Abstract
Ciliopathies are a class of diseases caused by the loss of a ubiquitous, microtubule-based organelle called a primary cilium. Ciliopathies commonly result in defective development of the craniofacial complex, causing midfacial defects, craniosynostosis, micrognathia and aglossia. Herein, we explored how the conditional loss of primary cilia on neural crest cells (Kif3af/f;Wnt1-Cre) generated aglossia. On a cellular level, our data revealed that aglossia in Kif3af/f;Wnt1-Cre embryos was due to a loss of mesoderm-derived muscle precursors migrating into and surviving in the tongue anlage. To determine the molecular basis for this phenotype, we performed RNA-seq, in situ hybridization, qPCR and Western blot analyses. We found that transduction of the Sonic hedgehog (Shh) pathway, rather than other pathways previously implicated in tongue development, was aberrant in Kif3af/f;Wnt1-Cre embryos. Despite increased production of full-length GLI2 and GLI3 isoforms, previously identified GLI targets important for mandibular and glossal development (Foxf1, Foxf2, Foxd1 and Foxd2) were transcriptionally downregulated in Kif3af/f;Wnt1-Cre embryos. Genetic removal of GLI activator (GLIA) isoforms in neural crest cells recapitulated the aglossia phenotype and downregulated Fox gene expression. Genetic addition of GLIA isoforms in neural crest cells partially rescued the aglossia phenotype and Fox gene expression in Kif3af/f;Wnt1-Cre embryos. Together, our data suggested that glossal development requires primary cilia-dependent GLIA activity in neural crest cells. Furthermore, these data, in conjunction with our previous work, suggested prominence specific roles for GLI isoforms; with development of the frontonasal prominence relying heavily on the repressor isoform and the development of the mandibular prominence/tongue relying heavily on the activator isoform.
Keywords: Aglossia, Primary cilia, Craniofacial, Neural crest cell, Ciliopathies, Tongue development, Mouse, Sonic Hedgehog, Gli
Introduction
Primary cilia are ubiquitous microtubule-based cellular projections that are specialized for transducing extracellular signaling cues. Functional disruptions to the primary cilia are associated with a spectrum of complex human genetic disorders known as ciliopathies (Badano et al., 2006; Baker and Beales, 2009; D’Angelo and Franco, 2010; Goetz and Anderson, 2010). Craniofacial dysmorphologies are common characteristics of the ciliopathic disease spectrum. Approximately 30% of known human ciliopathies are primarily characterized by their craniofacial defects (Chang et al., 2015; Tobin et al., 2008; Zaghloul and Brugmann, 2011). Oral malformations, including those affecting the development of the tongue, are among the most common phenotypes present in craniofacial ciliopathies. Ciliopathic conditions such as Oral-facial-digital syndrome, Meckel-Gruber syndrome, and Joubert syndrome frequently present with glossal abnormalities including: an abnormally small tongue (microglossia), bifid or cleft tongue, anterior marginal hamartomas or cysts of the tongue, and tongue tumors (Chang et al., 2015; Gai et al., 2012; Moran-Barroso et al., 1998; Parisi, 2009). Although glossal abnormalities are common occurrences among ciliopathies and have a significant impact on the feeding and speech of patients, the underlying developmental mechanisms that affect glossal development in ciliopathies have not been explored.
The tongue and several other facial structures affected in ciliopathies are derivatives of, or have a substantial contribution from neural crest cells (NCCs). NCCs are a migratory, multipotent cell population that migrate from the dorsal neural tube to populate the facial prominences, including the pharyngeal arches from which the tongue is derived (Noden et al., 1999). Development of the tongue begins with the emergence of a swelling composed of NCCs on the floor mandible called the median lingual swelling (Parada et al., 2012; Salles et al., 2008). During this stage, the vast majority of the tongue anlage is composed of NCC-derived mesenchymal cells (Han et al., 2012; Parada and Chai, 2015; Parada et al., 2012). Subsequently, bilateral elevations called the lateral lingual swellings emerge on either side of the medial lingual swelling. Simultaneously, mesoderm-derived mesenchymal cells migrate from the occipital somites into the lingual swellings to give rise to the intrinsic glossal musculature (Han et al., 2012; Noden and Francis-West, 2006). It is believed that reciprocal interactions between NCCs and myogenic precursor cells play an essential role in regulating tongue development, whereby NCCs initiate and direct the proliferation and differentiation of myoblasts into muscle (Han et al., 2012; Hosokawa et al., 2010).
Several signaling pathways have been implicated in glossal development. Non-canonical and canonical Transforming Growth Factor-β (TGF-β) signaling in NCCs have been reported to control the proliferation and organization of glossal muscles, after the formation of the tongue bud (Hosokawa et al., 2010; Parada and Chai, 2015). Hand2, via the negative regulation of Dlx5 and Dlx6 expression in the distal arch ectomesenchyme, patterns the distal portion of the mandible and is essential in the initiation of the tongue mesenchyme morphogenesis (Barron et al., 2011). Finally, canonical Fox mediated Hh signaling in NCCs is essential for normal patterning and growth of the face, including the mandible and the tongue (Jeong et al., 2004). Primary cilia are important for the transduction of these, and several other signaling pathways during development.
In mammals, the primary cilium is considered a hub for signal transduction. The cilium has previous been associated with transduction of several pathways including Wnt (Corbit et al., 2008; McDermott et al., 2010; Tran et al., 2014), PDGF (Clement et al., 2013; Schneider et al., 2010; Schneider et al., 2005; Schneider et al., 2009), FGF (Hong and Dawid, 2009; Neugebauer et al., 2009; Tabler et al., 2013) and BMP (Xie et al., 2016). Of these pathway, however; the strongest association has been with the Hh pathway (Briscoe and Therond, 2013). Hh activity is mediated by the GLI transcription factors, GLI1–3. GLI1 is a transcriptional activator which primarily acts to potentiate the pathway (Ingham and McMahon, 2001), whereas GLI2 and GLI3 can act as either a transcriptional activator or repressor of Hh target genes (Hui and Angers, 2011; Niewiadomski et al., 2014). Primary cilia are necessary for the post-translational modification which transform GLI2/3 full-length (GLI2/3FL) proteins into GLI activator (GLIA) or GLI repressor (GLIR) isoforms (Sasai and Briscoe, 2012). When the Hh ligand is absent, GLI2/3FL proteins are cleaved into truncated repressor forms (GLI2/3R) via proteolytic cleavage. When the Hh ligand is present, Patched suppression of Smoothened (SMO) is alleviated and SMO translocates into the primary cilium (Corbit et al., 2005). SMO inhibits GLI2/3R production and GLI2/3FL proteins are converted into transcriptional activators (GLI2/3A) via sequential phosphorylation, acetylation and sumoylation events (Kim et al., 2009). Interestingly, when SMO is deleted from NCCs, the resulting embryos lack Hh responsiveness on NCCs and the tongue does not form (Jeong et al., 2004).
We have previously reported a similar aglossia phenotype when the intraflagellar protein (IFT), KIF3A is conditionally ablated on NCCs using the Wnt1-Cre driver (Kif3af/f;Wnt1-Cre) (Brugmann et al., 2010). KIF3A is a subunit of the kinesin-2 motor protein, which is responsible for moving molecular cargo towards the plus ends of microtubules (anterograde direction) (Yamazaki et al., 1995). Loss of KIF3A prevents ciliogenesis (Kondo et al., 1994). Despite previously reporting this phenotype, we did not examine the mechanisms behind the onset of the aglossia. Here, we examined the cellular behaviors and molecular changes associated with ciliopathic aglossia. Taken together, our data add mechanistic insights into glossal development under normal and disease conditions.
Results
Conditional ablation of Kif3a in neural crest cells results in aglossia
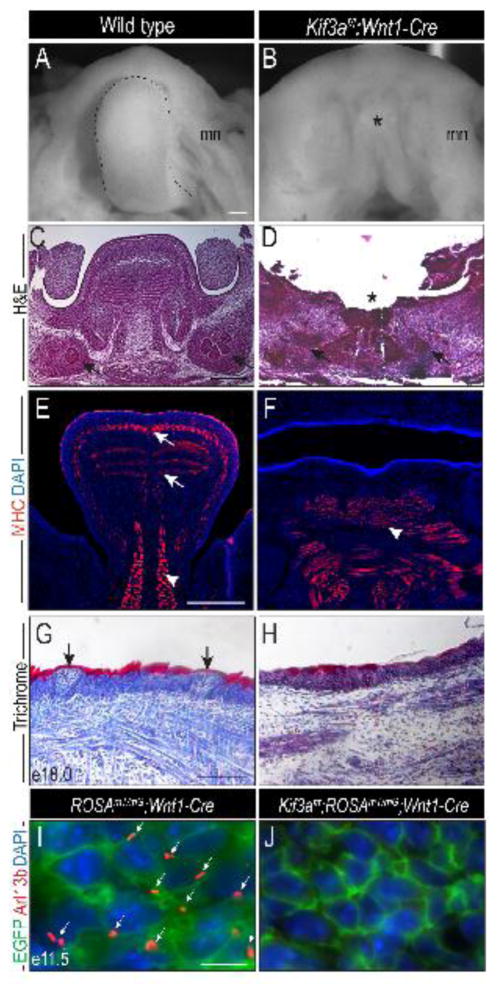
We previously characterized the midfacial defect in Kif3af/f;Wnt1-Cre mutants, which consisted of midfacial widening, bilateral cleft palate and duplication of the nasal septum (Brugmann et al., 2010; Chang et al., 2016). In addition to midfacial phenotypes, we also observed aglossia in these mutants. To understand the basis for ciliopathic aglossia, we began our analysis by characterizing the glossal phenotype. Despite the presence of the NCC-derived mandible, at e13.5 the tongue was completely absent (Fig. 1A, B; asterisk in B). H&E staining on frontal sections through the e13.5 tongue and mandible revealed that the entire body of the tongue, as well as the submandibular glands were absent (Fig. 1C, D; asterisk in D, arrows). Immunostaining for myosin heavy chain (MHC) revealed that the body of tongue at e13.5 is composed of highly organized intrinsic muscle fibers (Fig. 1E; arrows), anchored to the mandible by the extrinsic genioglossus (Fig. 1E, arrowhead). In Kif3af/f;Wnt1-Cre embryos the intrinsic muscle fibers were lost and the extrinsic muscles, including the genioglossus were severely disorganized (Fig. 1F). In e18.0 wild-type embryos the lingual epithelium was keratinized and taste buds were observed (Fig. 1G; arrows). Kif3af/f;Wnt1-Cre embryos had reduced keratinization in the epithelium and lacked developed taste buds (Fig. 1H). To confirm the loss of cilia was NCC-specific, we performed immunostaining for Arl13b, a small GTPase of the Arf/Arl family proteins that marks the ciliary axoneme (Larkins et al., 2011), on embryos carrying the tdTomato-EGFP (ROSAmT/mG) dual reporter transgene. Arl13b staining was observed in EGFP positive NCCs of the ROSAmT/mG;Wnt1-Cre mandibular prominence, but was absent from EGFP positive NCCs in Kif3af/f;ROSAmT/mG;Wnt1-Cre mandibular prominence (Fig. 1I–J; arrows). These data suggest a specific non-cell autonomous requirement for primary cilia in NCCs during glossal development.
Figure 1. Loss of primary cilia in NCCs results in aglossia.
Dorsal view of the tongue (dotted black lines) or tongue remnant (asterisk) in e13.5 (A) wild-type and (B) Kif3af/f;Wnt1-Cre embryos. (C, D) Hematoxylin and eosin (H&E) stained frontal sections of wild-type and Kif3af/f;Wnt1-Cre embryos. Black arrows indicate the salivary glands. (E, F) Anti-MHC immunostaining (red) on frontal sections of e13.5 wild-type and Kif3af/f;Wnt1-Cre embryos. White arrows indicate intrinsic musculature. White arrow heads mark extrinsic musculature. (G, H) Trichrome staining on e18.0 wild-type and Kif3af/f;Wnt1-Cre embryos. Black arrows indicate developing taste buds. (I, J) Anti-Arl13b immunostaining (red) in ROSAmT/mG;Wnt1-Cre and Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos. NCCs are marked by EGFP (green). White arrows mark ciliary axonemes (red). Nuclei stained with DAPI counterstain. mn, mandible. Scale bars = (A, B) 500um; (C–F) 250um; (G, H) 100um; (I, J) 10um.
To determine if the observed aglossia was due specifically to the loss of cilia in NCCs, we conditionally deleted Kif3a on myoblasts using the MyoDi-Cre driver (Kanisicak et al., 2009). Kif3af/f;MyoDi-Cre embryos did not have aglossia (Fig. S1A–B). The length of the tongue was not significantly different between wild-type and mutant embryos (n=3, p<0.5; Fig. S1C) and glossal muscle differentiation and organization in Kif3af/f;MyoDi-Cre was comparable to wild-type embryos (Fig. S1D, E). The presence or absence of Arl13b immunostaining on MyoD positive cells in wild-type and Kif3af/f;MyoDiCre embryos, respectively, confirmed that the loss of primary cilia was confined to myoblasts (Fig. S1F–G). Thus, the loss of primary cilia on NCCs, but not myogenic progenitor cells, resulted in aglossia.
Loss of cilia on neural crest cells prevents the migration of mesodermally-derived muscle precursors into the tongue anlage
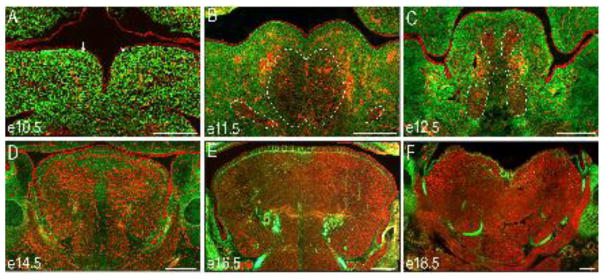
To understand why the aglossia phenotype arose when primary cilia were ablated on NCCs rather than myogenic precursors, we analyzed the developing tongue using the previously described dual reporter mice, ROSAmT/mG. The ROSAmT/mG;Wnt1-Cre reporter mice allows for simultaneous visualization of NCC-derived cells (EGFP-positive) and non-NCC-derived cell types (tdTomato-positive) in the developing tongue (Fig. 2). At e10.5 the overwhelming majority of mesenchymal cells in the paired tongue buds were EGFP-positive NCCs (Fig. 2A). We hypothesized based on previous studies of cranial mesoderm (Noden, 1983; Nogueira et al., 2015; Parada et al., 2012), that the small population of punctate tdTomato-positive cells within the mesenchyme were mesodermal contributions to the developing vasculature (Fig. 2A). By e11.5, both EGFP-positive NCCs and tdTomato-positive cell types were present in the merged tongue buds (Fig. 2B), indicating that mesoderm-derived myoblasts migrated into the tongue anlage. Intriguingly, between e12.5–e16.5 as the tongue matured, there was a marked reduction in the EGFP-positive NCC population accompanied by a striking increase in the tdTomato-positive cell population (Fig. 2C–F). By e14.5, the tdTomato-positive myogenic progenitor cells matured into myofibril-like structures and became organized into the extrinsic (genioglossus, hypoglossus and styloglossus) and intrinsic (superior longitudinal, inferior longitudinal and transverse) muscles of the tongue (Fig 2D). By e18.5, EGFP-positive NCCs only contributed to the connective tissue and tendons of the developing tongue (Fig 2F). Collectively, these data supported a model in which the NCC-derived mesenchyme were the first to populate the tongue primordia, followed by the invasion and subsequent differentiation of mesodermally-derived mesenchyme into the intrinsic tongue musculature. Since the ciliopathic aglossia phenotype in Kif3af/f;Wnt1-Cre embryos was generated by the loss of mesodermally-derived muscle tissue, we next examined the migration of muscle precursors into the tongue anlage in Kif3af/f;Wnt1-Cre embryos.
Figure 2. NCC contribution to the glossal mesenchyme.
(A–F) Frontal sections through the developing tongue of ROSAmT/mG;Wnt1-Cre embryos between e10.5 and e18.5. NCCs are green and non-NCCs are red. (A) At e10.5 the lateral lingual swellings (white arrows) are primarily composed of NCCs. (B–C) Between e11.5 and e12.5 non-NCC-derived presumptive myoblasts (red) migrate into the tongue anlage (dotted white lines). (D–F) Between e14.5–e18.5 the non-NCC-derived muscle fibers organize and populate the majority of the tongue. Scale bars = 250um.
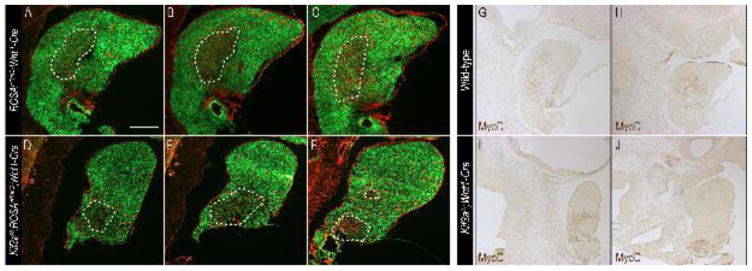
Using ROSAmT/mG;Wnt1-Cre and Kif3af/f;ROSAmT/mG;Wnt1-Cre, we examined invasion of the mesodermally-derived muscle precursor cells into the dorsal aspect of the mandibular prominence (the tongue anlage) in a sagittal section plane at e11.5 (Fig. 3). In ROSAmT/mG;Wnt1-Cre embryos a population of tdTomato-positive cells was detected interspersed among EGFP within the tongue anlage (Fig. 3A–C; n=3). We repeated this experiment using Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos and failed to detect any tdTomato-positive cells in the tongue anlage; however we did detect a population of tdTomato-positive cells that remained at the base of the mandibular prominence (Fig. 3D–F; n=3). To definitively test if the tdTomato-positive cells that failed to migrate into the tongue anlage were muscle precursors, we performed immunostaining for MyoD on sagittal sections of e11.5 wild-type and Kif3af/f;Wnt1-Cre embryos (Fig. 3G–J). As expected a population of MyoD-positive cells were detected in the tongue anlage in a similar pattern to tdTomato-positive cells in ROSAmT/mG;Wnt1-Cre embryos (Fig. 3G, H). Despite detecting MyoD positive cells in Kif3af/f;Wnt1-Cre embryos, these cells were confined to the base of the mandibular prominence and never detected in the tongue anlage, similar to what was observed in tdTomato-positive cells in Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos (Fig. 3I, J). In sum, these results suggested that loss of cilia on NCCs impaired the invasion of mesodermally-derived muscle precursors of the intrinsic glossal musculature into the tongue anlage.
Figure 3. Mesodermally-derived muscle precursors fail to migrate into the tongue anlage.
Sagittal sections through e11.5 (A–C) ROSAmT/mG;Wnt1-Cre and (D–F) Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos. NCCs are marked with EGFP (green) and non-NCCs are marked with red fluorescent protein. MyoD immunostaining on e11.5 sagittal sections of (G, H) wild-type and (I, J) Kif3af/f;Wnt1-Cre embryos. Scale bars = 250um.
Loss of cilia in NCCs causes cell autonomous and non-cell autonomous apoptosis in Kif3af/f;Wnt1-Cre embryos
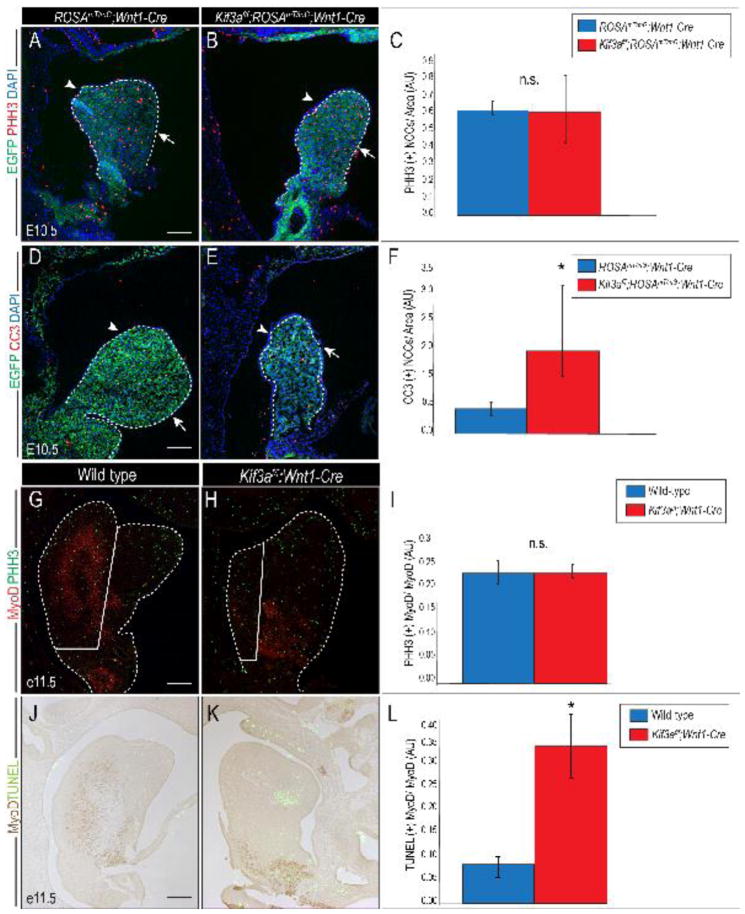
Another possible explanation for the observed aglossia could be reduced proliferation and/or increase cell death within the cells that give rise to the tongue anlage. To determine if NCC proliferation was affected in Kif3af/f;Wnt1-Cre embryos, we performed Phosphohistone H3 (pHH3) immunostaining on e10.5 ROSAmT/mG;Wnt1-Cre and Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos. The number of proliferating NCCs per mandibular area was statistically similar between ROSAmT/mG;Wnt1-Cre control and Kif3af/f;ROSAmT/mG;Wnt1-Cre mutant embryos (Fig. 4A–C; p=0.9; n=3). Examination of cell death using anti-cleaved caspase 3 (CC3) immunostaining revealed a significant increase in the number of apoptotic NCCs in Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos, compared to ROSAmT/mG;Wnt1-Cre control embryos (Fig. 4D–F; p=0.0007; n=3).
Figure 4. Loss of primary cilia in NCCs results in cell autonomous and non-cell autonomous apoptosis.
(A, B) Anti-pHH3 (red) immunostaining on sagittal sections of e10.5 ROSAmT/mG;Wnt1-Cre and Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos. Dotted white lines outline the mandibular prominence and tongue anlage. Lingual side is marked by an arrowhead, mandibular side is marked by and arrow. (C) Quantification of proliferation in EGFP-positive NCCs (green). (D, E) Anti-cleaved caspase3 (red) immunostaining on sagittal sections of e10.5 ROSAmT/mG;Wnt1-Cre and Kif3af/f;ROSAmT/mG;Wnt1-Cre embryos. (F) Quantification of apoptosis in EGFP-positive NCCs. (G, H) Anti-pHH3 (green) and anti-MyoD1 (red) double immunostaining on sagittal sections of e11.5 wild-type and Kif3af/f;Wnt1-Cre embryos. Solid white line divides glossal region and mandibular region. (I) Quantification of proliferation in MyoD1 immuno-positive myogenic cells. (J–K) TUNEL (green) and MyoD (brown) staining on sagittal sections of e11.5 wild-type and Kif3af/f;Wnt1-Cre embryos. (L) Quantification of apoptosis in wild-type and Kif3af/f;Wnt1-Cre embryos. White dotted lines outline the tongue and mandible. Scale bars = 250um.
We next examined proliferation and apoptosis of muscle progenitor cells. Double immunostaining for MyoD and pHH3 on e11.5 wild-type and Kif3af/f;Wnt1-Cre embryos confirmed a reduced number of MyoD positive cells in the tongue anlage of Kif3af/f;Wnt1-Cre embryos; however, the number of MyoD positive cells that were also immune-positive for pHH3 was statistically similar between wild-type and Kif3af/f;Wnt1-Cre tongue regions (Fig. 4G–I; n=3). Double immunostaining for MyoD and TUNEL on e11.5 embryos revealed increased apoptosis in MyoD-positive cells in Kif3af/f;Wnt1-Cre, relative to wild-type embryos (Fig. 4J–L; p<0.0007; n=3). Together, our results suggested that on a cellular level aglossia in Kif3af/f;Wnt1-Cre embryos was caused by increased apoptosis in both NCCs and mesodermally-derived muscle precursor cells, coupled with a failure of intrinsic glossal muscle precursors to invade the NCCs populated tongue anlage. We next went on to examine the possible molecular basis for the ciliopathic aglossia observed in Kif3af/f;Wnt1-Cre embryos.
Hh signaling is disrupted in the developing mandible of Kif3af/f;Wnt1-Cre embryos
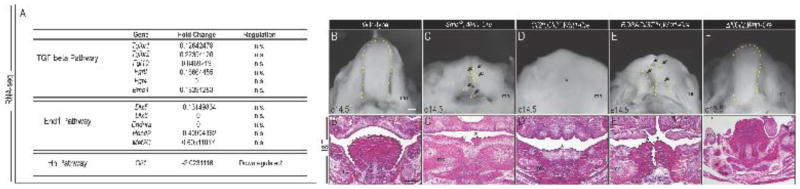
To identify the molecular basis for aglossia in the Kif3af/f;Wnt1-Cre ciliopathic mutant, we performed RNA-seq on e11.5 mandibular prominences from wild-type and Kif3af/f;Wnt1-Cre embryos. We analyzed our RNA-seq data for the expression of key gene regulatory pathways previously shown to be required for glossal development. We quantified gene expression as the total number of mapped reads for a given reference gene or transcript (Marioni et al., 2008). TGF-β mediated FGF and BMP signaling pathways are required for glossal muscle proliferation and differentiation (Han et al., 2014; Hosokawa et al., 2010). Based on our RNA-seq data, transcript levels of TGF-β pathway members such as Tgfbr2, Tgfbr1, Fgf4, Fgf8, Fgf10 and Bmp4 were not significantly altered in Kif3af/f;Wnt1-Cre mandibles (Fig. 5A). The Endothelin (Edn1) pathway has also been implicated in the patterning of mandibular NCCs and subsequent initiation of glossal development (Barron et al., 2011; Verzi et al., 2007). Our RNA-seq analysis revealed that transcript levels of Edn1 pathway members such as Hand2, Dlx5, Dlx6 and Mef2C were also not significantly altered in Kif3af/f;Wnt1-Cre mandibles (Fig. 5A).
Figure 5. Kif3af/f;Wnt1-Cre embryos have impaired Hh signaling and phenocopy loss of Hh phenotype.
(A) Expression changes in genes previously implicated in glossal development in e11.5 wild-type and Kif3af/f;Wnt1-Cre mandibular prominences, as determined by RNA-seq. (B–F′) Dorsal whole mount view and frontal sections through the developing tongue and mandible in e14.5 (B, B′) wild-type, (C,C′) Smon/c;Wnt1-Cre (D, D′) Gli2f/f;Gli3f/f;Wnt1-Cre, (E, E′) ROSAGli3TFlag;Wnt1-Cre and (F, F′) ΔNGli2;Wnt1-Cre embryos. (B–F) Dotted yellow lines and black arrows mark tongue or tongue remnants. (B′-F′) Dotted black lines and asterisk mark tongue and tongue remnants). mc, Meckle’s cartilage. Scale bars = (A, B) 500um; (C, D) 250um.
Given the close association between the Hh signaling pathway and the primary cilium, coupled with the aglossic phenotype in Smon/c;Wnt1-Cre embryos (Jeong et al., 2004), we examined our RNA-seq data to determine whether the Hh pathway was disrupted in Kif3af/f;Wnt1-Cre mandibles. We found that transcript levels of the key downstream Hh effector and pathway activity readout gene, Gli1, was downregulated 2-fold in Kif3af/f;Wnt1-Cre mandibles (Fig. 5A). In light of the reduced Gli1 expression in Kif3af/f;Wnt1-Cre mandibles, we examined several transgenic lines with aberrant Hh activity in NCCs (Fig. 5B–F′) and examined glossal development. As previously reported, Smon/c;Wnt1-Cre embryos, which lack one allele of the Smo in NCCs and the other in all cells, exhibited aglossia (Jeong et al., 2004) (Fig. 5C–C′). Gli2f/f;Gli3f/f;Wnt1-Cre embryos, which lack the downstream transcriptional mediators of the Hh pathway, Gli2 and Gli3, in NCCs (Fig. 5D–D′) also displayed aglossia strikingly similar to that of Kif3af/f;Wnt1-Cre and Smon/c;Wnt1-Cre embryos. These results strongly suggested Hh signaling in NCCs was essential for tongue development.
GLI transcription factors can function as both transcriptional activators and transcriptional repressors. Our previous work determined that development of the midface was predominantly driven by GLI repressor (GLIR) activity (Chang et al., 2016). To explore if GLIR and/or GLIA activity were important for glossal development we examined ROSAGli3TFlag;Wnt1-Cre and ΔNGli2;Wnt1-Cre embryos, which conditionally overexpress GLI3R or GLI2A, respectively (Mill et al., 2003; Vokes et al., 2008). Over expression of the GLI3R in NCCs resulted in the complete loss of the distal portion of the tongue and bifid or cleft, proximal tongue rudiment (Fig. 5E–E′); however, constitutive activation of GLI2A in NCCs did not produce an aberrant tongue phenotype (Fig. 5F–F′). Together, these results suggest that Hh signaling with in NCCs plays an important role in glossal development and that the observed ciliopathic aglossia in Kif3af/f;Wnt1-Cre mutants could be a result of aberrant Hh signaling activity; specifically a loss of GLIA activity. To further explore this hypothesis, we next examined the expression of GLI protein isoforms in the Kif3af/f;Wnt1-Cre embryos, comparing it against another aglossia mutant.
GLI protein production is disrupted in aglossia mutants
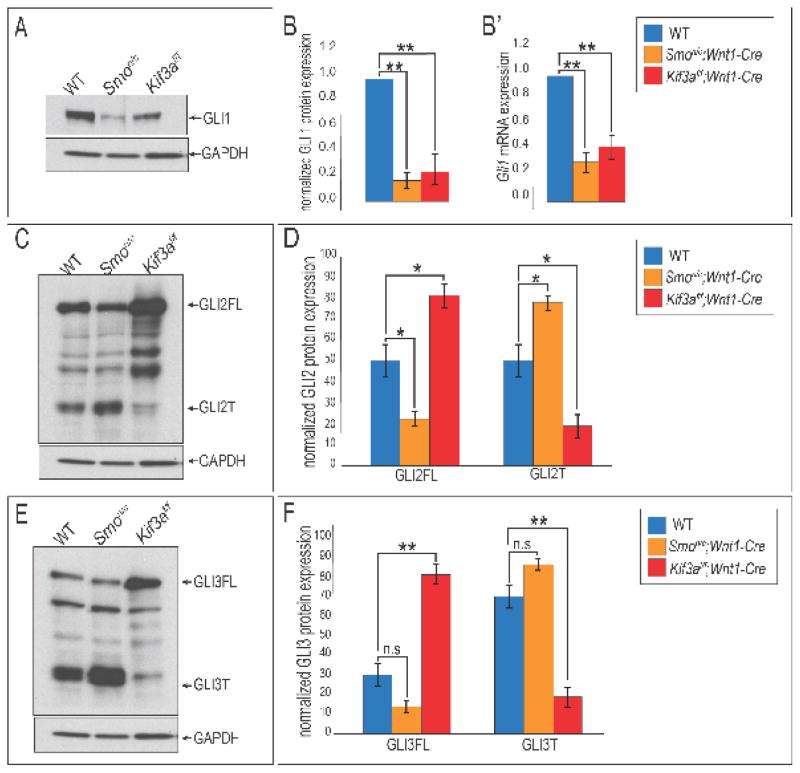
GLI proteins are the transcriptional mediators of the Hh pathway. To determine if disruptions in GLI protein production correlated with aglossia in Kif3af/f;Wnt1-Cre embryos, we examined expression of GLI1, 2 and 3 via Western blot analysis. GLI1 potentiates the Hh signal, and its mRNA expression is commonly used as a read out of Hh activity. In wild-type mandibles we detected a robust about of GLI1 protein (Fig. 6A, B). The amount of GLI1 protein detected in the Smon/c;Wnt1-Cre developing mandible was significantly reduced compared to wild-type controls (Fig. 6A, B), confirming the previously published finding that aglossia in these mutants was due to a loss of Hh function (Jeong et al., 2004). We repeated this analysis on Kif3af/f;Wnt1-Cre mandibular prominences and found that GLI1 protein levels were also significantly downregulated in our ciliopathic mutant (Fig. 6A, B; p<0.01; n=3). Furthermore, we performed qPCR on wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre mandibles and observed a significant reduction in the Gli1 transcript, confirming our RNA-seq results (Fig. 6B′). These results suggested that Hh pathway activity was impaired in the developing mandible via reduced GLI1 protein and mRNA expression in both Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre aglossia mutants.
Figure 6. GLI protein expression is altered in aglossia mutants.
(A) Western blot analysis for GLI1 on wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre e10.5 mandibular prominences. (B) Quantification of GLI1 Western blot analysis. (B′) qPCR for Gli1 transcript levels in wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre e10.5 mandibular prominences. (C) Western blot analysis for GLI2 on wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre e10.5 mandibular prominences. (D) Quantification of GLI2 Western blot analysis. (E) Western blot analysis for GLI3 on wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre e10.5 mandibular prominences. (D) Quantification of GLI3 Western blot analysis.
Functional primary cilia are required for the post-translational processing of GLI2 and GLI3 transcription factors from a full-length (GLI2FL and GLI3FL) isoforms to truncated (GLI2T and GLI3T) isoforms (Haycraft et al., 2005; Humke et al., 2010). This processing is important for transduction of a Hh signal as GLIFL isoforms can go on to act as activators (GLIA) and truncated isoforms can go on to function as repressors (GLIR). To examine the production of GLIFL to GLIT isoforms in aglossic mutants, we harvested e10.5 mandibular prominences from wild-type, Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre mandibles and performed Western blot analyses. We first analyzed the post-translational processing of GLI2. In wild-type embryos GLI2FL and GLI2T were both readily detected in the developing mandible (Fig. 6C, D). We repeated this analysis on Smon/c;Wnt1-Cre mandibles and found that relative to wild-type embryos there was a significant reduction in the amount of GLI2FL and a significant increase in the amount of GLI2T present in Smon/c;Wnt1-Cre mandibular prominences (Fig. 6C, D; p<0.05; n=3). Interestingly, changes in levels of GLI2FL and GLI2T were not conserved between Smon/c;Wnt1-Cre and Kif3af/f;Wnt1-Cre embryos. Western blot analysis on Kif3af/f;Wnt1-Cre mandibular prominences revealed a significant increase in the amount of GLI2FL and a reduced amount of GLI2T (Fig. 6C, D; p<0.05; n=3).
We next examined the level of GLI3 proteins in wild-type, Smon/c;Wnt1-Cre and Kif3a f/f;Wnt1-Cre mandibular prominences (Fig. 6E, F). In wild-type mandibles we detected both GLI3FL and GLI3T isoforms; however, the ratio of activator to repressor was skewed in favor of the GLI3T. Levels of both GLI3FL and GLI3T did not appear to be significantly different in Smon/c;Wnt1-Cre, relative to wild-type mandibular prominences (Fig. 6E, F; p=0.07; n=3). Conversely, we observed a significant increase in GLI3FL proteins in Kif3af/f;Wnt1-Cre mandibular prominence (Fig. 6E, F; p<0.01; n=3). In contrast to what we observed in Smon/c;Wnt1-Cre mandibular prominences, GLI3T protein levels were significantly reduced in Kif3af/f;Wnt1-Cre prominence (Fig. 6E, F; p<0.01; n=3). We noted two important conclusions from these results. First, GLI2 production, but not GLI3 production, was significantly impacted in Smon/c;Wnt1-Cre embryos. We took note of this because GLI2 functions as the predominant activator of the Hh pathway (Matise et al., 1998) and these results suggest it is the loss of GLIA, rather than the gain of GLIR which causes impaired Hh signal transduction. Second, despite having reduced GLI1 protein and mRNA expression, coupled with an aglossia phenotype indicative of a loss of Hh function, production of both GLI2FL and GLI3FL isoforms were increased in Kif3af/f;Wnt1-Cre mandibular prominence. Our previous work, and the work of others, suggested that GLIFL isoforms produced in ciliary mutants are unable to function as full-length GLIA because they fail to undergo cilia-dependent dissociation from the Suppressor of Fused (SUFU) (Barnfield et al., 2005; Chang et al., 2016; Eggenschwiler and Anderson, 2007; Humke et al., 2010; Li et al., 2012; Rohatgi et al., 2007; Tukachinsky et al., 2010; Wang et al., 2010). We next tested if the loss of functional GLIA alone could phenocopy ciliopathic aglossia.
Loss of GLIA in NCCs morphologically and molecularly phenocopies ciliopathic aglossia
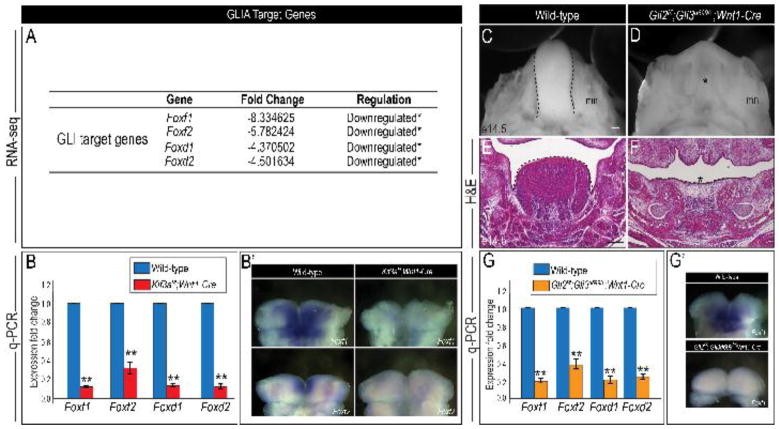
To test if aglossia in Kif3af/f;Wnt1-Cre embryos is attributed a loss of GLIA, we first examined the literature to identify genes that were direct targets of GLI activation within the craniofacial complex. Fox genes encode a family of transcription factors that regulate craniofacial development and are direct targets of GLI proteins (Madison et al., 2009; Teh et al., 2002). The expression of Foxf1, Foxf2, Foxd1 and Foxd2 in the mandibular prominence defines the mandible, incisors and tongue (Jeong et al., 2004). Aglossia in the Smon/c;Wnt1-Cre mutant was attributed to a downregulation of Fox genes in the mandibular prominence (Jeong et al., 2004). Based on these findings, we analyzed our RNA-seq data for Fox gene expression and found there was a significant downregulation of Foxf1, Foxf2, Foxd1 and Foxd2 transcripts in Kif3af/f;Wnt1-Cre mandibular prominences, compared to wild-type mandibles (Fig. 7A). We confirmed our RNA-seq data by performing qPCR analysis on e10.5 wild-type and Kif3af/f;Wnt1-Cre mandibular prominences. Relative to wild-type, we found a significant reduction in Foxf1, Foxf2, Foxd1 and Foxd2 transcripts in Kif3af/f;Wnt1-Cre mandibular prominences via both qPCR and in situ hybridization (Fig. 7B–B′; p<0.01; n=3). These results suggested that despite producing increased levels of GLIFL protein, GLIA activity was reduced in Kif3af/f;Wnt1-Cre embryos, resulting in a significant downregulation of GLI target genes in the mandibular arch.
Figure 7. Loss of GLIA activity recapitulates aglossia phenotype morphologically and molecularly.
(A) Gene expression changes for GLIA target genes in wild-type and Kif3af/f;Wnt1-Cre mandibular prominences, as per RNA-seq analysis. (B) q-PCR for Foxf1, Foxf2, Foxd1 and Foxd2 in wild-type and Kif3af/f;Wnt1-Cre mandibular prominences. (B′) Dorsal views of whole mount in situ hybridization for Foxf1 and Foxf2 in wild-type and Kif3af/f;Wnt1-Cre mandibular prominences. (C, D) Dorsal view of the tongue (dotted black lines) in e14.5 wild-type and Gli2f/f;Gli3d699/+;Wnt1-Cre embryos. (E, F) Hematoxylin and eosin-staining (H&E) on frontal sections through the developing tongue and mandible of e14.5 wild-type and Gli2f/f;Gli3d699/+;Wnt1-Cre embryos. (G) q-PCR for Foxf1, Foxf2, Foxd1 and Foxd2 in wild-type and Gli2f/f;Gli3d699/+;Wnt1-Cre mandibular prominences. (G′) Dorsal views of whole mount in situ hybridization for Foxf1 in wild-type and Gli2f/f;Gli3d699/+;Wnt1-Cre mandibular prominences. Scale bars = (C, D) 500um; (E, F) 250um.
To test if loss of GLIA alone was sufficient to induce an aglossic phenotype, we aimed to recapitulate aglossia by removing GLIA from NCC cells of wild-type mice. Although GLI2 is the predominant Hh pathway activator (Matise et al., 1998), previous studies showed that GLI3 functioned as a weak activator and compensated for the loss of GLI2 in certain scenarios (McDermott et al., 2005; Mo et al., 1997; Sasaki et al., 1997). To genetically delete GLIA from NCC cells, we opted for an approach whereby we impaired both GLI2A and GLI3A in NCC cells. We generated Gli2f/f;Gli3d699/+;Wnt1-Cre embryos, in which we conditionally deleted Gli2 in NCC cells and introduced the Gli3d699 allele. Gli3d699 murine mutants only express the truncated N-terminal repressor domain of GLI3, GLI3R (Bose et al., 2002). Independently, Gli2f/f;Wnt1-Cre and Gli3d699 homozygous mutants exhibited no obvious glossal defects (Fig. S2A–B) (Chang et al., 2016). Relative to wild-type mandibles, e14.5 Gli2f/f;Gli3d699/+;Wnt1-Cre mandibles exhibited aglossia (Fig. 7C–F). Furthermore, our qPCR analysis on e10.5 Gli2f/f;Gli3d699/+;Wnt1-Cre mandibular prominences showed that Foxf1, Foxf2, Foxd1, and Foxd2 mRNA expression was significantly reduced compared to wild-type, in a similar fashion to that of Kif3af/f;Wnt1-Cre embryos (Fig. 7G–G′; p<0.01; n=3). Taken together, these results indicate that glossal development requires GLIA activity in NCC cells. Our results further support the hypothesis that aglossia in Kif3af/f;Wnt1-Cre ciliopathic mutants is caused by the failure to produce functional GLIA and the subsequent down-regulation of mandibular Fox genes.
Overexpressing GLIA in NCCs partially rescues the aglossia phenotype in Kif3af/f;Wnt1-Cre embryos
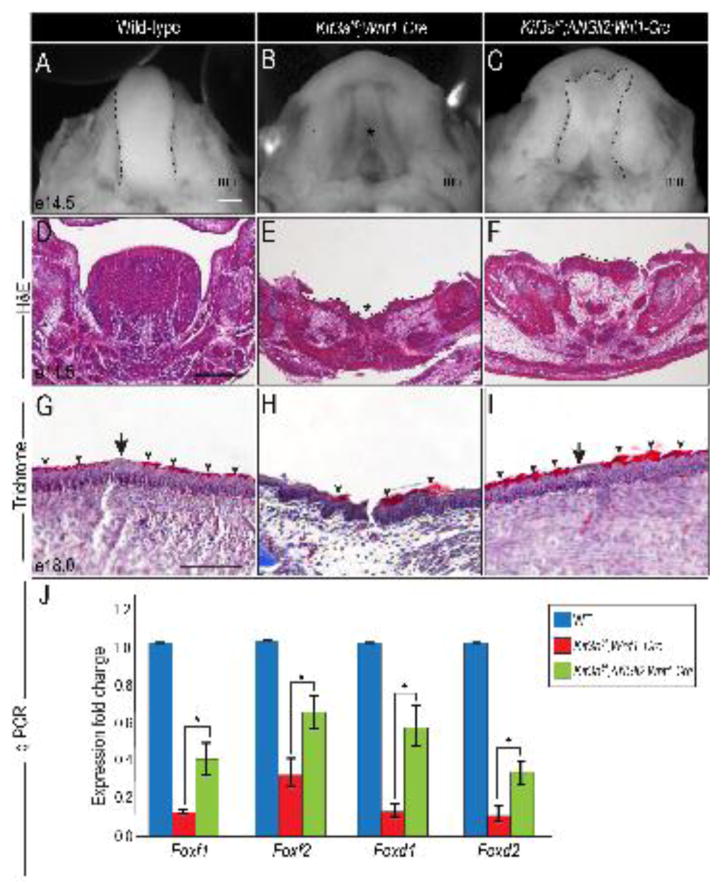
To determine if GLIA-deficiency in NCC cells is causal for aglossia in Kif3af/f;Wnt1-Cre embryos, we aimed to rescue the aglossia phenotype and restore mandibular Fox gene expression by overexpressing GLIA in NCCs. To do so, we utilized ΔNGli2 mice (Mill et al., 2003), because the processing and activation potential of ΔNGli2 is independent of the presence of primary cilia (Han et al., 2009). At e14.5, the aglossic phenotype was clear in Kif3af/f;Wnt1-Cre embryos (Fig. 8A, B; asterisk in B). Interestingly, Kif3af/f;ΔNGli2;Wnt1-Cre embryos formed a partial tongue-like structure on the floor of the mandible (Fig. 8B, C; black dotted lines in C). Examining the developing tongue and mandible in section revealed that aglossia in Kif3af/f;Wnt1-Cre embryos generated a concave depression in the developing mandible (Fig. 8D, E). The addition of the ΔNGli2 allele in Kif3af/f;ΔNGli2;Wnt1-Cre embryos partially restored the concave depression in the mandible and formed a rudimentary glossal outgrowth, relative to Kif3af/f;Wnt1-Cre embryos (Fig. 8E, F; dotted lines). Furthermore, we observed that the lingual epithelial differentiation defects in Kif3af/f;Wnt1-Cre embryos were also partially rescued with the addition of the ΔNGli2 allele (Fig 8G–I). The epithelial keratinization pattern and the presence of a taste bud-like structure in Kif3af/f;ΔNGli2;Wnt1-Cre epithelium more closely resembled that of the wild-type tongue (Fig. 8G, I; arrow and arrowheads). These results indicate that morphologically, addition of the ΔNGli2 allele partially restores a tongue-like structure in Kif3af/f;Wnt1-Cre embryos.
Figure 8. Constitutively active GLI2A partially rescues aglossia in Kif3af/f;Wnt1-Cre embryos.
(A–C) Dorsal view of the developing tongue (dotted black lines) and mandibular prominence in e14.5 wild-type, Kif3af/f;Wnt1-Cre and Kif3af/f;ΔNGli2;Wnt1-Cre embryos. (D–F) Hematoxylin and eosin (H&E) staining on frontal sections through the developing tongue and mandible of e14.5 wild-type, Kif3af/f;Wnt1-Cre and Kif3af/f;ΔNGli2;Wnt1-Cre embryos. (G–I) Trichrome staining on frontal sections through the developing tongue of e14.5 wild-type, Kif3af/f;Wnt1-Cre and Kif3af/f;ΔNGli2;Wnt1-Cre embryos. (J) q-PCR analysis for Foxf1, Foxf2, Foxd1 and Foxd2 expression in mandibular prominences of e10.5 wild-type, Kif3af/f;Wnt1-Cre and Kif3af/f;ΔNGli2;Wnt1-Cre embryos. Scale bars = (A–C) 500um; (D–F) 250um; (G–I) 100um.
To determine if ΔNGli2 activity in Kif3af/f;Wnt1-Cre embryos rescued the glossal defects on a molecular level, we examined the expression of mandibular Fox genes in wild-type, Kif3af/f;Wnt1-Cre, and Kif3af/f;ΔNGli2;Wnt1-Cre embryos at e10.5 by qPCR. Relative to Kif3af/f;Wnt1-Cre mandibular prominences, the expression of Foxf1, Foxf2, Foxd1 and Foxd2 in Kif3af/f;ΔNGli2;Wnt1-Cre mandibular prominences was significantly increased (Fig. 8J p<0.05; n=6); however, the observed increased was not restored to wild-type levels. Concurrent with the partial morphological glossal rescue, these results suggest that ΔNGli2 partially rescues the ciliopathic glossal defect at the molecular level.
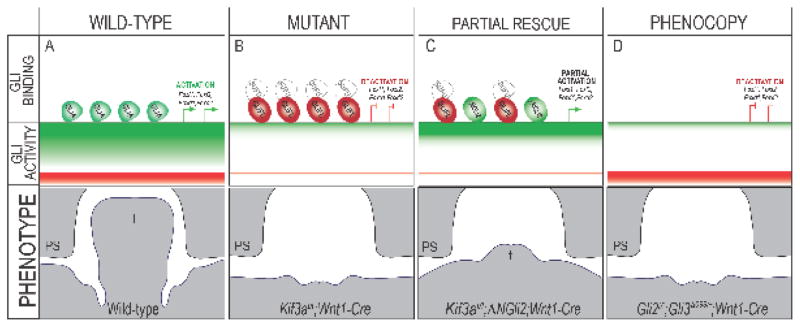
In sum, our data contributed to the proposal of the following mechanism for glossal development and the observed ciliopathic glossal phenotypes. In the wild-type mandibular prominence, cilia effectively process GLIFL into GLIA. GLIA, occupies the Gli binding regions (GBRs) within GLI target genes and a ratio of high GLIA binding relative to GLIR binding at GBRs of target genes (Foxf1, Foxf2, Foxd1, Foxd2) is established (Fig. 9A). Thus, high levels of GLIA activity are required for normal outgrowth of the mandible and tongue (Fig. 9A). The loss of cilia in NCCs (Kif3afl/fl;Wnt1-Cre) impairs the processing of GLIFL to GLIA (and GLIR), resulting in production of a GLIFL that is unable to function as an activator. Several studies, including our own, have reported that loss of cilia prevents the SMO-dependent dissociation of GLIFL from the Hh pathway inhibitor, SUFU (Barnfield et al., 2005; Chang et al., 2016; Eggenschwiler and Anderson, 2007; Humke et al., 2010; Li et al., 2012; Rohatgi et al., 2007; Tukachinsky et al., 2010; Wang et al., 2010). Thus, in ciliary mutants the GLIFL protein that is generated occupies GBRs, yet is not functional due to maintained association with SUFU (Fig. 9B). This disruption in processing causes a loss of required GLIA activity and a “deactivation” of GLI targets in the developing tongue and mandible, namely Foxf1, Foxf2, Foxd1 and Foxd2. Loss of GLI activation in the mandible and tongue results in the loss of Hh phenotype of micrognathia and aglossia. Genetic addition of the ΔNGli2 allele produces a cilia-independent, full-length, constitutively active GLI2A protein free from SUFU suppression. ΔNGLI2 can compete with ciliopathic GLIFL associated with SUFU to occupy GBRs within the regulatory regions of target genes (Fig. 9C). Binding of ΔNGLI2 at GBRs partially restores amount of GLIA enrichment and the GLI ratio shifts in favor of the GLIA, partially rescuing the aglossic phenotype (Fig. 9C). We suggest the rescue is only partial because some amount of GLIFL-SUFU complex still occupies GBRs (Fig. 9C). Finally, our hypothesized model also explained how Gli2f/f;Gli3d699/+;Wnt1-Cre embryos could phenocopy the Kif3afl/fl;Wnt1-Cre aglossic phenotype (Fig. 9D). Loss of GLI2 and GLI3A would prevent any GLIA from binding on GBRs within the regulatory regions of target genes, thus mimicking the Kif3afl/fl;Wnt1-Cre loss of GLIA and non-function GLIFL-SUFU complex binding. Loss of GLI2 and GLI3A in NCCs had the same effect on the GLI production in the developing tongue and mandible as loss of cilia (i.e., loss of the predominant activator resulting in a deactivation of target genes). Loss of GLI activation in the mandibular prominence then results in the loss of Hh phenotype of micrognathia and aglossia (Fig. 9D). Together, these data supported the hypothesis that GLIA activity plays an essential role in glossal development.
Figure 9. Schematic of hypothesized model for GLI-dependent glossal development.
In (A) wild-type, (B) Kif3afl/fl;Wnt1-Cre, (C) Kif3af/f;ΔNGli2;Wnt1-Cre, (D) Gli2f/f;Gli3d699/+;Wnt1-Cre embryos, GLI binding represents GLI proteins sitting on GLI binding regions (GBRs) sequences, GLI activity represents overall ratio of GLIA activity versus GLIR activity, green indicates GLIA activity and red indicates GLIR activity, white indicates loss of activity. Phenotype represents the growth of the tongue schematized as a frontal section. Green half ovals (GLIA) indicates fully functional GLI full-length activator, processed via ciliary trafficking. Red full oval (GLIFL) indicates a full-length isoform lacking ciliary processing and thus unable to function as an activator. White circle (SUFU) indicate SUFU protein that was not dissociated from GLIFL due to lack of ciliary processing. Green full oval (ΔNGLI2) indicates the constitutively active GLI2 activator that did not require ciliary processing. PS, palatal shelf; T, tongue; t, rescued tongue.
Discussion
Despite being a vital organ for vertebrate communication and feeding, little is known about the development of the tongue. We previously reported that loss of cilia, via conditional knock-out of the kinesin motor protein KIF3a, resulted in micrognathia and aglossia (Brugmann et al., 2010). Herein we characterized the glossal deficit (Fig. 1) and explored the cellular (Figs. 2–4) and molecular (Figs. 5–7) basis for this phenotype. From a cellular perspective, the loss of cilia on NCCs, resulted in increased apoptosis in both NCCs and mesodermally-derived muscle precursors. Additionally, the mesodermally-derived muscle precursors failed to invade the tongue anlage. Molecularly, NCCs that lacked cilia failed to potentiate a Hh signal due to a loss of GLIA activity. The loss of GLIA activity subsequently prevented the transcription of direct GLI targets important for mandibular and glossal development, including members of the Fox gene family. Taken together, these data established the foundation for several interesting hypotheses regarding the role of different signaling pathways during mandibular patterning and glossal outgrowth, as well as possibility that facial prominences required unique input from GLIA and GLIR isoforms for proper development.
A variety of glossal mutants suggests several synergistic and parallel pathways function during tongue and mandibular development
Aglossia is a rare condition frequently associated with syndromes that affect development of the craniofacial complex (Emmanouil-Nikoloussi and Kerameos-Foroglou, 1992), including ciliopathies (Chang et al., 2015; Schock et al., 2016; Zaghloul and Brugmann, 2011). Interestingly, there are now several transgenic lines with impaired transduction of major signaling pathways that results in glossal anomalies (Barron et al., 2011; Han et al., 2012; Jeong et al., 2004; Parada and Chai, 2015; Parada et al., 2012). Aglossia in Smon/c;Wnt1-Cre embryos arises from the loss of the Hh signaling component, SMOOTHENED (SMO) on NCCs. SMO plays an essential role in ciliary-dependent Hh signal transduction. When Hh ligand is present, PATCHED mediated repression of SMO is alleviated and SMO is trafficked into the cilium. Activated SMO, in conjunction with KIF7, then functions to dissociate GLIFL isoforms from the pathway repressor SUFU, allowing for GLIFL to function as an activator. Absence of SMO or loss of cilia prevents SMO localization and subsequent production of functional GLIA (Jia et al., 2009). According to this mechanistic model, it is clear why the Kif3afl/fl;Wnt1-Cre and Smon/c;Wnt1-Cre embryos mandibular phenotypes are so similar. Both embryos exhibited increased apoptosis, a failure of muscle precursors to occupy the tongue anlage and reduced expression of several Fox genes (Figs. 3, 4, 7) (Jeong et al., 2004). Interestingly, Smon/c;Wnt1-Cre embryos also reported decreased cell proliferation (cell origin was not specified) (Jeong et al., 2004), which was not observed within either NCCs or mesodermally-derived muscle precursor cells of Kif3afl/fl;Wnt1-Cre embryos (Fig. 4). Identifying and understanding differences like this between these two mutants may offer a unique opportunity to distinguish between the roles of cilia-dependent and cilia-independent Hh signaling during craniofacial development.
Another aglossia mutant, Hand2f/f;Wnt1-Cre, in which the transcription factor HAND2 was deleted from NCCs has a less similar phenotype to Kif3afl/fl;Wnt1-Cre embryos. First, Hand2f/f;Wnt1-Cre embryos fail to form the earliest morphological indication of a tongue, the lateral lingual swelling (Barron et al., 2011). Second, unlike what was observed in Kif3afl/fl;Wnt1-Cre embryos, no change in cell death within the glossal mesenchyme was reported in Hand2f/f;Wnt1-Cre embryos. Furthermore, molecularly, aglossia in Hand2f/f;Wnt1-Cre embryos was due to a failure to repress distal Dlx5 and Dlx6 expression. Foxf1 expression, which was significantly downregulated in Kif3afl/fl;Wnt1-Cre embryos, was unchanged in Hand2f/f;Wnt1-Cre mutants. Despite these significant differences, both mutants displayed severely disorganized glossal musculature coupled with and a partially differentiated lingual epithelium. Thus, notwithstanding completely separate cellular and molecular mechanisms, both Kif3afl/fl;Wnt1-Cre and Hand2f/f;Wnt1-Cre mutants have similar aglossic phenotypes. HAND2 and GLI3 have previously been reported to have an antagonistic relationship in the developing limb (Galli et al., 2010; te Welscher et al., 2002; Vokes et al., 2008; Zhulyn et al., 2014). Given the GLI processing defects in Kif3afl/fl;Wnt1-Cre mutants, it will be interesting to explore how GLI proteins in ciliopathic mutants interact with factors such as HAND2 during craniofacial development.
Aglossia in ciliary mutants was caused by increased cell death and a failure of mesoderm invasion
One of the most intriguing aspects of the aglossia phenotype in Kif3afl/fl;Wnt1-Cre embryos is the loss of the intrinsic glossal musculature, a cell population not derived from NCCs. The intrinsic tongue muscles are derived from the occipital somites, which are mesodermal in origin. These cells migrate into mandible via the hypoglossal cord where they proliferate and differentiate into muscle fibers (Noden and Francis-West, 2006; Noden and Trainor, 2005). These results suggest two possible scenarios for which the NCCs could be influencing the muscle precursors in a non-cell autonomous fashion. First, since we failed to detect muscle precursors migrating into the mandible, it is possible that the NCCs provide some chemotactic signal that directs the cells within the hypoglossal cord to invade the tongue anlage. Second, since we observed apoptosis within the myogenic precursor cells in the posterior aspect of the mandible, it is also possible that NCCs are providing a survival signal for the muscle precursors. Even though it has previously been hypothesized that NCCs provide signals that release the myogenic populations from differentiation inhibitors (Noden and Trainor, 2005; Tzahor et al., 2003), our data do not favor this mechanism within the developing tongue because other muscle precursor in Kif3afl/fl;Wnt1-Cre mutants adjacent to unciliated NCCs (those that give rise to the extrinsic musculature) differentiated into muscle fibers. Identifying and understanding the cilia-dependent factors that murine NCCs produce to allow for the survival and invasion of the cells within the hypoglossal cord is a topic of our ongoing research.
Ciliary phenotypes suggested distinct modes of function for GLI proteins within facial prominences
GLI proteins are bimodal transcription factors that are responsible for the activation or repression of the Hh pathway. The bimodal actions of these transcription factors allow for two GLI-dependent scenarios to alter net Hh pathway activity. A gain of Hh pathway activity can be caused by an increase in GLIA or a loss of GLIR. Alternatively, a loss of Hh pathway activity can be caused by a loss of GLIA or a gain of GLIR. Our study generated several pieces of data that strongly suggest normal development of the tongue relied heavily on high GLIA activity, and that the loss of GLIA is the molecular basis for ciliopathic aglossia. Our first piece of evidence supporting a role for GLIA in glossal development was that aglossia in Smon/c;Wnt1-Cre embryos correlated with a loss of the predominant activator of the Hh pathway, GLI2FL, and not in GLI3FL production. Secondly, mRNA and protein levels of the GLI1, the potentiator of Hh pathway activity, were significantly downregulated via RNA-seq, qPCR and Western blot analyses in Kif3afl/fl;Wnt1-Cre mutants. Third, we were able to generate the aglossia phenotype by genetically removing only GLIA (Gli2f/f;Gli3d699/+;Wnt1-Cre; Fig. 7). Finally, we could partially rescue ciliopathic aglossia by genetically introducing a constitutively active GLI2A (Kif3af/f;ΔNGli2;Wnt1-Cre; Fig. 8). Thus, we concluded that the GLIA isoform is the key factor during mandibular and glossal development.
Our previous work examined the midfacial phenotype of the Kif3afl/fl;Wnt1-Cre mutant (Chang et al., 2016). The midface is derived from the frontonasal prominence, which is distinct in location and cellular makeup from the mandibular prominence (Brugmann et al., 2006). Loss of cilia in Kif3af/f;Wnt1-Cre mutants causes severe midfacial widening such that the internasal distance was increased approximately 120% relative to wild-type embryos (Brugmann et al., 2010). Midline widening has long been tightly associated with a gain of Hh function via the loss of GLI3R activity (Cordero et al., 2004; Hu and Helms, 1999; Marcucio et al., 2005; Vortkamp et al., 1992; Vortkamp et al., 1991). Thus, when taken together with our work herein, these results suggest that one facial prominence utilizes the GLIR isoform for patterning (frontonasal prominence), whereas another facial prominence utilizes the GLIA isoform (mandibular prominence).
The possibility that facial prominences function as individual developmental fields, utilizing unique mechanisms to modulate Hh signaling is intriguing. Pinpointing the GLI isoform necessary for mandibular versus frontonasal growth could be extremely useful in formulating therapeutic option for craniofacial anomalies. Utilizing ciliary mutants, rather than pathway mutants, may be a powerful way to bring clarity to how Hh signaling drives craniofacial development. Our ongoing studies address these issues using genetic and biochemical based approaches.
Materials and Methods
Mouse strains
The Wnt1-Cre, Gli3f/f (Gli3tm1Al/j), Smof/f (Smotm2/Amc/J), Smonull, RosaGli3TFlag c/c and ROSAmT/mG mouse strains were purchased from Jackson Laboratory. MyoDi-Cre mice were acquired from Dr. David Goldhamer at University of Connecticut; Kif3af/f, from Dr. Bradley Yoder at University of Alabama at Birmingham; Gli2f/f from Dr. Alexandra Joyner at Memorial Sloan-Kettering Cancer Center and Gli3d699 from Dr. Chi-Chung Hui at the Hospital for Sick Kids, Canada. Animal usage was approved by the Institutional Animal Care and Use Committee (IACUC) and maintained by Veterinary Services at Cincinnati Children’s Hospital Medical Center. All transgenic lines were maintained on a mixed (CD1) background.
Histological analysis and immunostaining
Hematoxylin and eosin (H&E), Trichrome staining and immunohistochemistry and immunofluorescence experiments were performed per standard protocols. Immunostainings were performed using primary antibodies against the following: anti-Arl13b (Proteintech), anti-MyoD1 (clone 5.8A, from Novus bio), anti-Myogenin (F5D, from abcam) myosin heavy chain (MF20, from R&D), and anti-active Caspase3 (clone Asp175, from Cell Signaling). For phospho-Histone H3 (pHH3) staining (Ser10, Santa Cruz) and TUNEL staining (11684795910, Roche), e11.5 heads were fixed in 4% PFA, paraffin embedded and cut transversely or sagittally. Staining was done according to manufacturer’s instruction. Secondary antibodies with fluorescence tags were applied and incubated at room temperature for 1 hour. Slides were then stained with 4′,6-diamino-2-phenylindone (DAPI; 5 μg/mL; Invitrogen) and mounted with Prolong gold mounting media from Invitrogen. Cell counts were performed by manually counting double labelled cells within the outlined areas. Counts were normalized to the area of the outlined object. Area of the outlined object was quantified using ImageJ software. Area of all objects was quantified using images obtained at the same magnification.
Mandible and tongue morphology
For whole mandible images, e13.5 and e14.5 embryos were fixed in Bouin’s fixative at 4° Celsius overnight. The mandibles were dissected and imaged using Leica imaging software in gray scale mode.
In situ hybridization
Sectioned and whole mount in situ hybridizations on mouse embryos were performed following procedures modified Gallus Expression in situ hybridization Analysis (GEISHA) protocols (Darnell et al., 2007). Digoxigenin-labeled anti-sense riboprobes for Shh, Patched, and Foxd1 were created from mouse cDNA templates that were amplified from primer sequences acquired from Gene Paint (www.genepaint.org). The digoxigenin-labeled anti-sense riboprobes for Foxf1, Foxf2 and Foxd2 were generated from plasmid DNA provided by Dr. Yu Lan at Cincinnati Children’s Hospital Medical Center.
Western blot analysis
Combined mandibular prominences were sonicated in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1 % NP-40, 0.25 % sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) containing protease inhibitors (Roche), phosphatase inhibitors (1 mM PMSF, 1 mM Na3VO4, 10 mM NaF, 60 mM β-glycerophosphate). Protein concentration was measured by BCA protein assay (Thermo Scientific, # 23227). 10μg protein was loaded to a 6% SDS-PAGE gel for GLI proteins and 5ug protein on a 10% SDS-PAGE gel for GAPDH. Goat polyclonal anti-GLI2 antibody (R&D, AF3635; primary concentration 1:500, secondary concentration 1:5,000) and goat polyclonal anti-GLI3 antibody (R&D, AF3690; primary concentration 1:1,000, secondary concentration 1:5,000) were used to detect both full-length and truncated isoforms of GLI2 and GLI3. Rabbit polyclonal anti-GAPDH antibody (Santa Cruz) was used to detect GAPDH (FL335, Santa Cruz; primary concentration 1:10,000, secondary concentration 1:10,000). An electrochemi-luminescence (ECL) assay (ECL prime, Amersham, Pittsburg, PA, USA) was performed to develop the chemiluminescence signals. Western blot films were quantified using Image J. Raw expression levels for GLI1, GLI2/3FL, GLI2/3T proteins were obtained and normalized to GAPDH. Experiments were conducted in triplicate (n=3). Student’s t-test was performed, p < 0.05 was used to determine statistical significance.
RNA-seq analysis
For RNA-seq analysis, mandibular prominences were dissected and pooled from approximately 10 wild-type and Kif3af/f;Wnt1-Cre e11.5 embryos. RNA was prepared for RNA-seq. RNAs were processed according to recommended procedures, using the Illumina TruSeq and Nugen Ovation RNA-Seq System V2 methods. Sequencing was carried out using the Illumina HiSeq 2000 system according to Illumina protocols. BAM files underwent whole genome RNA-seq analysis using Strand-NGS. The reference mouse genome was taken from Ensembl (4/9/2012) and the program was set to interpret data obtained from Illumina single-end sequencing. Quality inspection was run for the data set and revealed that the data was acceptable for further analysis. Partial reads were considered and the detection of novel genes and exons was allowed for. Counts were normalized to one, with the baseline median to all samples. The Audic-Claverie algorithm (no correction) was used to identify differentially expressed genes between wild-type and experimental samples with a p-value ≤ 0.05.
Supplementary Material
Dorsal view of the tongue (dotted black lines) and mandible in e13.5 (A) wild-type and (B) Kif3af/f;MyoDi-Cre embryo. (C) Quantification of tongue length in wild-type and Kif3af/f;MyoDi-Cre embryos (p>0.05, n=3). (D, E) Anti-MHC immunostaining (red) in wild-type Kif3af/f;MyoDi-Cre embryos. (F, G) Arl13b (red) and MyoD (green) co-immunostaining in wild-type and Kif3af/f;MyoDi-Cre embryos. Scale bars = (A, B) 500um; (D, E) 250um; (F, G) 10um.
Dorsal view of the tongue (dotted black lines) and mandible in e14.5 (A) wild-type and (B) Gli2f/f;Wnt1-Cre embryos. Scale bars = 500um.
Highlights.
Both neural crest and mesodermally-derived muscle precursors play an important role in glossal development
Increased apoptosis in both neural crest and mesodermally-derived muscle precursors contributes to aglossia in Kif3af/f;Wnt1-Cre mutant embryos
Mesodermally-derived muscle precursors fail to migrate into the tongue anlage of Kif3af/f;Wnt1-Cre mutant embryos
GLI activator activity is lost in the developing mandible of Kif3af/f;Wnt1-Cre mutant embryos
Restoration of GLI activator activity can partially rescue glossal development
Acknowledgments
We thank members of the Brugmann lab for helpful comments and suggestions, Jaime Struve for genotyping, Bradley Yoder, Alex Joyner and C.C. Hui for sharing transgenic mouse lines, and Jill Murphy for critical reading of the manuscript. This research was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grant R01DE023804 (S.A.B). G.M. was partially funded by T32 ES007051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baker K, Beales P. Making sense of cilia in disease: the human ciliopathies. American journal of medical genetics. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation; research in biological diversity. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- Barron F, Woods C, Kuhn K, Bishop J, Howard MJ, Clouthier DE. Downregulation of Dlx5 and Dlx6 expression by Hand2 is essential for initiation of tongue morphogenesis. Development. 2011;138:2249–2259. doi: 10.1242/dev.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Grotewold L, Ruther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet. 2002;11:1129–1135. doi: 10.1093/hmg/11.9.1129. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA. A primary cilia-dependent etiology for midline facial disorders. Hum Mol Genet. 2010;19:1577–1592. doi: 10.1093/hmg/ddq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Tapadia MD, Helms JA. The molecular origins of species-specific facial pattern. Current topics in developmental biology. 2006;73:1–42. doi: 10.1016/S0070-2153(05)73001-5. [DOI] [PubMed] [Google Scholar]
- Chang CF, Chang YT, Millington G, Brugmann SA. Craniofacial Ciliopathies Reveal Specific Requirements for GLI Proteins during Development of the Facial Midline. PLoS genetics. 2016;12:e1006351. doi: 10.1371/journal.pgen.1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Schock EN, Attia AC, Stottmann RW, Brugmann SA. The ciliary baton: orchestrating neural crest cell development. Current topics in developmental biology. 2015;111:97–134. doi: 10.1016/bs.ctdb.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, Pedersen SF, Christensen ST. PDGFRalpha signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. Journal of cell science. 2013;126:953–965. doi: 10.1242/jcs.116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. The Journal of clinical investigation. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo A, Franco B. The primary cilium in different tissues-lessons from patients and animal models. Pediatr Nephrol. 2010 doi: 10.1007/s00467-010-1650-7. [DOI] [PubMed] [Google Scholar]
- Darnell DK, Kaur S, Stanislaw S, Davey S, Konieczka JH, Yatskievych TA, Antin PB. GEISHA: an in situ hybridization gene expression resource for the chicken embryo. Cytogenetic and genome research. 2007;117:30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annual review of cell and developmental biology. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouil-Nikoloussi EN, Kerameos-Foroglou C. Developmental malformations of human tongue and associated syndromes (review) Bull Group Int Rech Sci Stomatol Odontol. 1992;35:5–12. [PubMed] [Google Scholar]
- Gai PB, Hiremath SV, Joshi R, Goudar PHK. Mohr syndrome: A rare case of oro-facial-digital syndrome type II with congenital heart disease. International Journal of Case Reports and Images. 2012;3:32. [Google Scholar]
- Galli A, Robay D, Osterwalder M, Bao X, Benazet JD, Tariq M, Paro R, Mackem S, Zeller R. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS genetics. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Zhao H, Li J, Pelikan R, Chai Y. ALK5-mediated transforming growth factor beta signaling in neural crest cells controls craniofacial muscle development via tissue-tissue interactions. Mol Cell Biol. 2014;34:3120–3131. doi: 10.1128/MCB.00623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Zhao H, Parada C, Hacia JG, Bringas P, Jr, Chai Y. A TGFbeta-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development. 2012;139:1640–1650. doi: 10.1242/dev.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nature medicine. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS genetics. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SK, Dawid IB. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc Natl Acad Sci U S A. 2009;106:2230–2235. doi: 10.1073/pnas.0812880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa R, Oka K, Yamaza T, Iwata J, Urata M, Xu X, Bringas P, Jr, Nonaka K, Chai Y. TGF-beta mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue-tissue interactions during tongue morphogenesis. Developmental biology. 2010;341:186–195. doi: 10.1016/j.ydbio.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Hui CC, Angers S. Gli proteins in development and disease. Annual review of cell and developmental biology. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Developmental biology. 2009;330:452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Developmental biology. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. The Journal of cell biology. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22:4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZJ, Nieuwenhuis E, Nien W, Zhang X, Zhang J, Puviindran V, Wainwright BJ, Kim PC, Hui CC. Kif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesis. Development. 2012;139:4152–4161. doi: 10.1242/dev.081190. [DOI] [PubMed] [Google Scholar]
- Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Developmental biology. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome research. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- McDermott A, Gustafsson M, Elsam T, Hui CC, Emerson CP, Jr, Borycki AG. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development. 2005;132:345–357. doi: 10.1242/dev.01537. [DOI] [PubMed] [Google Scholar]
- McDermott KM, Liu BY, Tlsty TD, Pazour GJ. Primary cilia regulate branching morphogenesis during mammary gland development. Curr Biol. 2010;20:731–737. doi: 10.1016/j.cub.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Moran-Barroso V, Valdes Flores M, Garcia-Cavazos R, Kofman-Alfaro S, Saavedra-Ontiveros D. Oral-facial-digital (OFD) syndrome with associated features: a new syndrome or genetic heterogeneity and variability? Clinical dysmorphology. 1998;7:55–57. [PubMed] [Google Scholar]
- Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R. Gli protein activity is controlled by multisite phosphorylation in vertebrate Hedgehog signaling. Cell Rep. 2014;6:168–181. doi: 10.1016/j.celrep.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, Emerson CP., Jr Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Developmental Dynamics. 1999;216:96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. Journal of anatomy. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira JM, Hawrot K, Sharpe C, Noble A, Wood WM, Jorge EC, Goldhamer DJ, Kardon G, Dietrich S. The emergence of Pax7-expressing muscle stem cells during vertebrate head muscle development. Front Aging Neurosci. 2015;7:62. doi: 10.3389/fnagi.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Chai Y. Mandible and Tongue Development. Curr Top Dev Biol. 2015;115:31–58. doi: 10.1016/bs.ctdb.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Han D, Chai Y. Molecular and cellular regulatory mechanisms of tongue myogenesis. Journal of dental research. 2012;91:528–535. doi: 10.1177/0022034511434055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. American journal of medical genetics Part C, Seminars in medical genetics. 2009;151C:326–340. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Salles F, Anchieta M, Costa Bezerra P, Torres ML, Queiroz E, Faber J. Complete and isolated congenital aglossia: case report and treatment of sequelae using rapid prototyping models. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e41–47. doi: 10.1016/j.tripleo.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Sasai N, Briscoe J. Primary cilia and graded Sonic Hedgehog signaling. WIRES Dev Biol. 2012:753–772. doi: 10.1002/wdev.43. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schneider L, Stock CM, Dieterich P, Jensen BH, Pedersen LB, Satir P, Schwab A, Christensen ST, Pedersen SF. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-alpha in the primary cilium. The Journal of cell biology. 2009;185:163–176. doi: 10.1083/jcb.200806019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EN, Chang CF, Youngworth IA, Davey MG, Delany ME, Brugmann SA. Utilizing the chicken as an animal model for human craniofacial ciliopathies. Developmental biology. 2016;415:326–337. doi: 10.1016/j.ydbio.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler JM, Barrell WB, Szabo-Rogers HL, Healy C, Yeung Y, Perdiguero EG, Schulz C, Yannakoudakis BZ, Mesbahi A, Wlodarczyk B, Geissmann F, Finnell RH, Wallingford JB, Liu KJ. Fuz mutant mice reveal shared mechanisms between ciliopathies and FGF-related syndromes. Dev Cell. 2013;25:623–635. doi: 10.1016/j.devcel.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–4780. [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, Tada M, Mayor R, Burns AJ, Lupski JR, Hammond P, Beales PL. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Talbott GC, Turbe-Doan A, Jacobs DT, Schonfeld MP, Silva LM, Chatterjee A, Prysak M, Allard BA, Beier DR. Downregulating hedgehog signaling reduces renal cystogenic potential of mouse models. Journal of the American Society of Nephrology : JASN. 2014;25:2201–2212. doi: 10.1681/ASN.2013070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. The Journal of cell biology. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, Agarwal P, Brown C, McCulley DJ, Schwarz JJ, Black BL. The transcription factor MEF2C is required for craniofacial development. Dev Cell. 2007;12:645–652. doi: 10.1016/j.devcel.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Franz T, Gessler M, Grzeschik KH. Deletion of GLI3 supports the homology of the human Greig cephalopolysyndactyly syndrome (GCPS) and the mouse mutant extra toes (Xt) Mammalian Genome. 1992;3:461–463. doi: 10.1007/BF00356157. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137:2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YF, Shi WG, Zhou J, Gao YH, Li SF, Fang QQ, Wang MG, Ma HP, Wang JF, Xian CJ, Chen KM. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone. 2016;93:22–32. doi: 10.1016/j.bone.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. The Journal of cell biology. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Brugmann SA. The emerging face of primary cilia. Genesis. 2011;49:231–246. doi: 10.1002/dvg.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulyn O, Li D, Deimling S, Vakili NA, Mo R, Puviindran V, Chen MH, Chuang PT, Hopyan S, Hui CC. A switch from low to high Shh activity regulates establishment of limb progenitors and signaling centers. Dev Cell. 2014;29:241–249. doi: 10.1016/j.devcel.2014.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dorsal view of the tongue (dotted black lines) and mandible in e13.5 (A) wild-type and (B) Kif3af/f;MyoDi-Cre embryo. (C) Quantification of tongue length in wild-type and Kif3af/f;MyoDi-Cre embryos (p>0.05, n=3). (D, E) Anti-MHC immunostaining (red) in wild-type Kif3af/f;MyoDi-Cre embryos. (F, G) Arl13b (red) and MyoD (green) co-immunostaining in wild-type and Kif3af/f;MyoDi-Cre embryos. Scale bars = (A, B) 500um; (D, E) 250um; (F, G) 10um.
Dorsal view of the tongue (dotted black lines) and mandible in e14.5 (A) wild-type and (B) Gli2f/f;Wnt1-Cre embryos. Scale bars = 500um.