Introduction
KEY TEACHING POINTS
|
The availability of novel mapping systems and software in combination with or without dedicated multipolar diagnostic catheters allows for automatic high-density 3-dimensional electroanatomic mapping that enables detailed insight into atrial tachycardia mechanisms.1 For the accuracy of the activation map, the consistency of electrogram annotation is crucial.2 Depending on the system used, numerous algorithms (eg, peak amplitude or rapid downstroke of the unipolar signal) are currently implemented that can be selected for automatic signal annotation. For reliable automatic high-density mapping with thousands of points acquired within a few minutes, the robustness of the implemented algorithm is critical, especially for fractionated signals or double potentials.
Case Report
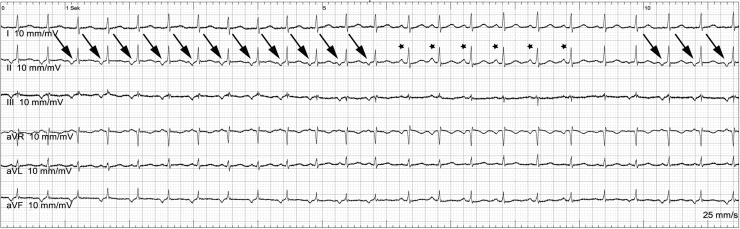
A 35-year-old female patient suffering from palpitations due to atrial arrhythmia was referred for catheter ablation. The electrocardiogram showed continuous alternation between sinus rhythm with 123 beats per minute (bpm) (asterisks) and an ectopic atrial tachycardia with 135 bpm (arrows), with negative P-waves in the inferior leads resulting in a heart rate of 127 bpm (Figure 1). The electrophysiological study was performed in combination with an electroanatomic mapping system (Rhythmia System; Boston Scientific, Natick, MA). A bidirectional deflectable decapolar catheter (Webster CS; Biosense Webster, Diamond Bar, CA) was advanced into the coronary sinus (CS) showing the earliest activation 16 mm distal from the CS ostium at the level of electrode 9–10.
Figure 1.
Surface electrocardiogram (limb leads) of the atrial tachycardia. The tachycardia shows negative P-waves in the inferior leads (arrows). The asterisks mark 5 sinus beats. After the sinus beats, there are 2 beats with fused P-waves.
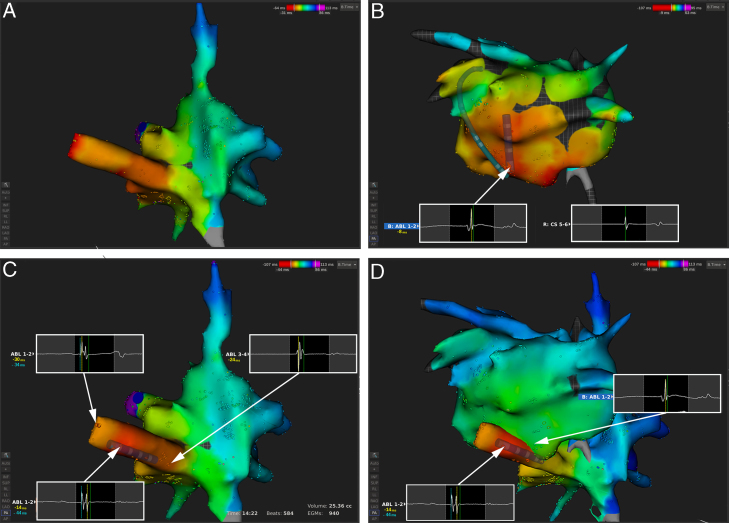
An automated electroanatomic map of the right atrium and the CS consisting of 940 electrograms was performed within 14 minutes in tachycardia using a sensor-based 4-mm irrigated-tip radiofrequency ablation catheter (IntellaNav OI; Boston Scientific, Natick, MA). Ectopic atrial tachycardia (EAT) beats were automatically differentiated by the system from the sinus beats based on the difference in signal propagation within the CS. The region of earliest activation was identified in 2 areas in the CS (Figure 2A), suggesting a focus in the left atrium. After single transseptal puncture, a left atrial activation map consisting of 634 electrograms was performed within 13 minutes without an earlier signal than the ones in the CS (Figure 2B). However, a round-shaped far-field component on the bipolar endocardial recording from the ablation catheter (Abl 1–2) preceding the local atrial signal at the level of the CS signal (CS 9–10) was observed (Figure 2B). We reviewed the automatically annotated signals in the map of the right atrium and CS and identified double-potential bipolar signals within the CS during EAT 30 ms before the onset of the P-wave (Figure 2C). Manual reannotation on this first potential, lasting together another 15 minutes, revealed the focal activation originating from the superior-posterior wall of the CS. Ablation with 25 W terminated the tachycardia within 3 seconds. Twenty-four-hour Holter monitoring 1 month after the ablation showed no recurrence of the arrhythmia.
Figure 2.
Bipolar activation map of the right atrium (RA) (A, C) and left atrium (LA) (B) and the combination of both (D). The intracardiac recordings at the different locations are superimposed (white boxes). B: A far-field signal preceding the earliest signal within the LA on ABL 1–2 could be observed. C: Reviewing the automatically annotated signals in the coronary sinus identified double-potential bipolar signals with a wrong automatic annotation (yellow line: -14 ms before CS 5–6) on the second component in the middle of the coronary sinus. Manual reannotation on the first potential (cyan line: -44 ms before CS 5–6) revealed the focal activation originating from the superior-posterior wall. D: Combination of the reannotated map of the RA and the intracardiac signal on the opposite side from within the LA.
Discussion
We describe a case of an EAT originating from the CS musculature with continuous alternation between sinus and ectopic atrial beats. This type of tachycardia has been described before, but is exceedingly rare.3 A sharp potential in the CS corresponding to the site of earliest activation is recorded during tachycardia (Figure 2C). The differentiation between sinus beats and EAT to create an activation map of the EAT poses a challenge to man and machine during activation mapping, but was reliably and automatically performed by the system based on the activation changes within the CS. However, signal annotation to create the correct bipolar activation map of the EAT using a 4-mm-tip ablation catheter still required some manual reannotation. The established algorithms currently implemented in the available 3-dimensional mapping system rely (among other parameters) on the steepest deflection of the unipolar signals (dV/dt), considered the most accurate marker of local tissue activation,4 to annotate the bipolar signal. This is highly reproducible and works well for macroreentrant and focal tachycardias without bipolar fractionated, double-potential, or prepotential signals. In the presented case of a sharp double potential at the site of origin within the CS, the system annotated the second, later signal based on the criterion of the unipolar signal from the 4-mm tip and ring electrodes. To our knowledge, onset annotation on the first sharp deflection of the bipolar signal with or without incorporation of the unipolar signal, as done manually in our and recommended by other groups,2 is also not implemented in other currently available electroanatomic mapping systems. Whether dedicated focal or multipolar catheters with smaller electrodes and interelectrode distances with less impact of far-field recordings and, consequently, more precise local tissue characterization are less susceptible to this issue needs to be determined. When ablation catheters with “large” electrodes are used, the far-field component contains important information and the distinction to near-field electrograms may be of paramount importance to identify the origin of the focal arrhythmias.
Conclusion
Despite the advancements in the development of the 3-dimensional electroantomic mapping systems, there is currently still the need for a plausibility check when using automatic annotation. Our case of a focal atrial tachycardia highlights the need for validation of automatically annotated signals with the currently available annotation algorithms.
Footnotes
Florian Spies is a former employee of VascoMed GmbH. Christian Sticherling is a member of Medtronic Advisory Board Europe and received educational grants from Biosense Webster and Biotronik. Michael Kühne discloses the following: educational grants from Biosense Webster; proctor for Medtronic; speakers bureau for Boston Scientific, St Jude Medical, and Biotronik.
References
- 1.Schaeffer B., Hoffmann B.A., Meyer C., Akbulak R.O., Moser J., Jularic M., Eickholt C., Nührich J.M., Kuklik P., Willems S. Characterization, mapping, and ablation of complex atrial tachycardia: initial experience with a novel method of ultra high-density 3D mapping. J Cardiovasc Electrophysiol. 2016;27:1139–1150. doi: 10.1111/jce.13035. [DOI] [PubMed] [Google Scholar]
- 2.Del Carpio Munoz F., Buescher T., Asirvatham S.J. Teaching points with 3-dimensional mapping of cardiac arrhythmias: taking points: activation mapping. Circ Arrhythm Electrophysiol. 2011;4:e22–e25. doi: 10.1161/CIRCEP.110.960351. [DOI] [PubMed] [Google Scholar]
- 3.Badhwar N., Kalman J., Sparks P.B., Kistler P., Attari M., Berger M., Lee R.J., Sra J., Scheinman M. Atrial tachycardia arising from the coronary sinus musculature: electrophysiological characteristics and long-term outcomes of radiofrequency ablation. J Am Coll Cardiol. 2005;46:1921–1930. doi: 10.1016/j.jacc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Biermann M., Shenasa M., Borggrefe M., Hindricks G., Haverkamp W., Breithardt G. The interpretation of cardiac electrograms. In: Shenasa M., Borggrefe M., Breithardt G., editors. Cardiac Mapping. 2nd ed. Blackwell Publishing/Futura Division; Elmsford, NY: 2003. pp. 15–39. [Google Scholar]