Introduction
KEY TEACHING POINTS
|
Determining the level of atrioventricular [AV] block noninvasively can be challenging (ie, AV nodal versus His-Purkinje system). Presyncope or syncope in the setting of AV block is often assumed to be due to progression of the conduction disease.
We describe a woman with second-degree AV block with narrow complex QRS with unclear level of AV block. She developed pause-dependent torsade de pointes in the setting of the AV block.
Case report
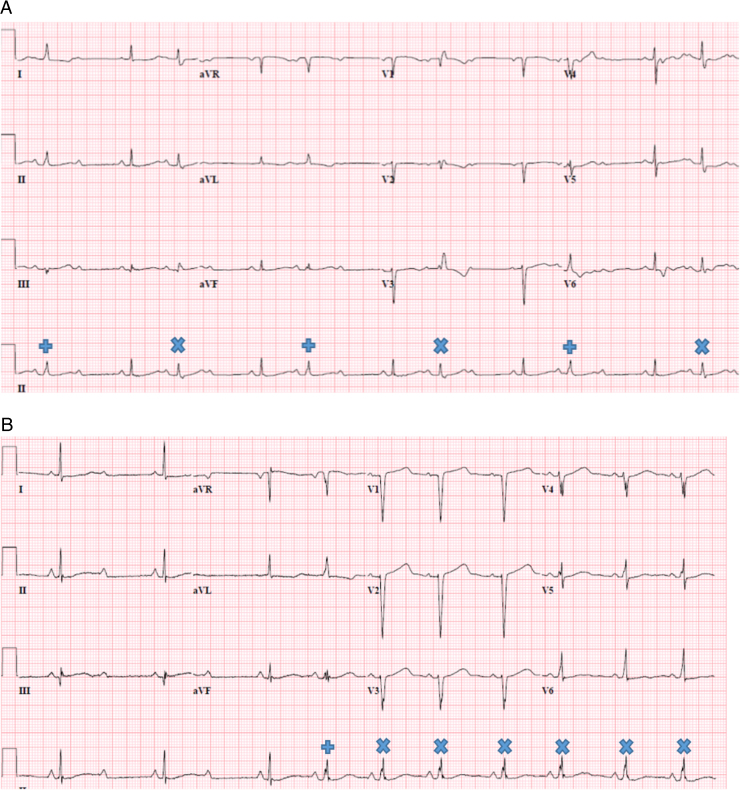
A 70-year-old female with a history of degenerative disease of the spine underwent laminectomy in October 2015 and developed a pulmonary embolism postoperatively. Echocardiography results showed an ejection fraction of 60%, no significant valvular disease, and mild-to-moderate pulmonary hypertension. Incidentally, electrocardiogram (Figure 1A) and telemetry results showed 2:1 AV and 3:2 AV conduction. There was a slight increase in the PR interval during the 3:2 block cycle. Furthermore, the second conducted beat in each grouping was associated with either a right bundle branch block (RBBB) morphologyx or an incomplete left bundle branch block (LBBB). + She was on no AV node–blocking agents. The prolongation of the PR interval, the narrow baseline QRS complex, and lack of symptoms led to the assumption that the block was at the level of the AV node, and the patient was discharged with close follow-up.
Figure 1.
Electrocardiograms (ECGs). A: ECG from October 2015 with 3:2 AV block. Initially, conducted QRS complexes are narrow with relatively short PR intervals. With subsequent conducted beats, there is slight PR prolongation and a change of QRS morphology to right bundle branch block. B: ECG from January 2016 with initial 2:1 AV block followed by 1:1 AV conduction. The first 3 QRS complexes are narrow. During 1:1 AV conduction, the first QRS complex is narrow and subsequent QRS complexes widen. PR slightly prolongs between the first and second conducted QRS complex and then remains constant.
In late December 2015 and early January 2016, the patient began having unheralded near syncope. She was admitted to the hospital with fatigue, dyspnea, and malaise. Telemetry results pointed to various degrees of block. With 2:1 AV conduction, QRS complexes remained narrow. However, with periods of 1:1 AV conduction (Figure 1B), there was slight PR prolongation after the first conducted beat, with subsequent conducted beats having widening of QRS complexes.x Her fatigue and malaise were believed to be related to bradycardia. The intermittent widening of the QRS complexes raised concern for infra- and intra-Hisian conduction disease that could have led to near syncope. Implantation of a permanent pacemaker was scheduled.
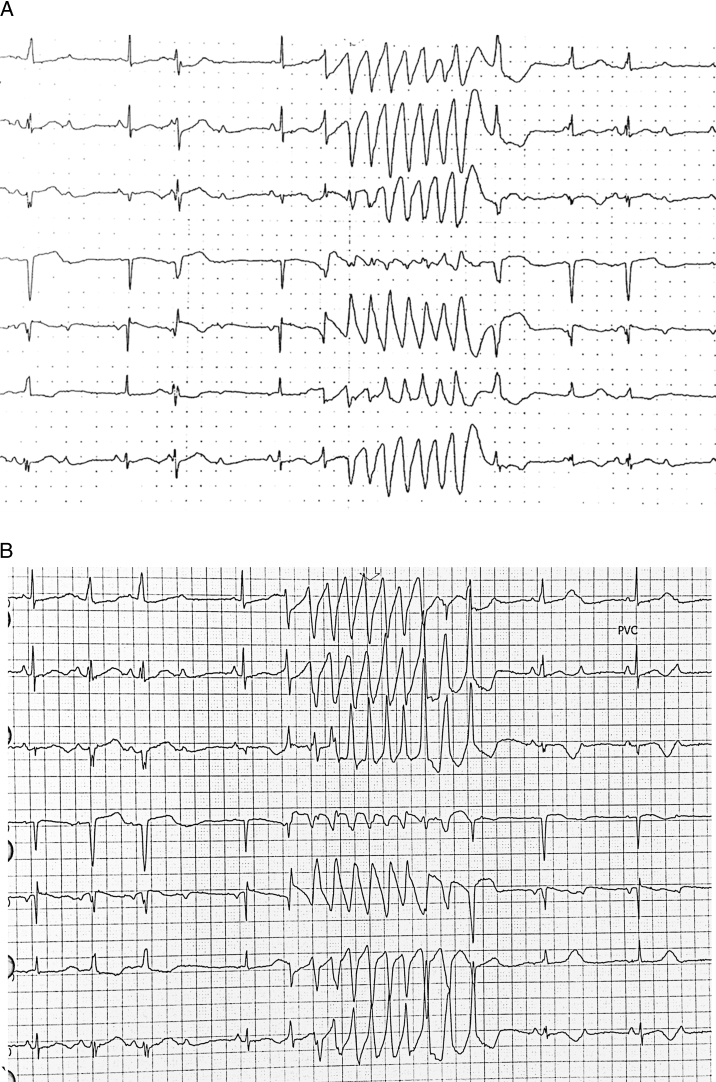
The following morning, prior to permanent pacemaker implantation, the patient had multiple episodes of nonsustained polymorphic ventricular tachycardia that showed in telemetry results (Figure 2). Her medication regimen included citalopram (20 mg daily), desipramine (25 mg nightly), pantoprazole (40 mg daily), valsartan (320 mg daily), and warfarin. She underwent implantation of a dual-chamber pacemaker without complications. We considered discontinuing desipramine, but it has been an important part of her medical regimen, and so it was continued.
Figure 2.
Telemetry results in January 2016 are representative of episodes of polymorphic ventricular tachycardia in a setting of short–long–short sequences. A: The pause leading to the run of nonsustained ventricular tachycardia is preceded by a premature ventricular contraction and a nonconducted P wave. B: The pause is preceded only by a nonconducted P wave.
Discussion
This case is unique in 2 respects. First, it highlights the difficulty of interpreting 2:1 AV block with a narrow QRS complex. Second, it is an excellent example of syncope related to torsade de pointes in the setting of heart block.
AV block
Management of AV block in the setting of narrow complex QRS can be challenging, particularly because determination of the level of block is not always straightforward. If the block is determined to be at the AV node level, symptoms guide whether a pacemaker is indicated. On the other hand, if the level of block is determined to be at the His-Purkinje level, even asymptomatic disease warrants a pacemaker.1
In this case, the initial electrocardiogram in October 2015 (Figure 1A) showed a slight PR prolongation following the P wave that occurred just prior to the blocked P wave. At the time, it was interpreted as evidence of block at the level of the AV node—in other words, AV Wenckebach physiology. In this scenario, the intermittent RBBB and incomplete LBBB (Figure 1A) would have to be attributable to a second level of block in the His-Purkinje system due to long–short sequence generated by AV nodal block (ie, phase 3 block).
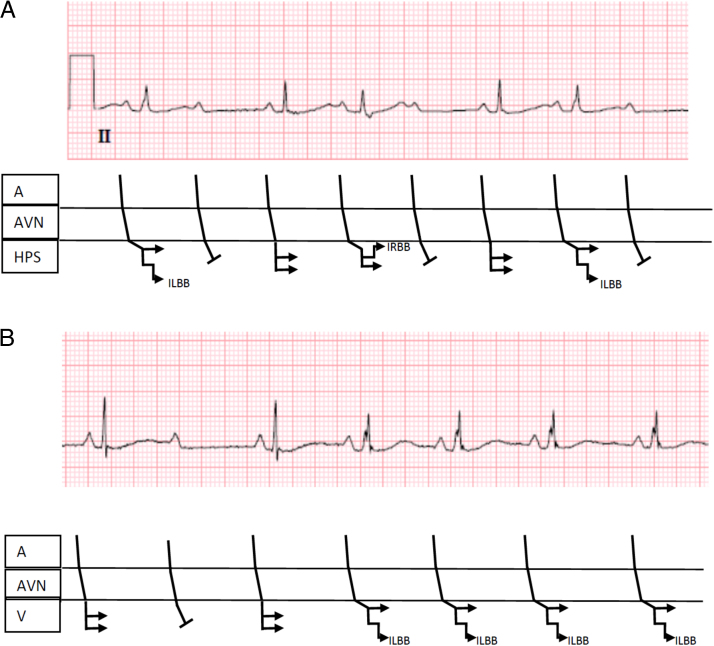
In retrospect, P waves conducting with a PR interval of <160 ms associated with the development of an RBBB or incomplete LBBB raise a possibility that the level of block is intra- or infra-Hisian.2 See Figure 3A for a ladder diagram illustrating the likely level of block.
Figure 3.
Ladder diagrams to explain most likely explanation of block in the electrocardiograms (ECGs) presented in Figure 1. A: Referring to the first ECG (Figure 1A), the atrioventricular (AV) prolongation and incomplete left and right bundle branch block are explained by a diseased His-Purkinje system. B: The second ECG (Figure 3B) shows a narrow QRS complex when there is 2:1 AV block (both left and right bundles have opportunity to recover at slower cycle length) but transitions to incomplete left bundle branch block with a faster rate (acceleration-dependent block). A = atrium; AVN = atrioventricular node; HPS = His-Purkinje system; ILBB = incomplete left bundle branch block; IRBB = incomplete right bundle branch block; V = ventricle.
In the more recent electrocardiogram, from January 2016 (Figure 1B), the patient went from 2:1 AV block to consistent 1:1 AV conduction. The first 3 conducted QRS complexes (Figure 1B) were narrow, whereas the subsequent QRS complexesx,+ were slightly widened. This probably represents acceleration-dependent block, an indication of disease in the His-Purkinje system.3 See Figure 3B for a ladder diagram illustrating the likely level of block. Less likely, this observed phenomenon could represent 2:1 block at the AV nodal level transitioning to 1:1 AV conduction. The long–short sequence could explain the initial incomplete LBBB. However, the continued intraventricular conduction delayx is challenging to explain. Perpetuation of block with retrograde invasion can cause continuation of LBBB or RBBB but seems less plausible with incomplete bundle branch block.
Although the level of block was not confirmed with an electrophysiology study, based on the short PR interval, presence of both RBBB and incomplete LBBB, and evidence of acceleration-dependent His-Purkinje block, this patient likely has AV block at the His-Purkinje level. It is important to recognize that 15%–40% of complete heart blocks are intra-Hisian,3 where the QRS complexes may not be wide. Thus, looking for wide QRS complexes alone can miss malignant heart block. An electrophysiology study can confirm this interpretation, based on a split His potential.3, 4
The medical team considered that intrinsic delay in infranodal conduction properties were augmented by concomitant therapy by pharmacologic agents known to delay infranodal conduction. It was believed, however, that the conduction disease was more intrinsic and there was limited value in discontinuing desipramine and citalopram given positive therapeutic effect on her psychiatric condition.
Torsade de pointes
When one approaches a patient with syncope in the setting of bradycardia, the possibility that a ventricular tachyarrhythmia such as TdP should also be entertained. Bradycardia or short–long–short sequences have been shown to be responsible for causing acquired long QT syndrome that can lead to torsade de pointes.5, 6, 7 In fact, when torsade de pointes was described for the first time by Dessertenne,8 it was in the setting of bradycardia.
Pauses, even more than stable bradycardia, predispose the heart to TdP.5, 7 First, the duration of repolarization in myocardial tissue is dependent on the preceding cycle length; hence, a pause will be followed by an increase in repolarization time. Second, the pause can precipitate an abnormally long QTU interval or duration (out of proportion just to the bradycardia).9 The prolonged repolarization is not homogenous and leads to the so-called dispersion of repolarization. This vulnerable period is a setup for early afterdepolarizations and for reentry.10
In our case, nonsustained TdP did not occur when the patient was in stable 2:1 block. Rather, episodes were always associated with a long–short sequence due to premature ventricular contraction (PVC) or from irregular AV conduction. In a study of 20 patients with TdP from 898 patients with AV block, episodes of TdP only occurred in the setting of long–short sequence precipitated by a PVC.11 In another article on AV block, patients with TdP often had advanced AV block with irregularity in AV conduction prior to TdP.12 Thus, either PVCs or irregular AV node conduction seem to be important for initiation of TdP, perhaps by affecting dispersion of refractoriness.
Studies have examined the QT just prior to the TdP and noted it to be prolonged.5, 9 In Figure 3A, the QT interval is difficult to measure because of the TdP obscuring the end of the QT interval. In Figure 3B, the QT interval is ≥550 ms.
Treatment for TdP in the setting of short–long–short sequences and bradycardia due to AV block immediately warrants correction of electrolyte abnormalities such as hypokalemia and the infusion of IV magnesium, pacing, or administration of isoproterenol to decrease the early afterdepolarizations (EADs) that precipitate TdP. It is also mandatory to exclude medications with the potential to prolong repolarization.9
Conclusion
In this case, the bradycardia was ultimately believed to be due to block at the His-Purkinje level, given the absence of a baseline prolonged PR interval and the slight PR prolongation and associated bundle branch block seen when 2:1 block occurred. We did not, however, rule out block at the AV nodal level with subsequent block at the His-Purkinje system (multilevel block). An electrophysiology study was not believed to be warranted, because the patient’s symptomatic bradyarrhythmia mandated the insertion of a permanent pacemaker irrespective of the findings of the study.
In retrospect, the near-syncopal episodes she experienced could have equally been due to polymorphic ventricular tachycardia/TdP or AV block. Nonsustained TdP did not occur when the patient was in 2:1 block. They occurred only in the setting of irregular AV conduction or with PVCs, highlighting the importance of dispersion of refractoriness generated by the short–long–short sequences in the precipitation of TdP. Moreover, desipramine, citalopram, and the patient’s female gender were likely contributors.13, 14
On follow-up, this patient is pacing 100% of the time in the right ventricle. The patient’s fatigue and dyspnea have resolved. She has had no further near-syncope episodes and has had no further documented runs of nonsustained polymorphic ventricular tachycardia recorded by her pacemaker.
Footnotes
Conflict of Interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Epstein A.E., DiMarco J.P., Ellenbogen K.A. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Josephson M.E. Clinical Cardiac Electrophysiology: Techniques and Interpretations. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA: 2008. Atrioventricular conduction; pp. 93–113. [Google Scholar]
- 3.Josephson M.E. Clinical Cardiac Electrophysiology: Techniques and Interpretations. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA: 2008. Intraventricular conduction disturbances; pp. 114–144. [Google Scholar]
- 4.Pasquié J.L., Grolleau R. Intrahisian block with Wenckebach phenomenon. Heart Rhythm. 2004;1:368. doi: 10.1016/j.hrthm.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Kay G.N., Plumb V.J., Arciniegas J.G., Henthorn R.W., Waldo A.L. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol. 1983;2:806–817. doi: 10.1016/s0735-1097(83)80226-5. [DOI] [PubMed] [Google Scholar]
- 6.Viskin S., Fish R., Zeltser D., Belhassen B., Heller K., Brosh D., Laniado S., Barron H.V. Arrhythmias in the congenital long QT syndrome: how often is torsade de pointes pause dependent? Heart. 2000;83:661–666. doi: 10.1136/heart.83.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayes de Luna A., Coumel P., Leclercq J.F. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 8.Dessertenne F. Ventricular tachycardia with 2 variable opposing foci. Arch Mal Coeur Vaiss. 1966;59:263–272. [PubMed] [Google Scholar]
- 9.Roden D.M. A practical approach to torsade de pointes. Clin Cardiol. 1997;20:285–290. doi: 10.1002/clc.4960200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roden D.M. Early after-depolarizations and torsade de pointes: implications for the control of cardiac arrhythmias by prolonging repolarization. Euro Heart J. 1993;14:56–61. doi: 10.1093/eurheartj/14.suppl_h.56. [DOI] [PubMed] [Google Scholar]
- 11.Cho M.S., Kim J., Kim J.H., Kim M., Lee J.H., Hwang Y.M., Jo U., Nam G.B., Choi K.J., Kim Y.H. Electrocardiographic predictors of bradycardia-induced torsades de pointes in patients with acquired atrioventricular block. Heart Rhythm. 2015;12:498–505. doi: 10.1016/j.hrthm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Moroe K., Saku K., Tashiro N., Hiroki T., Arakawa K. “Torsades de pointes” and atrioventricular block. Clin Cardiol. 1988;11:9–13. doi: 10.1002/clc.4960110111. [DOI] [PubMed] [Google Scholar]
- 13.Bednar M.M., Harrigan E.P., Ruskin J.N. Torsades de pointes associated with nonantiarrhythmic drugs and observations on gender and QTc. Am J Cardiol. 2002;89:1316–1319. doi: 10.1016/s0002-9149(02)02337-8. [DOI] [PubMed] [Google Scholar]
- 14.Makkar R.R., Fromm B.S., Steinman R.T., Meissner M.D., Lehmann M.H. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]