Abstract
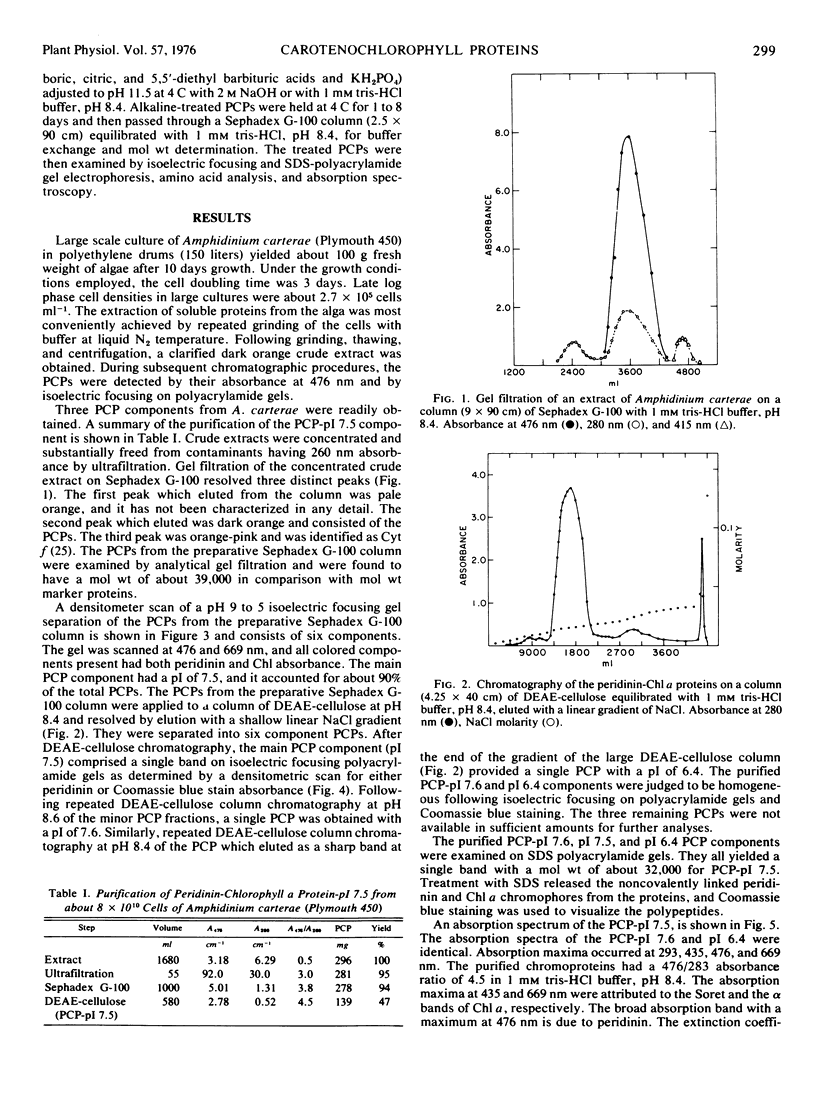
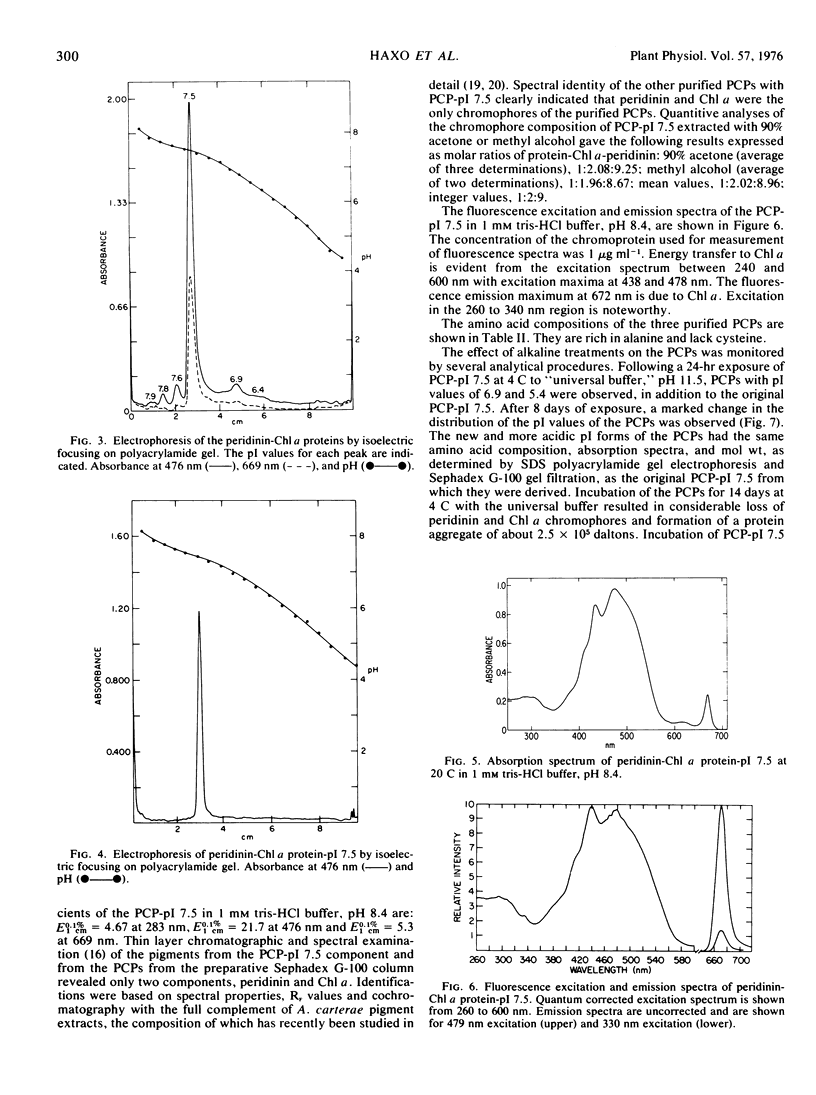
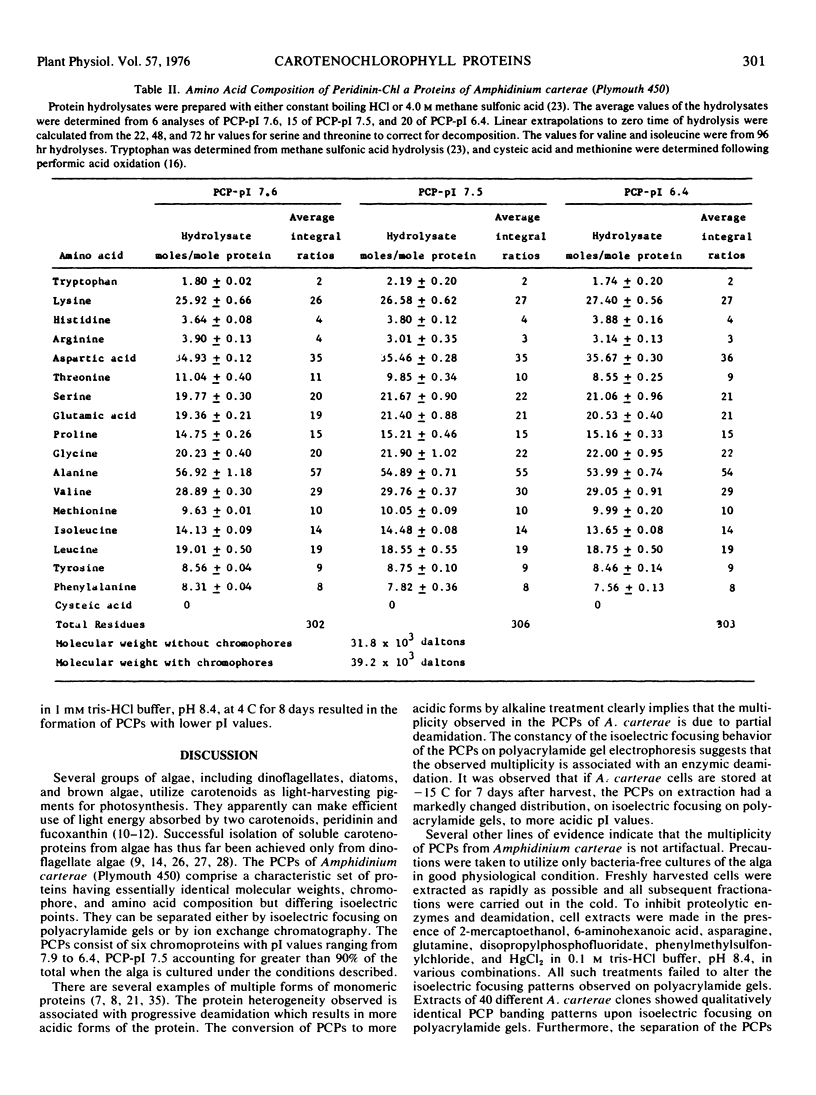
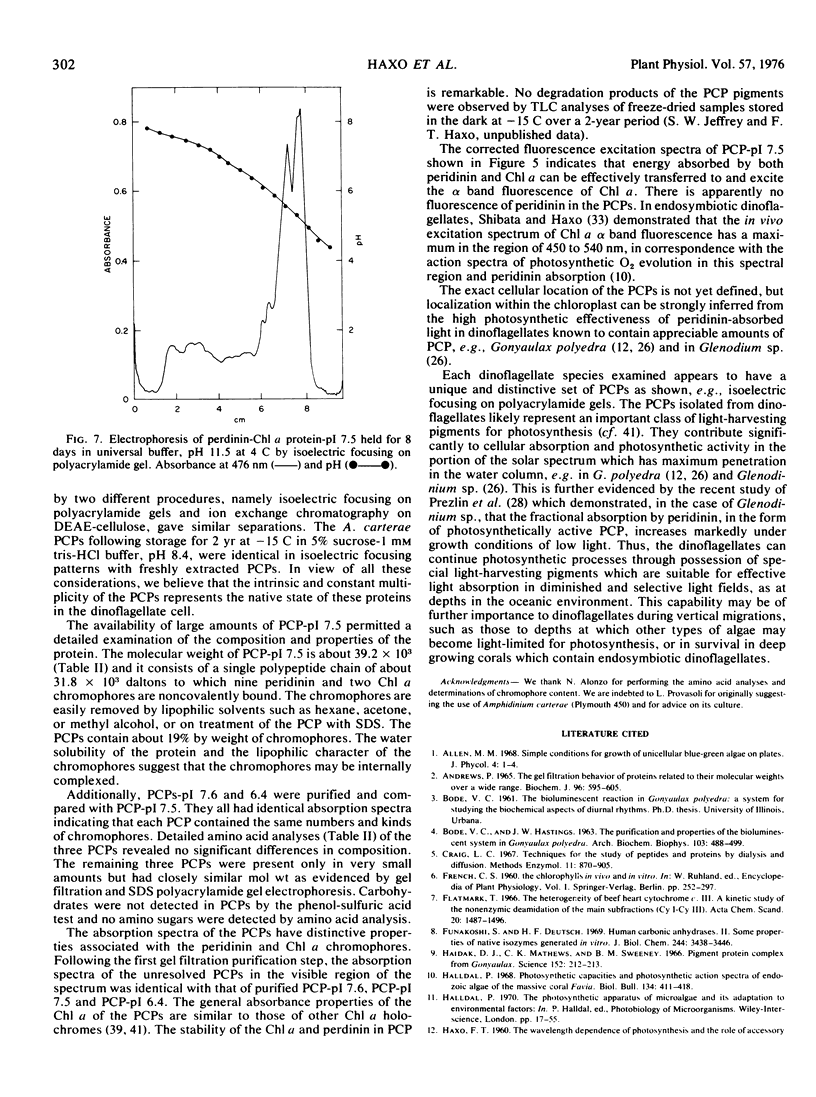
The marine dinoflagellate Amphidinium carterae (Plymouth 450) releases several water-soluble peridinin-chlorophyll a proteins after freezethawing. These chromoproteins have a molecular weight of 39.2 × 103 and are comprised of noncovalently bound peridinin and chlorophyll a and a nonoligomeric protein. They have distinct isoelectric points and may be resolved into six components by either isoelectric focusing on polyacrylamide gel or ion exchange chromatography. The predominant chromoprotein, which has a pI of 7.5, constitutes about 90% of the extractable peridinin-chlorophyll a protein. It consists of an alanine-rich apoprotein of molecular weight 31.8 × 103 stoichiometrically associated with 9 peridinin and 2 chlorophyll a molecules. Additionally, the peridinin-chlorophyll a proteins with pI values of 7.6 and 6.4 were purified and found to have amino acid and chromophore composition essentially identical with the pI 7.5 protein. Peridinin-chlorophyll a protein, pI 7.5, after treatment at alkaline pH was transformed into several more acid pI forms of the protein, strongly suggesting that the naturally occurring proteins are deamidation products of a single protein. Fluorescence excitation and emission spectra demonstrate that light energy absorbed by peridinin induces chlorophyll a fluorescence presumably by intramolecular energy transfer. The peridinin-chlorophyll a proteins presumably function in vivo as photosynthetic light-harvesting pigments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODE V. C., HASTINGS J. W. THE PURIFICATION AND PROPERTIES OF THE BIOLUMINESCENT SYSTEM IN GONYAULAX POLYEDRA. Arch Biochem Biophys. 1963 Dec;103:488–499. doi: 10.1016/0003-9861(63)90442-9. [DOI] [PubMed] [Google Scholar]
- Flatmark T. On the heterogeneity of beef heart cytochrome c. 3. A kinetic study of the non-enzymic deamidation of the main subfractions (Cy I-Cy 3). Acta Chem Scand. 1966;20(6):1487–1496. doi: 10.3891/acta.chem.scand.20-1487. [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- Haidak D. J., Mathews C. K., Sweeney B. M. Pigment protein complex from gonyaulax. Science. 1966 Apr 8;152(3719):212–213. doi: 10.1126/science.152.3719.212. [DOI] [PubMed] [Google Scholar]
- Jeffrey S. W. Quantitative thin-layer chromatography of chlorophylls and carotenoids from marine algae. Biochim Biophys Acta. 1968 Aug 20;162(2):271–285. doi: 10.1016/0005-2728(68)90109-6. [DOI] [PubMed] [Google Scholar]
- Leer J. C., Hammer-Jespersen K. Multiple forms of phosphodeoxyribomutase from Escherichia coli. Physical and chemical characterization. Biochemistry. 1975 Feb 11;14(3):599–607. doi: 10.1021/bi00674a021. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROVASOLI L., GOLD K. Nutrition of the American strain of Gyrodinium cohnii. Arch Mikrobiol. 1962;42:196–203. doi: 10.1007/BF00408175. [DOI] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Sodetz J. M., Brockway W. J., Castellino F. J. Multiplicity of rabbit plasminogen. Physical characterization. Biochemistry. 1972 Nov 21;11(24):4451–4458. doi: 10.1021/bi00774a005. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]



