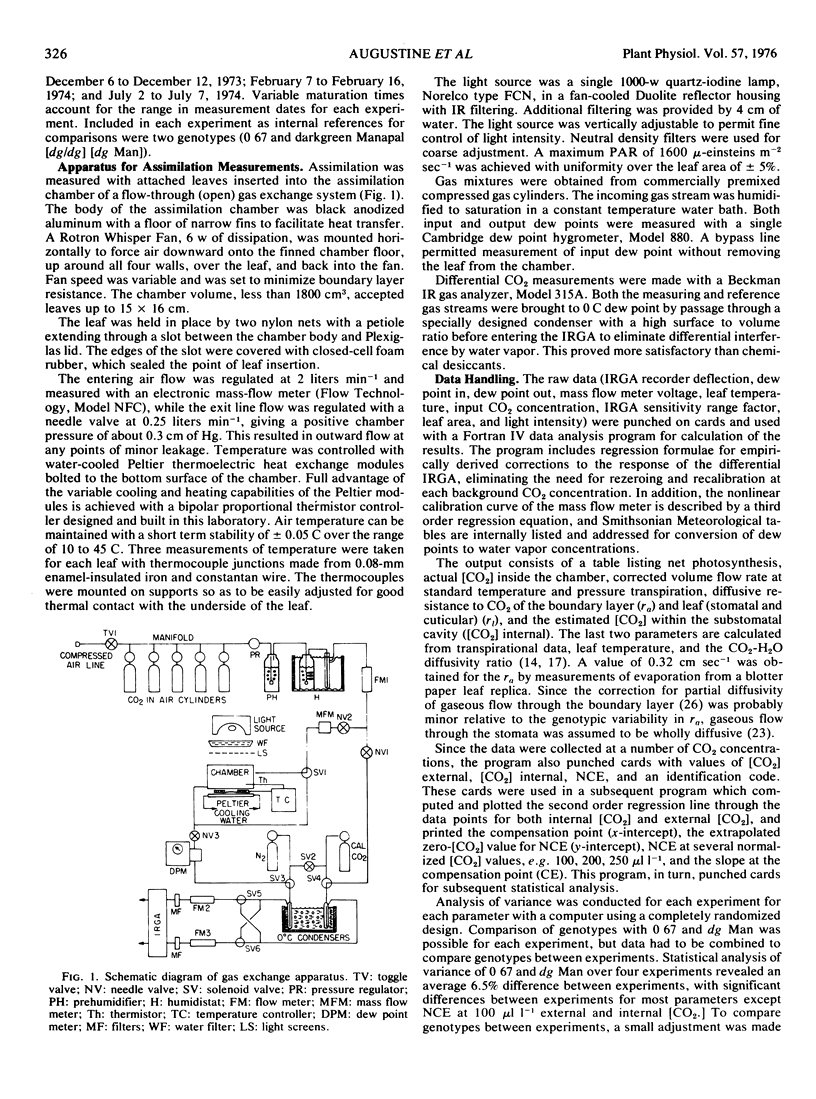
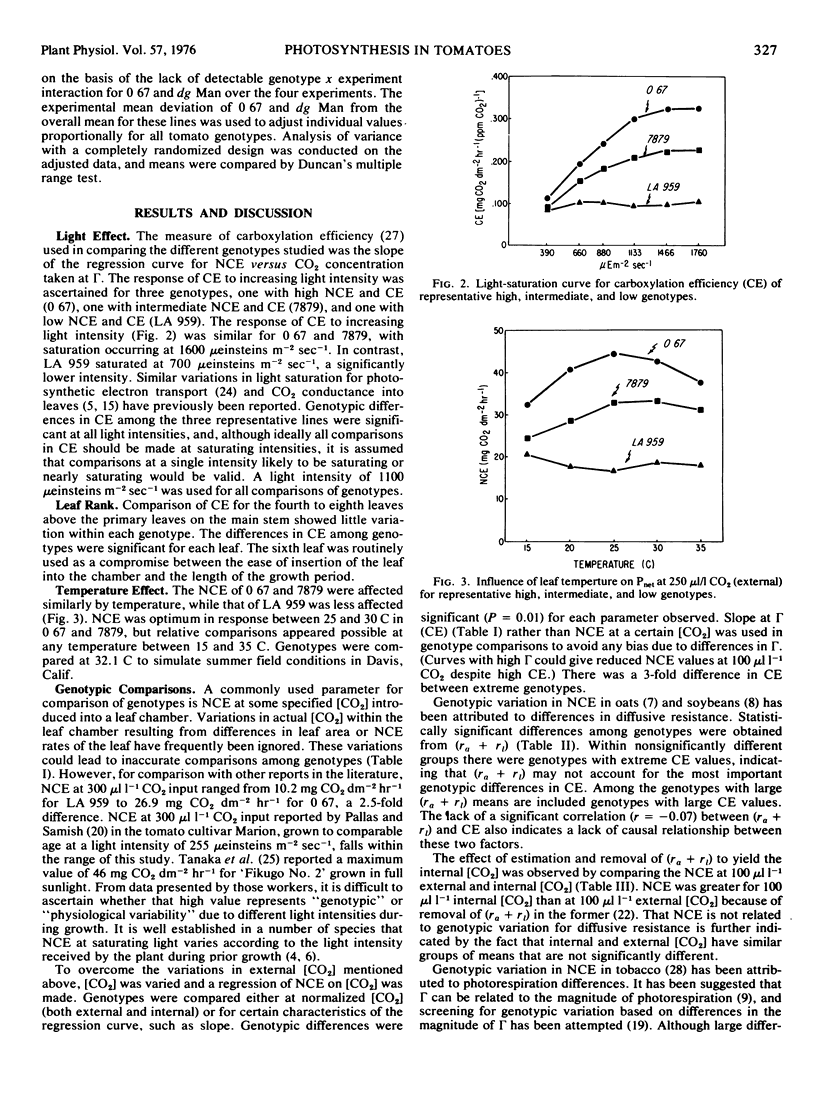
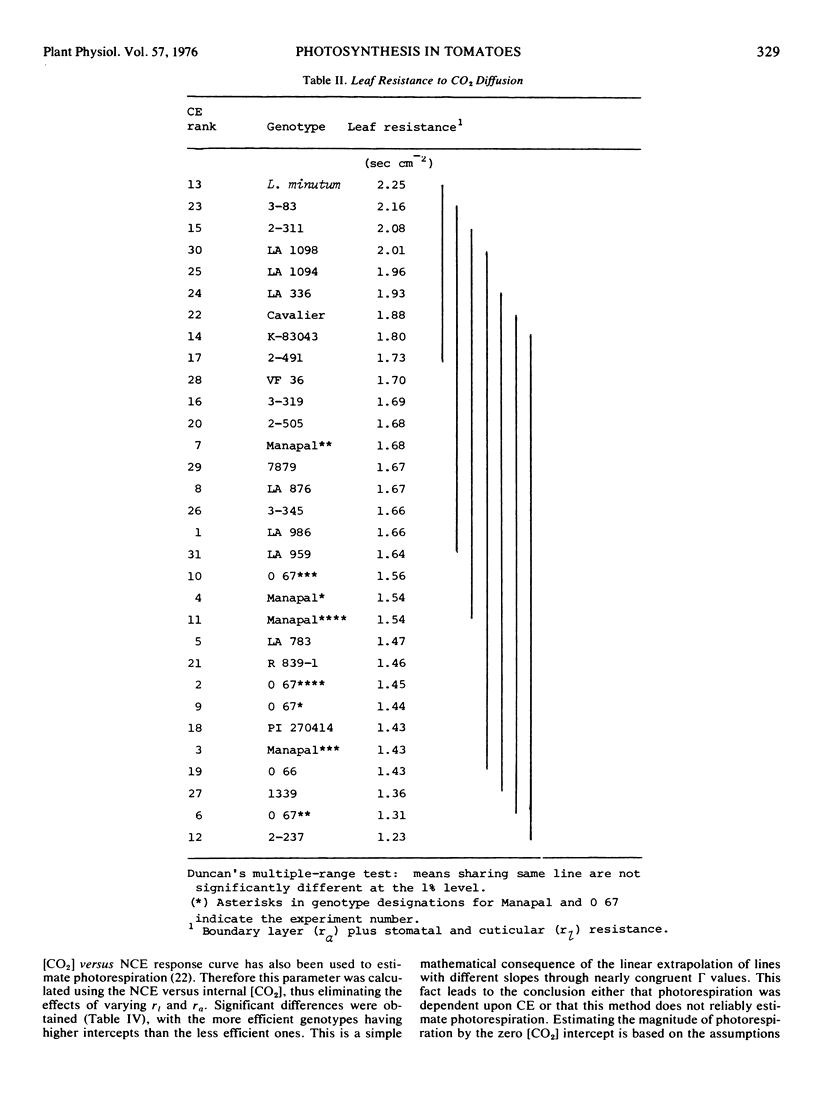
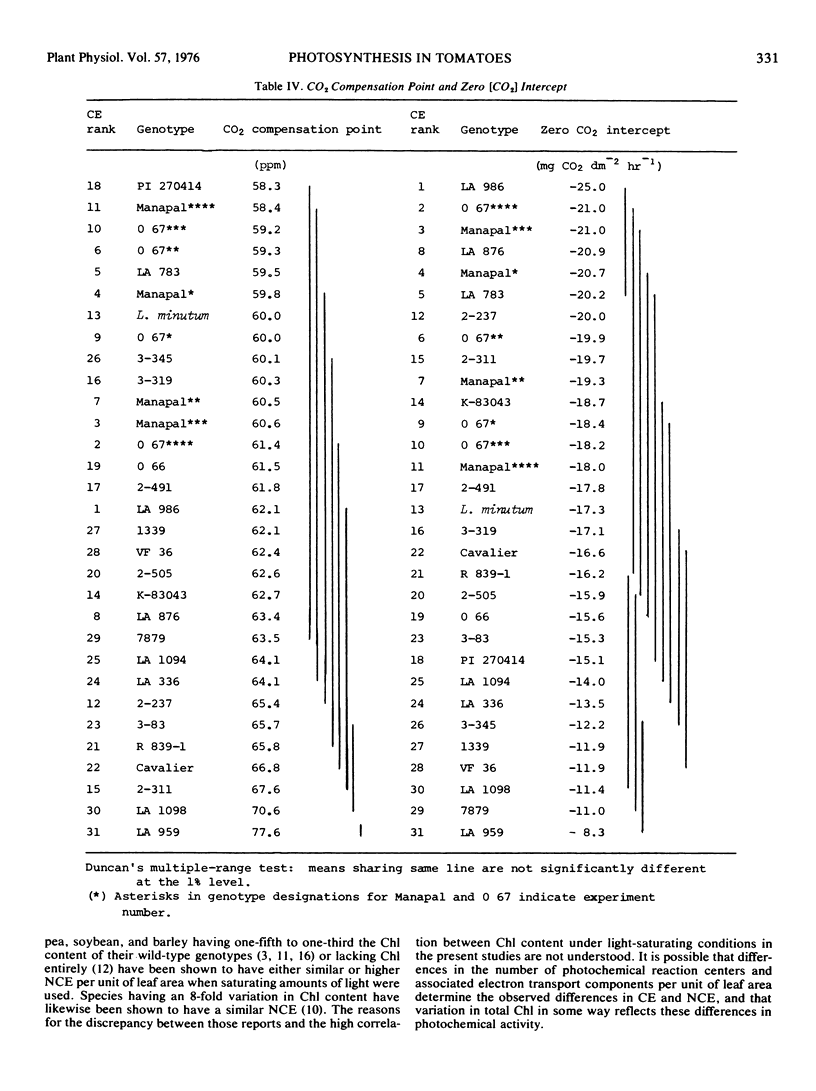
Abstract
The gas exchange characteristics of 24 genotypes of Lycopersicon esculentum Mill. and one of L. minutum were measured with an infrared gas analyzer and dew point hygrometer in an open system. Net carbon exchange (NCE) and transpiration rate were measured at 50, 100, 150, and 300 μ1 1−1 CO2, and a regression of NCE versus internal lead [CO2] estimates was calculated. The slope of the regression curve at the CO2 compensation point was used as the measure of carboxylation efficiency (CE). Significant genotypic differences for CE were obtained. Differences in CE did not appear to be due to differences in diffusive resistance defined as the sum of the boundary layer resistance (ra) and the stomatal plus cuticular resistance (r1). There was no correlation (r = -0.07) between (ra + r1) and CE. Within groups with nonsignificantly different means for (ra + r1) there were genotypes with extremes for CE.
The zero CO2 intercept has been used as an indication of photorespiration. Application of this method revealed a strong inverse relationship between CE and the intercept value, indicating either that photorespiration is related directly to CE or that this method is unreliable for estimating photorespiration. The fact that the variation in CE occurs at light saturation suggests that the observed differences in CE and rates of NCE are determined either by: (a) the concentration and/or kinetic properties of the photochemical reaction centers and associated electron transfer components as they affect the supply of NADPH and ATP and consequently the levels of Calvin cycle intermediates; or (b) the concentration and/or kinetic properties of ribulose 1,5-diphosphate carboxylase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen W. R., Wildner G. F., Criddle R. S. Ribulose diphosphate carboxylase. 3. Altered forms of ribulose diphosphate carboxylase from mutant tomato plants. Arch Biochem Biophys. 1970 Mar;137(1):84–90. doi: 10.1016/0003-9861(70)90413-3. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C. R., McCree K. J., Kohel R. J. High photosynthetic rate of a chlorophyll mutant of cotton. Plant Physiol. 1972 Jun;49(6):968–971. doi: 10.1104/pp.49.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Frenkel A. W. Studies of growth & metabolism of a barley mutant lacking chlorophyll b. Plant Physiol. 1962 Nov;37(6):814–820. doi: 10.1104/pp.37.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A., Ke B. Photochemical characteristics in a soybean mutant. Plant Physiol. 1970 Nov;46(5):699–704. doi: 10.1104/pp.46.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Variation in photorespiration. The effect of genetic differences in photorespiration on net photosynthesis in tobacco. Plant Physiol. 1968 Nov;43(11):1838–1844. doi: 10.1104/pp.43.11.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]


