Introduction
KEY TEACHING POINTS
|
KCNJ2 encodes for the potassium inward rectifier channel protein Kir2.1, and mutations in KCNJ2 are associated with various inherited arrhythmia syndromes, such as Andersen-Tawil syndrome type 1 (ATS1), short QT syndrome 3, familial atrial fibrillation, and catecholaminergic polymorphic ventricular tachycardia 3.1, 2, 3, 4, 5 ATS1 is an autosomal dominant inherited disease with a multisystem phenotype consisting of periodic paralysis, ventricular arrhythmias, and craniofacial dysmorphic features.6 Ventricular arrhythmias can include frequent ventricular ectopy, bigeminy, polymorphic ventricular tachycardia (VT), and, less commonly, bidirectional VT. ATS patients electrocardiographically can show a normal or slightly increased QT interval but more commonly will have prominent U-waves and long QTu.7 The diagnosis of ATS is challenging owing to incomplete penetrance, varying phenotype severity among gene carriers, and phenotypic mimicry.8 Additionally, not all symptoms of the ATS triad clinically manifest contemporaneously. Common or subtle abnormalities can be overlooked or presumed to be normal variants. Here we present a case of ATS1 related to a novel KCNJ2 mutation, initially diagnosed as idiopathic high burden of premature ventricular contractions (PVCs).
Case Report
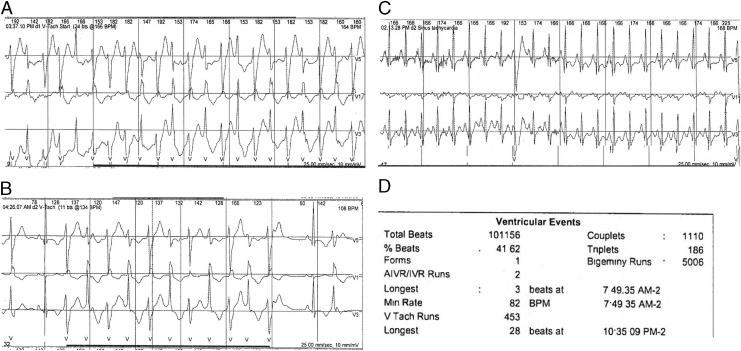
A 19-year-old woman presented to the University of Wisconsin Inherited Arrhythmias Clinic. Initially, she had undergone an extensive evaluation for complaints of palpitations at 9 years of age at an outside hospital. At that time, electrocardiogram (ECG) monitoring showed high burden of PVCs. At age 19 years she underwent an electrophysiology study at an outside hospital. It demonstrated polymorphic PVCs and the most commonly occurring (“dominant”) PVC was mapped to the region of the left ventricle anterior papillary muscle. Ablation resulted in transient suppression of this PVC morphology but no overall change in the frequency of the polymorphic PVCs. She was placed on verapamil and was referred to the University of Wisconsin Electrophysiology Clinic for possible repeat ablation. A Holter monitor after the ablation with the patient on verapamil ER (120 mg daily) demonstrated a 41% PVC burden, frequent bigeminy, and 453 runs of polymorphic VT (PMVT) and bidirectional VT. Examples of her PMVT and bidirectional VT are shown in Figure 1. A consultation with the University of Wisconsin Inherited Arrhythmias Clinic for consideration of an alternate diagnosis was requested.
Figure 1.
Example traces from 48 hour Holter monitor example traces. A: Run of polymorphic ventricular tachycardia (VT) that develops features of bidirectional VT; B: bidirectional VT; C: sinus tachycardia suppresses ventricular ectopy; D: summary data.
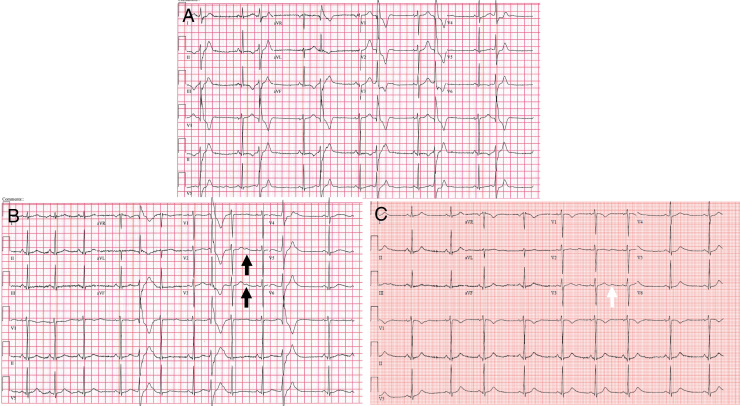
On presentation, she complained of palpitations and dyspnea but never had syncope. Physical examination revealed hypertelorism, micrognathia, and low-set ears. The presenting ECG showed ventricular bigeminy and repolarization assessment of the QTc or QTu was not feasible owing to ventricular bigeminy (Figure 2A). An exercise treadmill test was performed and sinus tachycardia suppressed ventricular ectopy at heart rates over 140 beats per minute.
Figure 2.
Resting electrocardiogram (ECG): A: ventricular bigeminy obscures QTc or QTu measurement; B: ECG on atenolol 12.5 mg twice daily (black arrows indicate postectopic U-wave accentuation); C: ECG on atenolol and flecainide (white arrows indicate U-wave).
Owing to her physical appearance, frequent ventricular ectopy, bidirectional VT, and ventricular arrhythmia suppression with sinus tachycardia, ATS was suspected. However, because she demonstrated bidirectional VT, which is also the signature arrhythmia for catecholaminergic polymorphic VT (CPVT), genetic testing via next-generation sequencing from a commercial laboratory was performed. Included in the panel are CPVT-related genes RYR2 and CASQ2, as well as KCNJ2, which may display phenotypic mimicry of CPVT. The data revealed no RYR2 or CASQ2 mutations but did identify a heterozygous variant (c.653 G>T) in exon 2 of KCNJ2, resulting in R218L. This variant was initially described as disease-causing but was reclassified as a variant of uncertain significance (VUS) under updated American College of Medical Genetics guidelines.9
Treatment of her arrhythmia was changed from verapamil to atenolol 12.5 mg twice daily (BID). On atenolol, a repeat ECG showed QTc of 447 ms and QTu of 558 ms (Figure 2B), with continued high burden of ventricular ectopy. An increased dose of atenolol was not tolerated owing to bradycardia and other beta-blockers were not tolerated owing to fatigue (metoprolol) or side effects (nadolol; major depression). Flecainide 50 mg BID was initiated and titrated up to 100 mg BID for PVC suppression. On combination therapy of flecainide and atenolol, she had resolution of her symptoms (Figure 2C). Repeat Holter monitoring showed that the PVC burden decreased to <1% with complete suppression of PMVT and bidirectional VT. She later experienced an episode of periodic paralysis, which spontaneously resolved. Serum potassium level at the time was unknown owing to the transient nature of the episode. She was evaluated neurologically and no additional therapy was required. She has otherwise been asymptomatic on stable antiarrhythmic therapy for 2 years.
Familial genetic testing revealed the KCNJ2 R218L variant in both her father and brother. Her brother has micrognathia and low-set ears but no neurologic phenotype. His ECG showed QTc of 385 ms and QTu of 560 ms and Holter monitor showed <1% PVC burden. Her father shows no cardiologic, neurologic, or craniofacial phenotype and is asymptomatic.
Discussion
This case highlights several aspects relevant to the management for genetic arrhythmia syndromes. First, ATS1 or other genetic arrhythmia syndrome should be considered as a clinical phenotype in patients presenting with a high burden of PVCs, particularly if the PVCs are polymorphic. Second, genetic variants reported as VUS may be disease-causing, and careful clinical evaluation is essential to determine the significance of a gene variant. Based on our report, R218L is disease-causing, despite the variant’s current categorization as a VUS.
The clinical diagnosis of genetic versus idiopathic arrhythmia can be insidious. In one study of ATS1 gene–positive individuals, only 58% expressed the full clinical triad, while at least 81% had 2 of the 3 classical characteristics, leaving 6% of individuals showing no penetrance of the gene.10 As in our case report, some ATS patients have associated bidirectional VT, and thus increase the diagnostic conundrum by mimicking other inherited syndromes such as CPVT.8 In our patient, the full triad did not manifest until the patient’s second decade of life. Her gene-positive brother only has dysmorphic features without cardiac or neurologic manifestations and her gene-positive father has no features of ATS. Owing to the small number of family members, we cannot determine if there is a sex dependence to the clinical phenotype associated with R218L mutation phenotype.11
Treatment of ATS1 is focused on suppression of ventricular ectopy. However, various trials of beta-blockers were not efficacious or were poorly tolerated in our patient. Flecainide is known to decrease automaticity by sodium channel blockade12 and has effectively been used in treatment of bidirectional VT related to CPVT13 and in ATS.14, 15 The mechanism of flecainide efficacy for ATS is not clear. As an additional mechanism, flecainide interacts with Kir2.1 amino acid C31116 to increase Ik1, thus increasing repolarization reserve. Ablative therapy for ventricular fibrillation (VF) (not polymorphic VT or bidirectional VT) has been documented to quell drug-resistant arrhythmic storms in cases of idiopathic VF, long QT syndrome, and Brugada syndrome.17 A single PVC morphology initiating each VF event is key to the success of this approach. Ablation was ineffective in our patient owing to the multiple morphologies of PVCs as well as the nature of her arrhythmia initiation.
Variants in KCNJ2 predominantly result in missense or deletion mutations with a variety of downstream effects on Kir2.1 channel protein function. Phosphatidylinositol 4,5-bisphosphate (PIP2) binding is necessary for Kir2.1 to be in the open conformation.18 ATS1 has been shown to be a result of KCNJ2 mutations that affect Kir2.1 PIP2 binding or allosteric conformational changes, leading to decreased frequency of open channels, effectively decreasing Kir2.1 current.19 Other ATS-causing KCNJ2 mutations include disturbances in the pore selectivity filter and misfolded or sequestered proteins, but these make up the minority of disease-causing mutations.19, 20 The C-terminus of Kir2.1, in addition to PIP2 binding sites, is home to the Kir2.1 endoplasmic reticulum export sequence and is implicated as a mechanism for Kir2.1 loss-of-function owing to protein trafficking defect.8 The effect of modifier genes and epigenetic factors such as environment on ATS phenotype and expression are not well understood.
The specific location of a disease-causing variant or the patient’s gender may affect expression variability.8, 11 Other mutations at R218 (R218Q and R218W) are associated with ATS1, highlighting the importance of this amino acid position near a PIP2 binding site. The R218L variant results in a charge change from arginine to leucine and may have an allosteric effect on PIP2 binding.
Conclusion
Flecainide and atenolol effectively suppressed bidirectional VT, PMVT, and high burden of PVCs in a patient harboring a novel ATS1-causing KCNJ2 variant R218L. This case highlights the importance of careful clinical phenotyping to help drive interpretation of genetic results and the approach to clinical management.
References
- 1.Plaster N.M., Tawil R., Tristani-Firouzi M. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen׳s syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 2.Priori S.G., Pandit S.V., Rivolta I. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 3.Tester D., Arya P., Will M., Haglund C., Farley A., Makielski J., Ackerman M. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm. 2006;3:800–805. doi: 10.1016/j.hrthm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Kalscheur M.M., Vaidyanathan R., Orland K.M., Abozeid S., Fabry N., Maginot K.R., January C.T., Makielski J.C., Eckhardt L.L. KCNJ2 mutation causes an adrenergic-dependent rectification abnormality with calcium sensitivity and ventricular arrhythmia. Heart Rhythm. 2014;11:885–894. doi: 10.1016/j.hrthm.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega A.L., Tester D.J., Ackerman M.J., Makielski J.C. Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2009;2:540–547. doi: 10.1161/CIRCEP.109.872309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen E.D., Krasilnikoff P.A., Overvad H. Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatr Scand. 1971;60:559–564. doi: 10.1111/j.1651-2227.1971.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 7.Tristani-Firouzi M., Jensen J.L., Donaldson M.R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) J Clin Invest. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H., Zhou J., Kawamura M. Phenotype variability in patients carrying KCNJ2 mutations. Circ Cardiovasc Genet. 2012;5:344–353. doi: 10.1161/CIRCGENETICS.111.962316. [DOI] [PubMed] [Google Scholar]
- 9.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tristani-Firouzi M., Etheridge S.P. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. 2010;460:289–294. doi: 10.1007/s00424-010-0820-6. [DOI] [PubMed] [Google Scholar]
- 11.Andelfinger G., Tapper A.R., Welch R.C., Vanoye C.G., George A.L., Jr, Benson D.W. KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet. 2002;71:663–668. doi: 10.1086/342360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hondeghem L.M., Katzung B.G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- 13.Priori S.G., Wilde A.A., Horie M. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:e85–108. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Fox D.J., Klein G.J., Hahn A., Skanes A.C., Gula L.J., Yee R.K., Subbiah R.N., Krahn A.D. Reduction of complex ventricular ectopy and improvement in exercise capacity with flecainide therapy in Andersen-Tawil syndrome. Europace. 2008;10:1006–1008. doi: 10.1093/europace/eun180. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K., Aiba T., Kimura H. Efficacy and safety of flecainide for ventricular arrhythmias in patients with Andersen-Tawil syndrome with KCNJ2 mutations. Heart Rhythm. 2015;12:596–603. doi: 10.1016/j.hrthm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Caballero R, Dolz-Gaiton P, Gomez R, et al. Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc Natl Acad Sci U S A;107:15631–15636. [DOI] [PMC free article] [PubMed]
- 17.Haissaguerre M., Extramiana F., Hocini M. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 18.Fan Z., Makielski J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson M.R., Jensen J.L., Tristani-Firouzi M. PIP2 binding residues of Kir2.1 are common targets of mutations causing Andersen syndrome. Neurology. 2003;60:1811–1816. doi: 10.1212/01.wnl.0000072261.14060.47. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen H.L., Pieper G.H., Wilders R. Andersen-Tawil syndrome: clinical and molecular aspects. Int J Cardiol. 2013;170:1–16. doi: 10.1016/j.ijcard.2013.10.010. [DOI] [PubMed] [Google Scholar]