Abstract
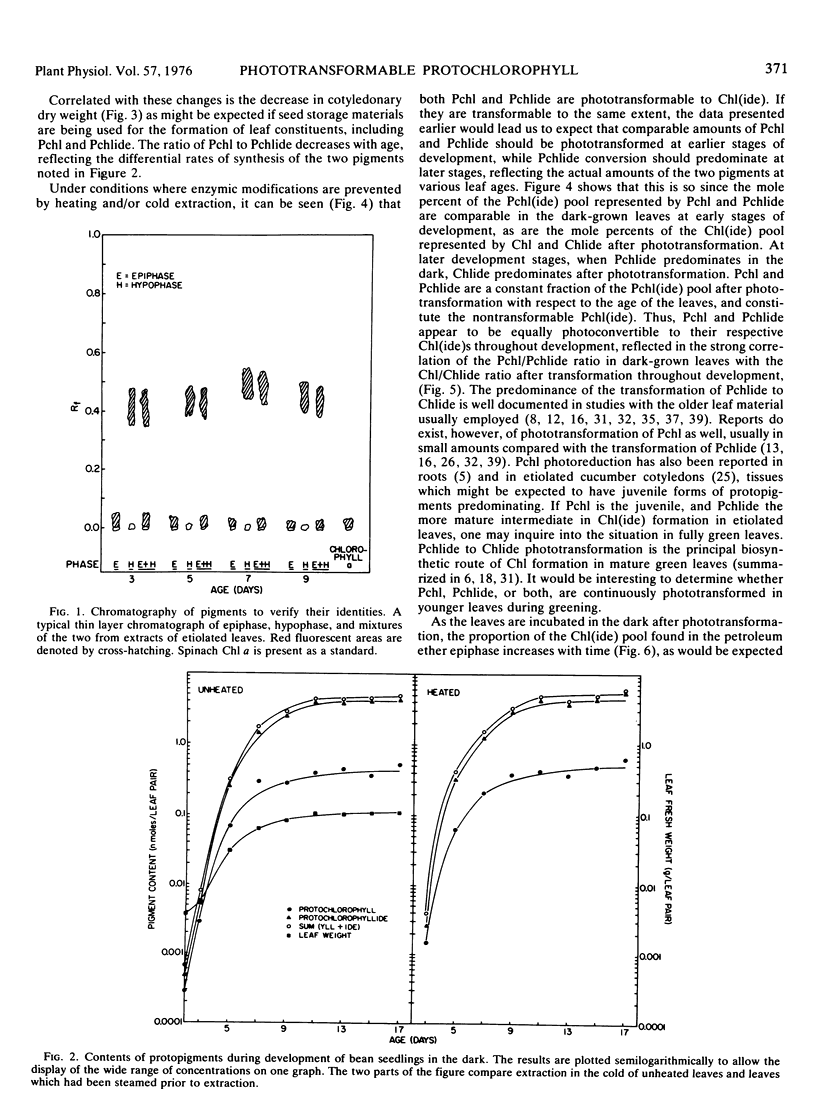
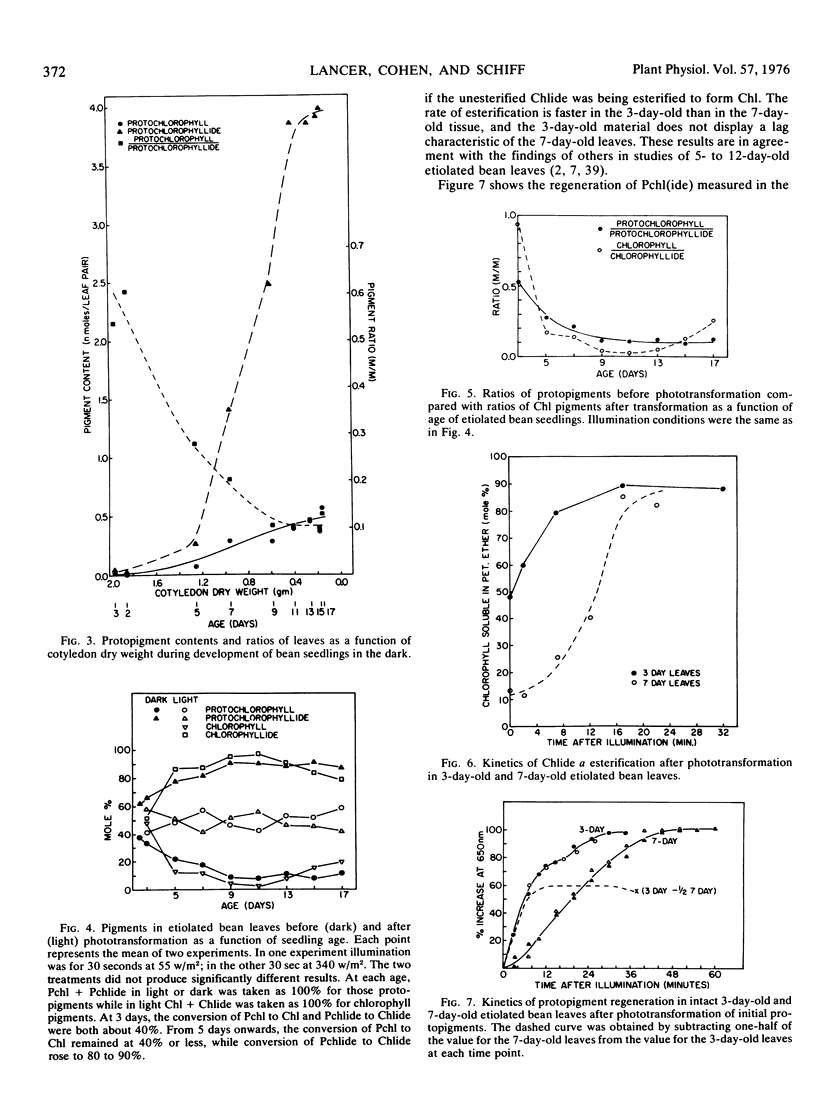
Protochlorophyll (Pchl) and protochlorophyllide (Pchlide) are at comparable levels in 2-day-old (young) etiolated bean leaves (Phaseolus vulgaris L. var. Red Kidney). During subsequent development in the dark, both pigments increase, but the rate of Pchlide increase is greater than that of Pchl, leading to the commonly observed predominance of Pchlide beyond 7 days (old leaves). Both protopigments are phototransformable to their respective chlorophyll(ide) photoproducts throughout dark development. The rate of protopigment regeneration in young leaves after illumination is rapid and displays no lag, whereas this process in old leaves begins slowly and achieves only about one-fifth the rate of younger leaves. The rate of chlorophyllide esterification is also faster in the younger tissue. Since the proplastid-related properties of young bean leaves are quite similar to those of Euglena, young leaves and Euglena may represent an evolutionarily primitive case compared with older bean leaves which contain etioplasts. Since Euglena and young beans green perfectly well when exposed to light, the extensive modifications associated with prolonged dark growth do not seem to be obligatory for plastid development. The properties of older beans are viewed as being the consequence of prolonged etiolation which may provide a faster rate of plastid development and appearance of photosynthesis as the plant nears the limits of its stored reserves.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Briggs W. R. The relation between structure and pigments during the first stages of proplastid greening. Biochim Biophys Acta. 1966 Jan 4;112(1):45–53. doi: 10.1016/s0926-6585(96)90006-0. [DOI] [PubMed] [Google Scholar]
- Ecklund P. R., Moore T. C. Correlations of Growth Rate and De-etiolation with Rate of Ent-Kaurene Biosynthesis in Pea (Pisum sativum L.). Plant Physiol. 1974 Jan;53(1):5–10. doi: 10.1104/pp.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M. Chlorophyll formation in seedlings of Zea mays L. Arch Biochem. 1950 Dec;29(2):339–343. [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A. The Correlated Appearance of Prolamellar Bodies, Protochlorophyll(ide) Species, and the Shibata Shift during Development of Bean Etioplasts in the Dark. Plant Physiol. 1972 Apr;49(4):619–626. doi: 10.1104/pp.49.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir I., Ben-Shaul Y. Structural organization of developing chloroplasts in Euglena. Protoplasma. 1974;80(1):109–127. doi: 10.1007/BF01666354. [DOI] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRONVAL C., MICHEL-WOLWERTZ M. R., MADSEN A. ON THE NATURE AND POSSIBLE FUNCTIONS OF THE 673- AND 684-MU FORMS IN VIVO OF CHLOROPHYLL. Biochim Biophys Acta. 1965 Mar 29;94:344–354. doi: 10.1016/0926-6585(65)90043-9. [DOI] [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]


