Abstract
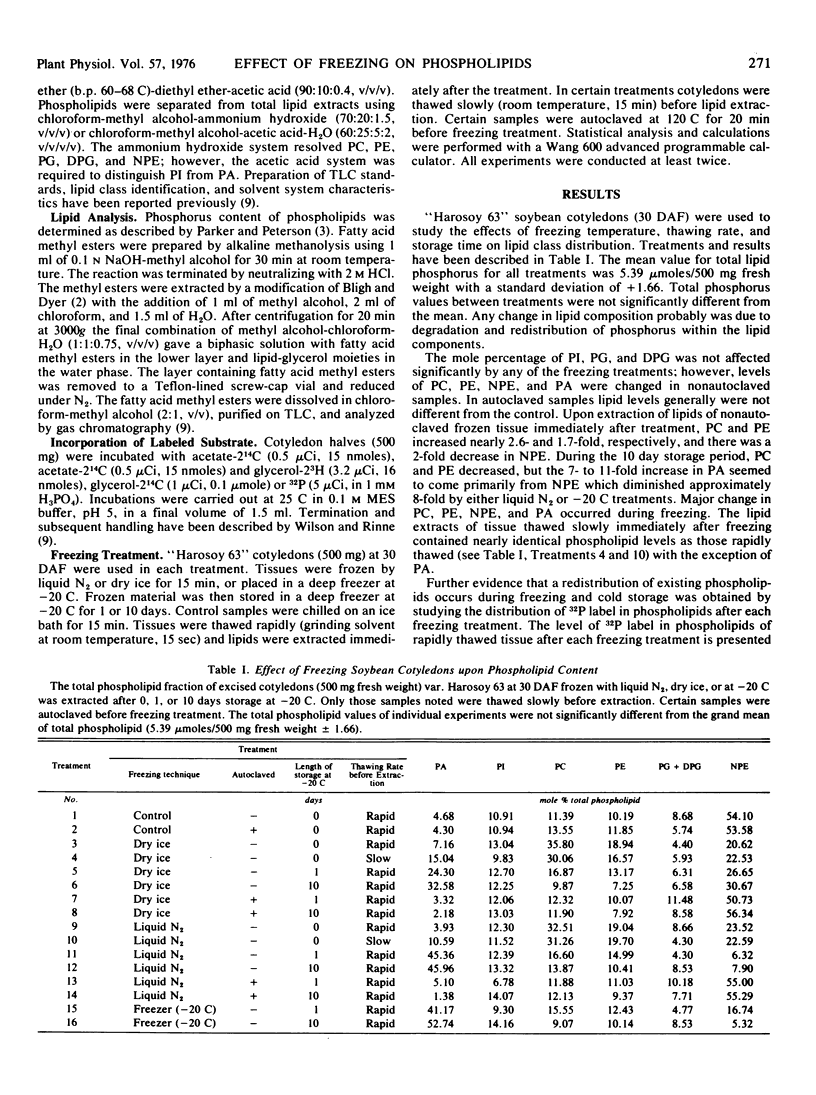
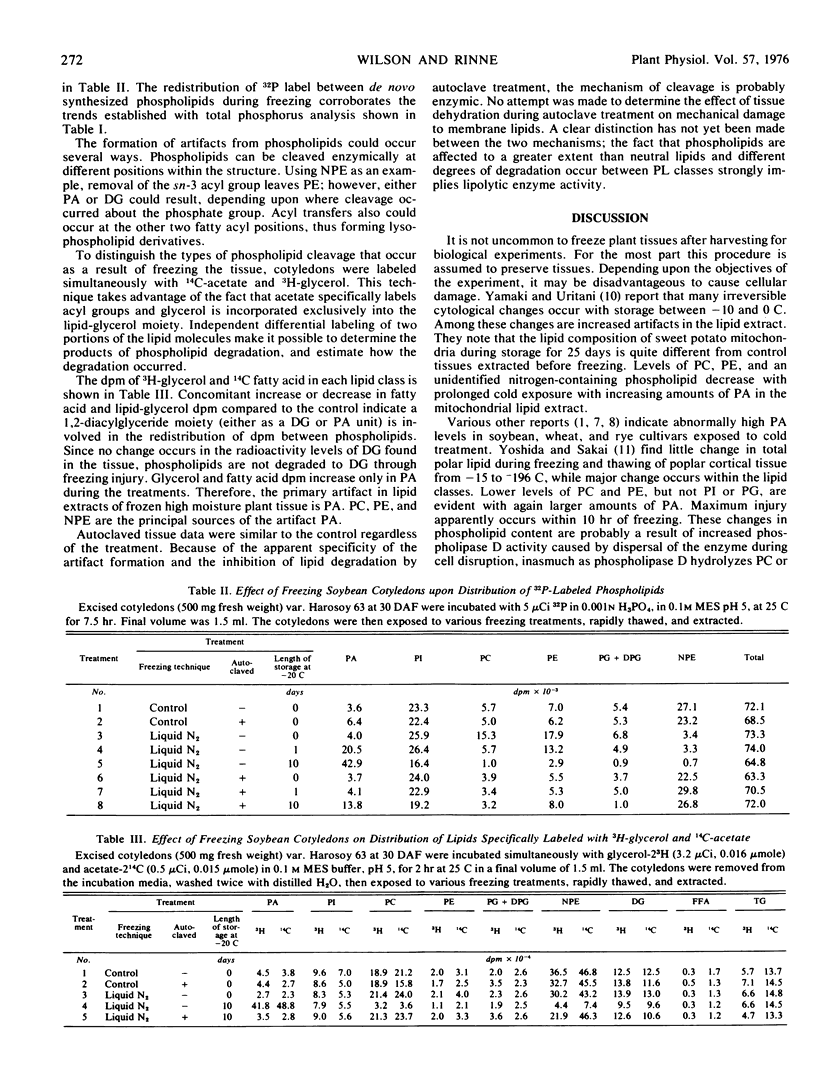
Freezing of plant tissue adversely affects lipid composition. Immature soybean cotyledons (Glycine max L. Merr.) var. “Harosoy 63” were frozen with liquid N2, dry ice, or stored in a freezer (−20 C) before lipid extraction. The effects of freezing temperature, thawing rate, and cold storage on the lipid composition of frozen tissue revealed significantly higher levels of phosphatidic acid, and diminished levels of phosphatidylcholine, phosphatidylethanolamine, and N-acylphosphatidylethanolamine from the control. Regardless of freezing temperature, phosphatidic acid levels increased from 4.7 mole% to nearly 50 mole% of the total phospholipid when frozen tissues were stored 10 days at −20 C. During the same period, N-acylphosphatidylethanolamine decreased from 54.1 mole% to 6.6 mole% phospholipid. At least 8 mole% of the phosphatidic acid increase occurred during slow thawing of the frozen tissues. In autoclaved samples, phosphatidic acid, phosphatidylcholine, phosphatidylethanolamine, and N-acylphosphatidylethanolamine levels were not different from the control. Labeling of the lipid-glycerol with 3H, and fatty acids with 14C, demonstrated the degradation product was primarily phosphatidic acid. Apparently enzymic destruction of the phospholipids occurred during freezing, cold storage, and thawing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Privett O. S., Dougherty K. A., Erdahl W. L., Stolyhwo A. Studies on the lipid composition of developing soybeans. J Am Oil Chem Soc. 1973 Dec;50(12):516–520. doi: 10.1007/BF02640523. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Privett O. S. Incorporation of 33P in soybean phosphatides. Biochim Biophys Acta. 1970 Feb 10;202(1):200–202. doi: 10.1016/0005-2760(70)90236-5. [DOI] [PubMed] [Google Scholar]
- Singh H., Privett O. S. Studies on the glycolipids and phospholipids of immature soybeans. Lipids. 1970 Aug;5(8):692–697. doi: 10.1007/BF02531436. [DOI] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Lipids in rye seedlings in relation to vernalization. Plant Physiol. 1973 Sep;52(3):268–273. doi: 10.1104/pp.52.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Phospholipids in the developing soybean seed. Plant Physiol. 1974 Nov;54(5):744–747. doi: 10.1104/pp.54.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sakai A. Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol. 1974 Mar;53(3):509–511. doi: 10.1104/pp.53.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]



