Abstract
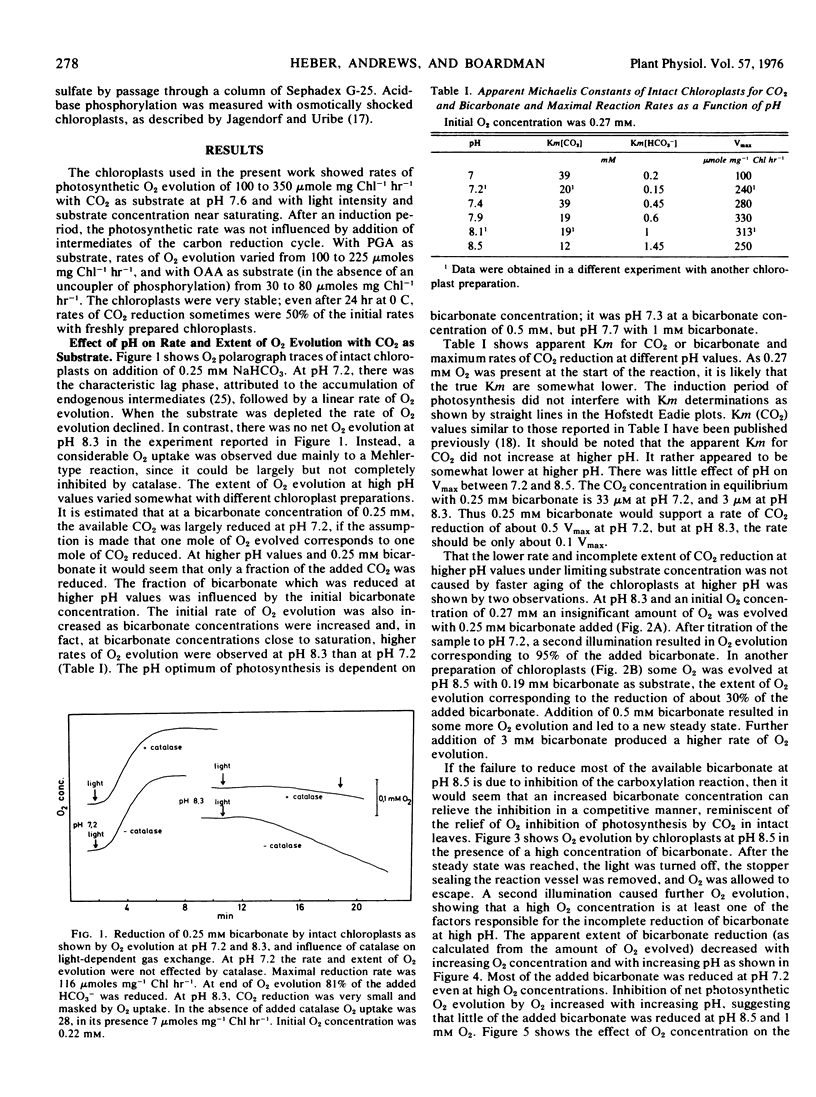
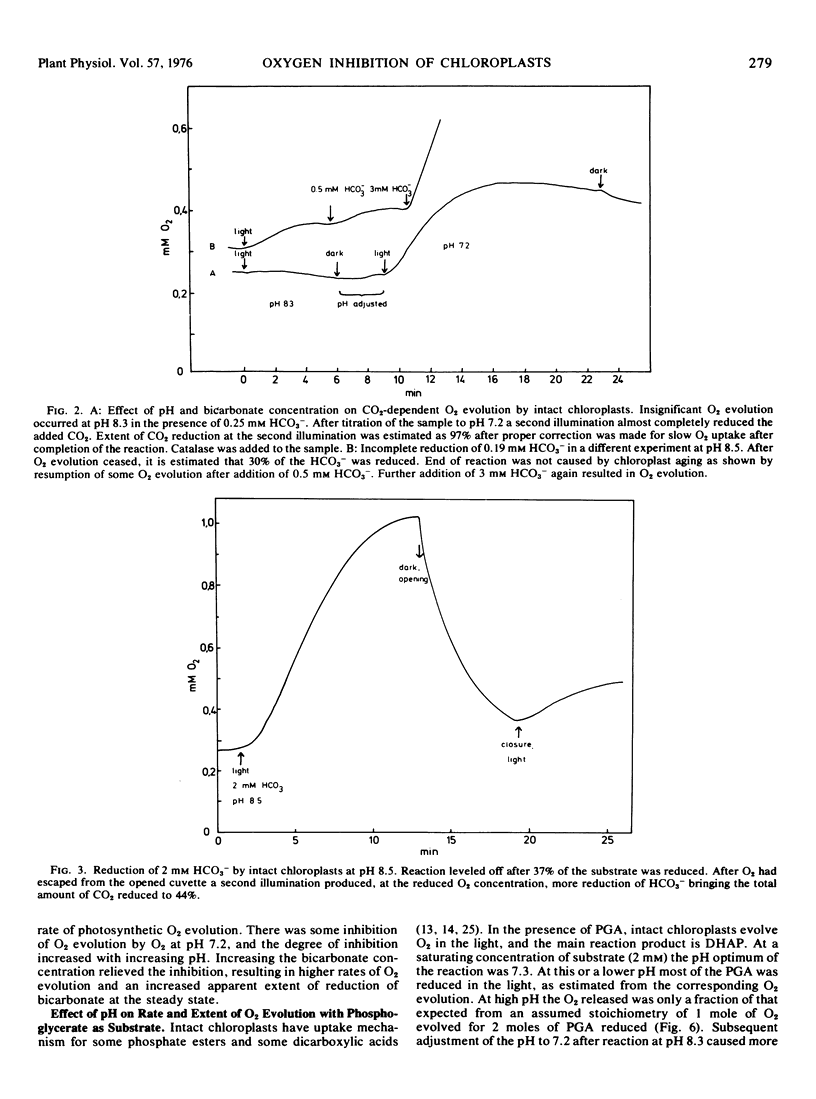
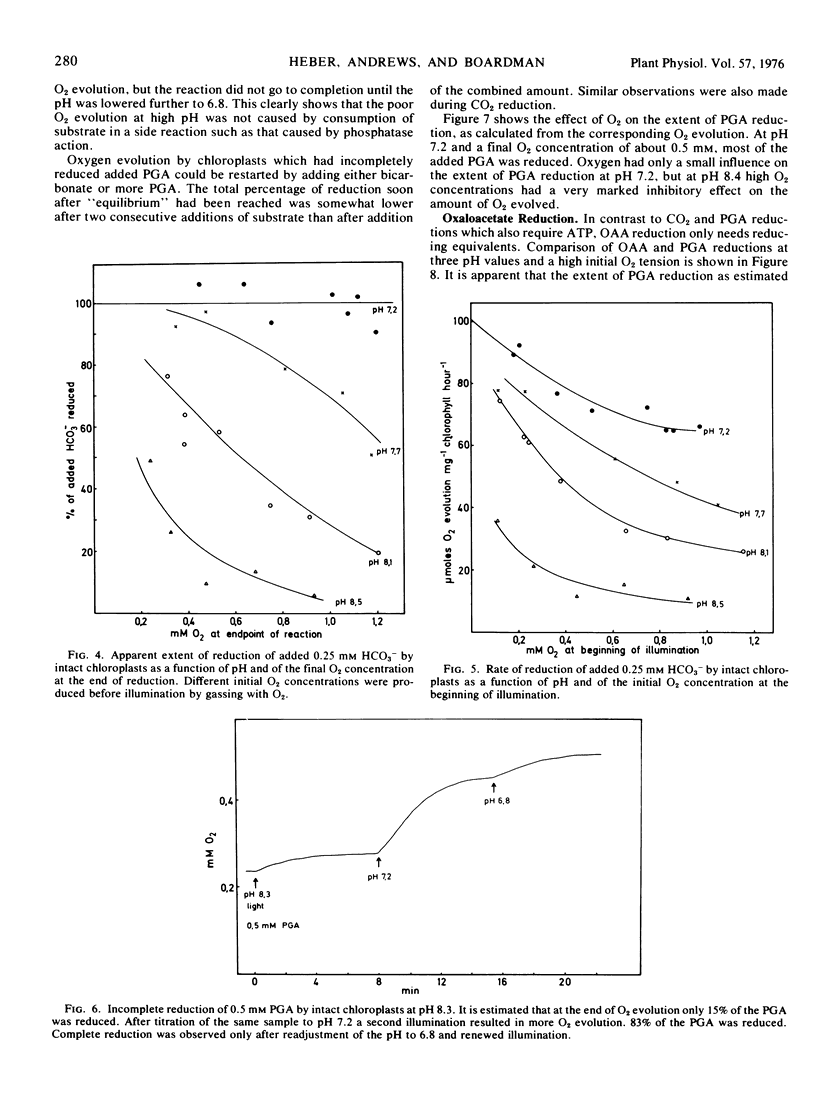
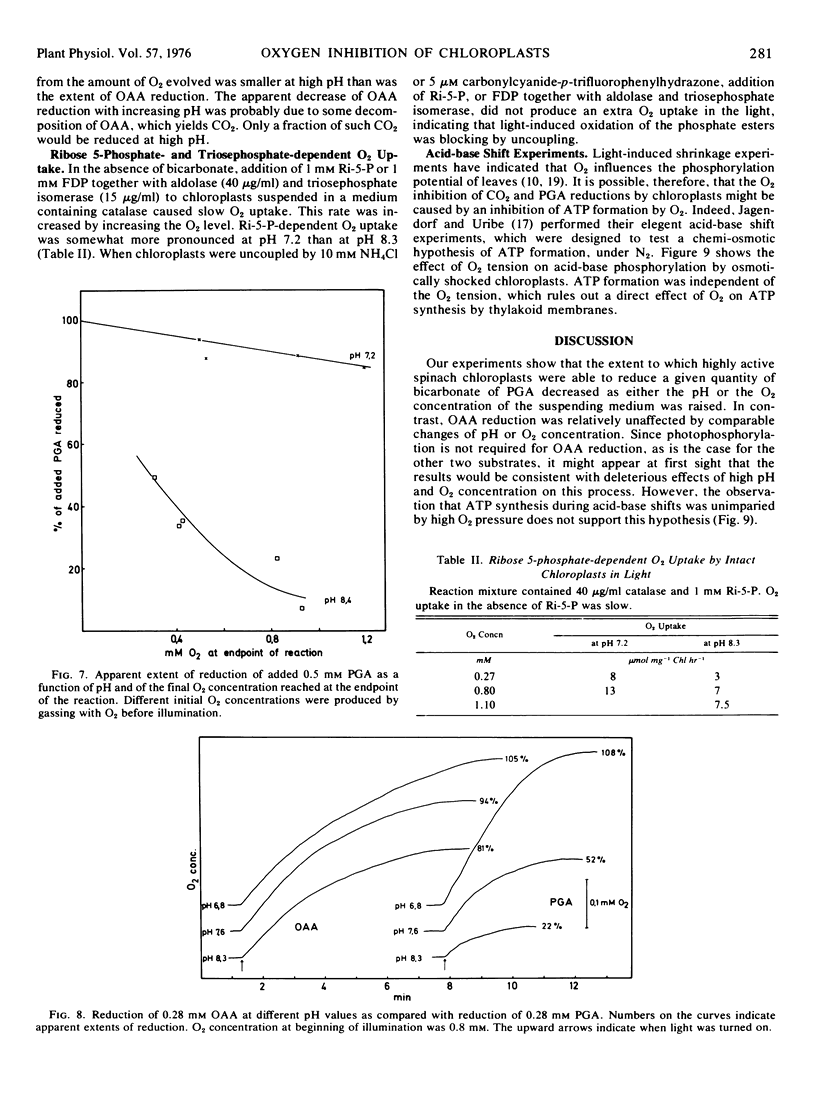
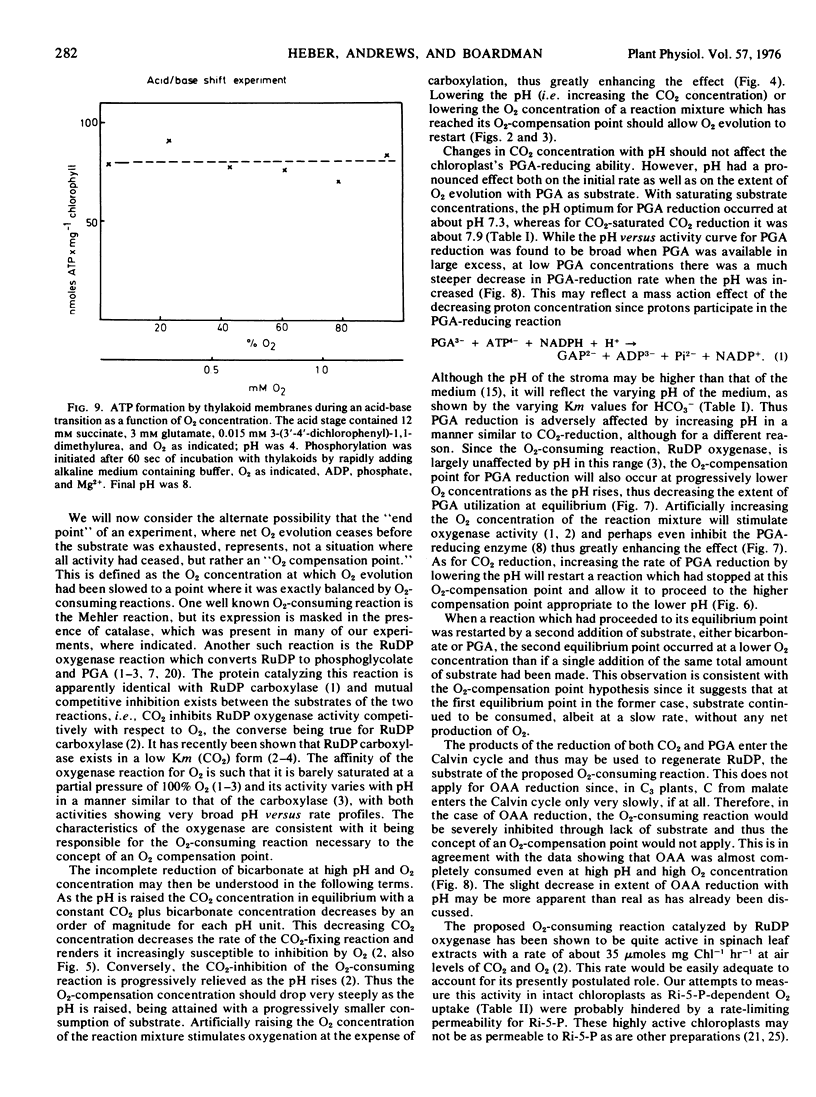
Oxygen inhibition of photosynthesis was studied with intact spinach (Spinacia oleracea L.) chloroplasts which exhibited very high rates of photosynthetic CO2 reduction and were insensitive to additions of photosynthetic intermediates when CO2 was available at saturating concentrations. Photosynthetic rates were measured polarographically as O2 evolution, and the extent of the reduction of substrate was estimated from the amount of O2 evolved. With CO2 as substrate, inhibition of photosynthesis by O2 was dependent on pH. At pH values above 8, rates of O2 evolution were strongly inhibited by O2 and only a fraction of the added bicarbonate was reduced before O2 evolution ceased. The extent of O2 evolution declined with increasing O2 concentration and decreasing initial bicarbonate concentration. At pH 7.2, the initial photosynthetic rate was inhibited about 30% at high O2 levels, but the extent of O2 evolution was unaffected and most of the added bicarbonate was reduced. Photosynthetic O2 evolution with 3-phosphoglycerate as substrate was similarly dependent on pH and O2 concentration. In contrast, there was little effect of O2 and pH on oxaloacetate-dependent oxygen evolution. Acid-base shift experiments with osmotically shocked chloroplasts showed that ATP formation was not affected by O2. The results are discussed in terms of a balance between photosynthetic O2 evolution and O2 consumption by the ribulose diphosphate oxygenase reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose bisphosphate oxygenase activity from freshly ruptured spinach chloroplasts. Arch Biochem Biophys. 1974 Oct;164(2):408–413. doi: 10.1016/0003-9861(74)90049-6. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H. The high-energy state of the thylakoid system as indicated by chlorophyll fluorescence and chloroplast shrinkage. Biochim Biophys Acta. 1973 Apr 5;292(3):715–728. doi: 10.1016/0005-2728(73)90019-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]



