominant periodicity, of which only 3.7% had a unipolar QS pattern. After PV antral ablation alone, AF recurrence was significantly greater in patients with extra-PV FS than in those with only PV FS, supporting their role in AF perpetuation. Thus, our parsimonious analysis strategy eliminated 98% of collected EGMs in the LA as non-FS sites, allowing the operator to focus on a small number of putative FS sites as potential ablation targets. In this report, we successfully applied our FS detection algorithm in real time and demonstrated persistent AF termination during FS ablation at the base of the LAA after PV antral ablation. Although AF termination has been associated with improved AF-free survival postablation,2 this end point is not yet a reproducible benchmark of long-term success. Therefore, we intend to complete 1-year clinical follow-up to assess the efficacy of our adjuvant ablation strategy.
Although the unipolar QS pattern can indicate the FS centroid with surrounding centrifugal wave propagation, other mechanisms may also produce this signature, such as epi- to endocardial wave propagation or epicardial breakthrough of a rotational source.8 In our patient, the presence of FS was supported by a careful analysis of adjacent bipolar and unipolar EGMs, which demonstrated centrifugal wave propagation on the basis of later activation times, bipolar EGM polarity reversal, and unipolar R-wave progression. This was evident for the FS at the base of the LAA where ablation terminated AF, thereby providing circumstantial evidence for its role in perpetuating AF. Because AF did not terminate after circumferential PV antral ablation, we speculate that a critical number of extra-PV FS were necessary in this patient to maintain AF once the PV FS were isolated with PVI.
Periodicity CL and FS
An interesting finding in our patient was the lack of relationship between periodicity CL and unipolar EGM morphology. Notably, sites with fast periodicity (CL 140 ms) did not necessarily manifest a unipolar QS pattern as evident in Figure 2B. In contrast, FS in the right upper PV and base of the LAA where ablation terminated AF had one of the slowest periodicity in the LA (190 and 195 ms, respectively) but demonstrated a unipolar QS pattern (Figures 2A and 2C). High DF sites have been proposed as AF drivers, particularly when surrounded by gradients of lower frequency. However, in a randomized controlled trial, DF ablation did not improve outcome in persistent AF compared to PVI alone.9 Based on our proposed hierarchal algorithm, some of these high DF sites may be passive to AF maintenance as evidenced by the presence of unipolar R waves. Our findings highlight the relevance of unipolar EGM morphology analysis in clarifying wave directionality in addition to periodicity detection.
Similarly, targeting possible AF drivers based on CFAE ablation has not been shown to improve clinical outcomes in patients with persistent or paroxysmal AF.1 An important limitation with CFAE mapping is the lack of consistent definitions and reproducibility among different commercial algorithms.10 Furthermore, CFAEs may arise from quite disparate electrophysiological processes including slow conduction or block, collision of wavefronts, and wave pivoting. In our case, CFAE map, based on widely accepted ICL >5, showed that most FS did not colocalize with CFAE zones. Rather, CFAEs were typically not periodic and had no unipolar QS features (Figure 2D). Because of their proximity to FS (within 1 cm), these CFAEs may simply be the result of passive wave collision in proximity to a high-frequency organized driver.
Atrial anatomy and FS
The relationship of FS relative to underlying low-voltage zones, a marker of atrial myopathy, merits further discussion. Our patient had substantial low-voltage substrate, and FS had a predilection for the border zones of low voltage with healthier myocardium. This spatially restricted pattern is consistent with optical mapping studies revealing microanatomic reentry in the border zones of fibrosis.11 Such locations have complex networks of fibrotic and nonfibrotic tissue with steep gradients of excitability and refractoriness that create a milieu for FS (micro anatomic reentry or triggered activations) formation. Ablation in such regions may homogenize the tissue to a more uniform fibrotic region, thus destabilizing or annihilating the FS.
The location of the FS where ablation terminated AF, at the anterior base of the LAA, is not an uncommon extra-PV site. In our series of 41 patients, 7% of FS were in a similar region.3 AF triggers have also been identified in the LAA in other observational studies7 such that LAA ablation and/or isolation has reduced AF recurrence.
Conclusion
We implemented a novel hierarchical analysis schema to identify and ablate FS in real time, which resulted in persistent AF termination after PV antral ablation. Our findings suggest that FS may be relevant to AF maintenance. The role of adjunctive FS ablation using our algorithm in reducing AF recurrence after PV antral ablation is currently being evaluated in a randomized controlled trial.
Acknowledgement
Open access publication of this article is supported by an unrestricted grant from Janssen Pharmaceuticals, a Johnson and Johnson Company. Janssen Pharmaceuticals was not involved in the selection, writing, or peer review of this article.
Footnotes
This study was supported by the Heart and Stroke Foundation of Ontario Career Award no. MC 7577, MaRS Innovation, and Biosense Webster Inc. (to Dr Chauhan). Dr Porta-Sánchez was supported by a fellowship award from “la Caixa” Foundation, Barcelona (Spain). Dr Nayyar was supported by a fellowship award from the Heart and Stroke Lewar Foundation.
Supplementary data associated with this article can be found in the online version at 10.1016/j.hrcr.2016.10.007.
Appendix. Supplementary data
Supplementary material
Supplementary material
Supplementary material
References
- 1.Verma A., Jiang C.Y., Betts T.R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 2.Lim H.S., Derval N., Komatsu Y., Zellerhoff S., Denis A., Shah A.J., Sacher F., Hocini M., Jaïs P., Haïssaguerre M. Is ablation to termination the best strategy for ablation of persistent atrial fibrillation? Persistent atrial fibrillation is best ablated by a strategy that terminates the arrhythmia: procedural termination is associated with improved long-term outcomes. Circ Arrhythm Electrophysiol. 2015;8:963–971. doi: 10.1161/CIRCEP.114.001721. [DOI] [PubMed] [Google Scholar]
- 3.Gizurarson S, Dalvi R, Das M, Ha ACT, Suszko A, Chauhan VS. Hierarchical schema for identifying focal electrical sources during human atrial fibrillation: implications for catheter-based atrial substrate ablation. JACC Clin Electrophysiol 2016;2:656–666. [DOI] [PubMed]
- 4.Kottkamp H., Berg J., Bender R., Rieger A., Schreiber D. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y-J, Lo M-T, Chang S-L, Lo LW, Hu Y-F, Chao T-F, Chung F-P, Liao J-N, Lin C-Y, Kuo H-Y. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC Clin Electrophysiol 2016;2:667–678. [DOI] [PubMed]
- 6.Verma A., Sanders P., Champagne J., Macle L., Nair G.M., Calkins H., Wilber D.J. Selective Complex Fractionated Atrial Electrograms Targeting for Atrial Fibrillation Study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol. 2014;7:55–62. doi: 10.1161/CIRCEP.113.000890. [DOI] [PubMed] [Google Scholar]
- 7.Lee S., Sahadevan J., Khrestian C.M., Cakulev I., Markowitz A., Waldo A.L. Simultaneous biatrial high-density (510-512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan S.M., Krummen D.E., Shivkumar K., Clopton P., Rappel W.-J., Miller J.M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atienza F., Almendral J., Ormaetxe J.M. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 10.Lau D.H., Maesen B., Zeemering S. Indices of bipolar complex fractionated atrial electrograms correlate poorly with each other and atrial fibrillation substrate complexity. Heart Rhythm. 2015;12:1415–1423. doi: 10.1016/j.hrthm.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Hansen B.J., Zhao J., Csepe T.A. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material