Introduction
KEY TEACHING POINTS
|
Neonatal accelerated idioventricular rhythm (AIVR) has been documented previously in neonates with congenital heart disease (CHD), although no cases have led to significant clinical deterioration or required placement of a permanent pacemaker.1, 2, 3 We present the first case of a neonate with complex single-ventricle physiology that developed AIVR after surgical correction of CHD, which degenerated to unstable wide complex tachycardia with multiple episodes of significant hemodynamic compromise. We discuss the management of this usually benign rhythm, which led to permanent pacing in the setting of complex CHD.
Case report
A newborn infant with a postnatal diagnosis of complex single-ventricle physiology including right atrial isomerism (RAI), unbalanced atrioventricular septal defect, dominant left ventricle (LV), tricuspid and pulmonary atresia, and total anomalous pulmonary venous connection was transferred from a referral hospital to our Cardiac Critical Care Unit for ongoing management. There were no initial rhythm concerns; an initial 12-lead electrocardiogram (ECG) revealed normal sinus rhythm (Figure 1A). After confirmation of the diagnosis by echocardiogram, which documented good systolic function, the infant underwent palliative first-stage surgery with ligation of the ductus arteriosus, placement of a modified Blalock-Thomas-Taussig shunt, and left pulmonary arterioplasty on the fourth day of life. An intraoperative echocardiogram revealed qualitatively good ventricular systolic function and the patient was successfully weaned from inotropic support of epinephrine and milrinone postoperatively. A postoperative ECG revealed a left atrial focus suggesting 2 sinus nodes, as the ECG on admission showed a right atrial focus, the presence of 2 sinus nodes being consistent with RAI. The initial procedure was complicated by 2 independent bradycardic arrests secondary to cardiac tamponade from a shunt seroma, chylothorax, and atrial ectopic tachycardia successfully treated with esmolol. An echocardiogram following recovery from the arrests revealed no change in the systolic function, which remained qualitatively good, although milrinone was restarted at 0.75 mcg/kg/min and maintained until the patient was transferred to the ward.
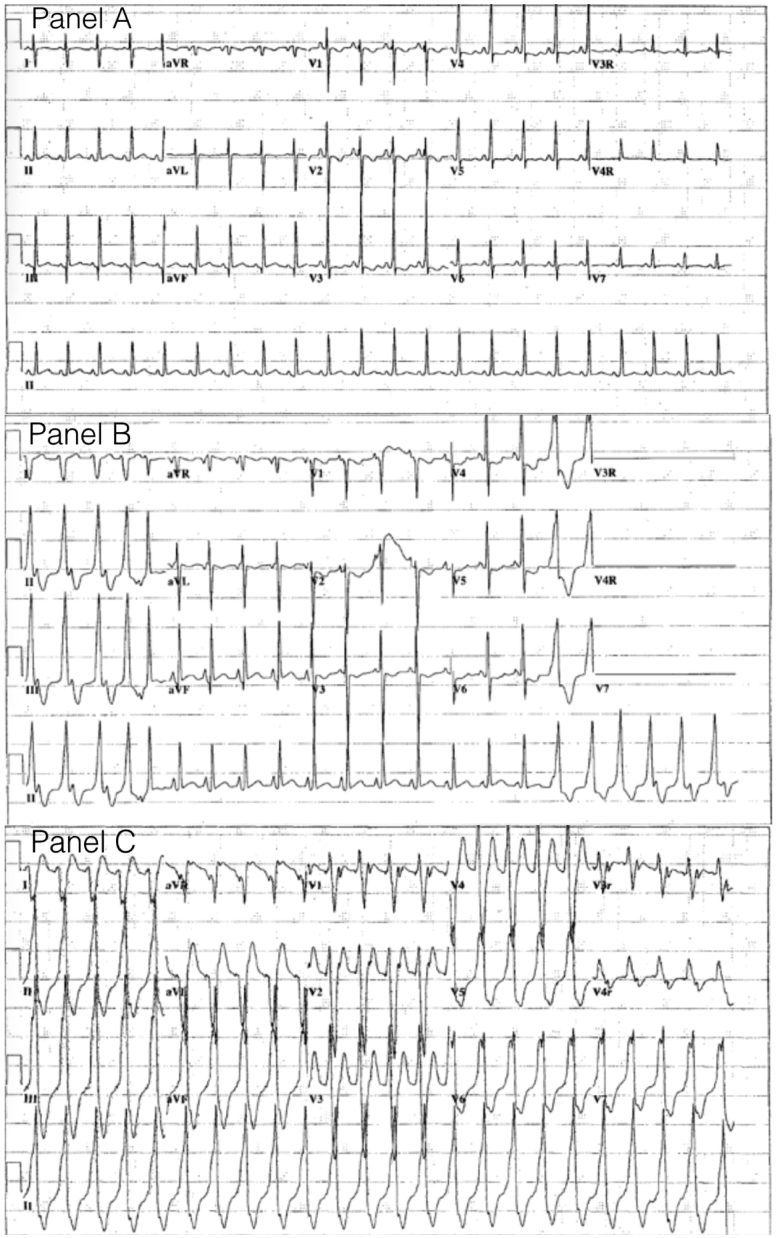
Figure 1.
A: A 12-lead electrocardiogram showing normal sinus rhythm. B: Accelerated idioventricular rhythm with wide QRS complexes, fusion beats, and ventricular-atrial dissociation, at rates similar to sinus rhythm. C: Ventricular tachycardia of similar QRS morphology to accelerated idioventricular rhythm.
Following extubation on day of life 22, an intermittent wide complex tachyarrhythmia at a rate of 140–150 beats per minute (bpm) (Figure 1B) was noted that was associated with hemodynamic compromise. This rhythm initially resolved with a 5 mg/kg bolus of amiodarone and atrial overdrive pacing at a rate slightly higher than the intrinsic rate. Atrial pacing successfully suppressed the ventricular rhythm, although intermittent ventricular capture was associated with decreased cardiac output. An echocardiogram done after stabilization revealed good ventricular function. This rhythm had a left bundle branch block pattern and was only 5–10 bpm faster than the baseline sinus rate, and there were intermittent fusion beats present. An atrial wire study revealed ventriculoatrial dissociation, and a diagnosis of AIVR was made. Lidocaine infusion was then initiated and atrial pacing was discontinued. The patient was then successfully transitioned, first to esmolol followed by propranolol at 2 mg/kg/day in divided doses. The arrhythmia intermittently recurred on this therapy but the patient remained hemodynamically stable and was transferred to the ward without further escalation of treatment.
While the patient was on the ward, recurrent episodes of AIVR at rates between 130 and 150 bpm, again with associated hemodynamic instability, led to a repeat admission to the Cardiac Critical Care Unit (Figure 1C). A Holter monitor was placed and a 19-hour recording showed AIVR 16% of the time with decreased near-infrared spectroscopy, a noninvasive continuous real-time monitoring system of cerebral perfusion where cerebral desaturations have been correlated with adverse outcomes, on 2 occasions of AIVR.4 The longest run of AIVR was 1289 beats, with the fastest rate recorded of 167 bpm. There was no evidence of sinus node dysfunction identified on the Holter. Propranolol was increased to 3 mg/kg/day and a lidocaine infusion was reinitiated. Persistent bursts of AIVR with impaired cardiac output then led to addition of an esmolol infusion and eventual transition to mexiletine at 3 mg/kg/dose 3 times per day. Hemodynamic stability was then reattained and the patient returned to the ward.
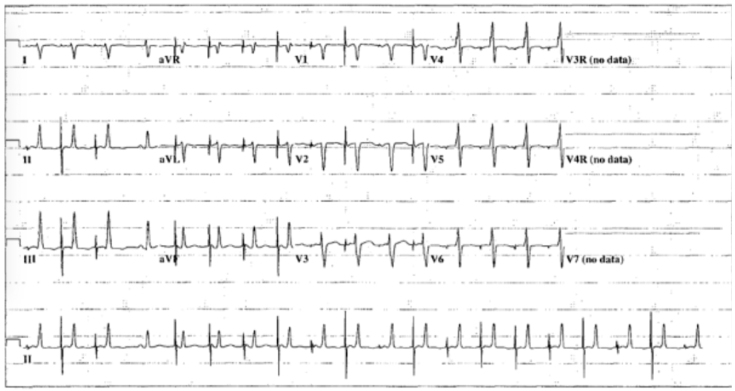
While on the ward, the patient had a bradycardic arrest during an echocardiogram, with no occurrence of AIVR prior to the arrest. During this arrest, the rhythm evolved to a wide complex rhythm and the patient was cannulated to extracorporeal membrane oxygenation (ECMO) for low cardiac output, with an echocardiogram revealing severely reduced LV systolic function. During cannulation, the patient had 2 episodes of wide complex tachycardia with a maximum rate of 180 bpm and QRS morphology distinct from the frequent AIVR rhythm for which 2 synchronized cardioversions were delivered, with reversion to normal sinus rhythm after the second cardioversion. While on ECMO the patient continued to have wide complex tachycardia with associated hypotension that required escalation in ECMO flow rates. Multiple drug combinations were used for attempted control of the ventricular arrhythmia, including intravenous lidocaine, esmolol, and amiodarone, as well as combinations of these medications. Ultimately, esophageal pacing was used for overdrive atrial pacing at a rate of 130 bpm, which resulted in improved hemodynamic stability. Esophageal pacing on ECMO with 1:1 atrioventricular conduction suppressed the ventricular rhythm, suggestive for a slow reentrant ventricular tachycardia. ECMO was discontinued after 5 days. An echocardiogram at this point revealed qualitatively good ventricular systolic function, which, when off ECMO, was unchanged from preclamping imaging. Given the continued recurrence of the AIVR with deterioration to unstable wide complex tachycardia and the beneficial response to esophageal pacing, temporary epicardial pacing leads were placed and the patient was paced in an AAI mode at 120 bpm. Owing to the successful management of AIVR using atrial pacing, a permanent pacemaker (PPM) was implanted and the patient was atrially paced at a rate of 120 bpm (Figure 2). Pharmacologic therapy was subsequently weaned and eventually discontinued. The patient was transferred back to the ward in stable condition.
Figure 2.
A 12-lead electrocardiogram showing an atrial paced rhythm with suppression of the accelerated idioventricular rhythm.
The AIVR intermittently recurred, requiring progressive escalation of the atrial pacing rate, and amiodarone was restarted. The patient subsequently developed another cardiac arrest unrelated to the AIVR and was successfully cannulated to ECMO, but required decannulation after a few hours secondary to poor cerebral perfusion. Review of the ECG prior to the arrest revealed first-degree heart block with intraventricular conduction delay and ST depression, suggesting myocardial ischemia or injury leading to progressive atrioventricular/interventricular conduction delay rather than deterioration secondary to the primary arrhythmia. The PPM was set at AAI 140 bpm with a backup external pacing rate at AAI 120 bpm to control the arrhythmia. Ultimately, owing to recurrence of the arrhythmia, final PPM setting that controlled the arrhythmia was at AAI 150 bpm. The patient continued to have intermittent episodes of hypoperfusion and cardiac arrest thought to be secondary to acidosis and hyperkalemia of unknown etiology that led to loss of pacing capture. A final echocardiogram revealed mildly reduced ventricular function in the context of significant atrioventricular valve regurgitation. After discussion with the family, a palliative care strategy was adopted and the patient died shortly after being transferred back to the referral hospital.
Discussion
The widely accepted definition of AIVR is a monomorphic wide complex tachycardia at a rate within 15% of the normal sinus rate that is asymptomatic and often demonstrates fusion beats.2 Hemodynamic compromise secondary to AIVR has not been reported and previous reports advocate for conservative management of this arrhythmia, given the usually benign outcome.2, 3, 5, 6, 7 This case initially appeared to be of typical AIVR, in which the rate was within 10% of the sinus rhythm, QRS morphology was monomorphic with left bundle branch block pattern, and there were fusion beats at the initiation of the rhythm. However, this case was unique because of significant hemodynamic compromise during the AIVR and deterioration to unstable ventricular tachycardia, which led to the treatments described above. A trial of multiple antiarrhythmic medications was unsuccessful and permanent pacing was required to circumvent the associated hemodynamic deterioration related to the AIVR.
We present the first case in the literature of AIVR in the setting of complex CHD that led to hemodynamic decompensation, which was treated with permanent atrial pacing and multiple antiarrhythmic medications. The hemodynamic deterioration noted in this patient may have been related to the loss of atrioventricular synchrony and dyssynchronous ventricular contractions related to the AIVR.
No previous cases have reported AIVR in a patient with similar complex single-ventricle physiology. Previous reports of this rhythm in cases of CHD are limited to septal defects, 1 case of Ebstein’s anomaly, 2 cases of tetralogy of Fallot, and 2 cases of double-outlet right ventricle.2, 3, 8 Our case of RAI, unbalanced atrioventricular septal defect, dominant LV, tricuspid and pulmonary atresia, and total anomalous pulmonary venous connection is the most complex case of single-ventricle physiology with AIVR in the literature. Given the usually benign course of AIVR, the outcome of our case suggests that as the complexity of CHD increases, so may the risk for hemodynamic compromise associated with AIVR.
Conclusion
AIVR is usually a benign and self-limited arrhythmia, although it can contribute to hemodynamic deterioration in the setting of complex CHD. We present a case of AIVR in a patient with complex single-ventricle physiology that was treated with atrial pacing in order to restore atrioventricular and interventricular synchrony. We recommend approaching AIVR from the context of any wide complex tachycardia with first establishing clinical stability in a given patient. If complex single-ventricle physiology is present, early consideration of atrial pacing to prevent hemodynamic deterioration could be considered.
References
- 1.Reynolds J.L., Pickoff A.S. Accelerated ventricular rhythm in children: a review and report of a case with congenital heart disease. Pediatr Cardiol. 2001;22:23–28. doi: 10.1007/s002460010146. [DOI] [PubMed] [Google Scholar]
- 2.Freire G., Dubrow I. Accelerated idioventricular rhythm in newborns: a worrisome but benign entity with or without congenital heart disease. Pediatr Cardiol. 2008;29:457–462. doi: 10.1007/s00246-007-9024-z. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa M., Yoshihara T., Matsumura A., Fusaoka T., Hamaoka K. Accelerated idioventricular rhythm in three newborn infants with congenital heart disease. Chest. 1993;104:322–323. doi: 10.1378/chest.104.1.322. [DOI] [PubMed] [Google Scholar]
- 4.Murkin J.M., Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 5.Van Hare G.F., Stanger P. Ventricular tachycardia and accelerated ventricular rhythm presenting in the first month of life. Am J Cardiol. 1991;67:42–45. doi: 10.1016/0002-9149(91)90096-4. [DOI] [PubMed] [Google Scholar]
- 6.MacLellan-Tobert S.G., Porter C.J. Accelerated idioventricular rhythm: a benign arrhythmia in childhood. Pediatrics. 1995;96:122–125. [PubMed] [Google Scholar]
- 7.Anatoliotaki M., Papagiannis J., Stefanaki S., Koropouli M., Tsilimigaki A. Accelerated ventricular rhythm in the neonatal period: a review and two new cases in asymptomatic infants with an apparently normal heart. Acta Paediatr. 2004;93:1397–1400. doi: 10.1080/08035250410027643. [DOI] [PubMed] [Google Scholar]
- 8.Massumi R.A., Ali N. Accelerated isorhythmic ventricular rhythms. Am J Cardiol. 1970;26:170–185. doi: 10.1016/0002-9149(70)90777-0. [DOI] [PubMed] [Google Scholar]