Abstract
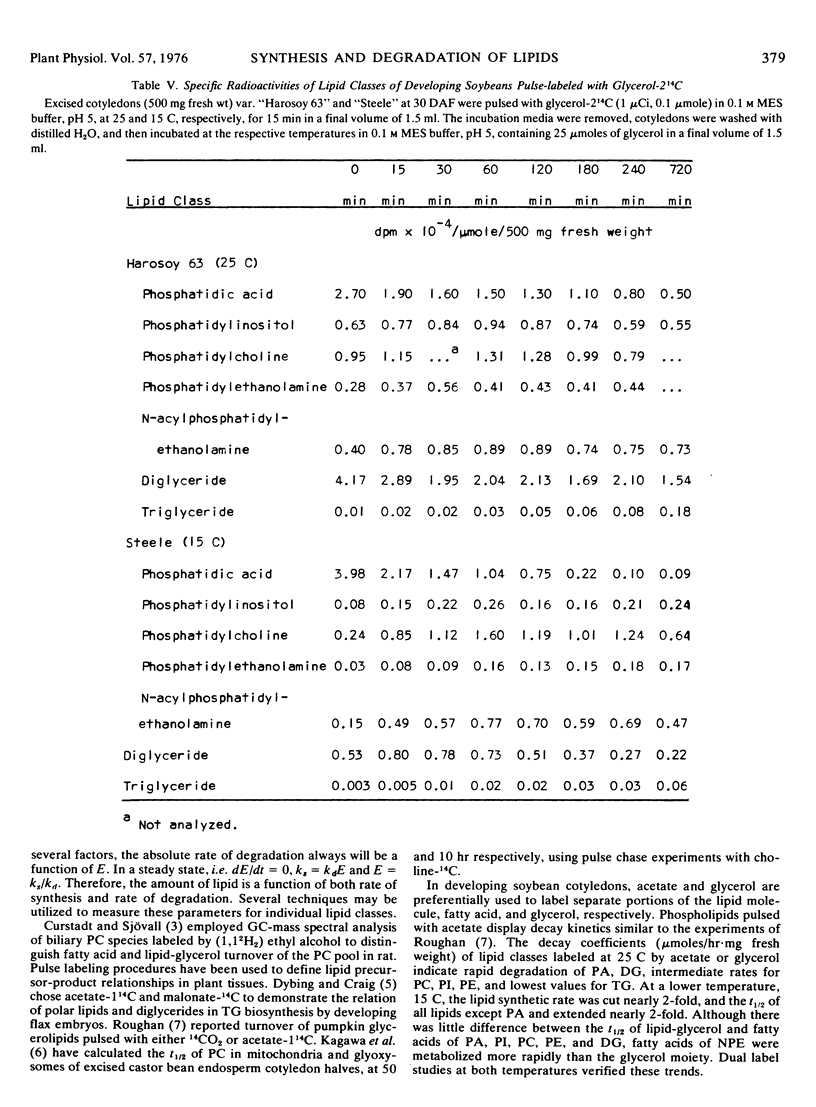
The metabolic activity of individual lipid classes found in developing soybean cotyledons (Glycine max.) is estimated by determining the degradation rate of the compound under given conditions. Pulse-labeling and dual substrate labeling are used to evaluate this parameter. These studies indicate first order decay kinetics for phosphatidic acid, phosphatidylinositol, phosphatidylcholine, phosphatidylethanolamine, N-acyl-phosphatidylethanolamine, diglyceride, and zero order kinetics for triglyceride in cotyledons var. “Harosoy 63” at 30 days after flowering. Decay coefficients for acyl groups and lipid-glycerol moieties within specific lipid classes from either method are comparable. Half-life (t½) calculations from the decay coefficients indicate extremely rapid turn-over rates (0.08 to 3.4 hours at 25 C) and suggest similar turnover rates of acyl groups and lipid-glycerol in diglyceride and all phospholipids except N-acylphosphatidylethanolamine where acyl groups are replaced independent of the glycerol moiety. These experiments reveal not only different metabolic activity between lipid components of soybean cotyledons, but also describe a new method for measuring lipid turnover in plants.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Curstedt T., Sjövall J. Biosynthetic pathways and turnover of individual biliary phosphatidylcholines during metabolism of (1,1-2H2)ethanol in the rat. Biochim Biophys Acta. 1974 Nov 18;369(2):173–195. doi: 10.1016/0005-2760(74)90250-1. [DOI] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Effect of freezing and cold storage on phospholipids in developing soybean cotyledons. Plant Physiol. 1976 Feb;57(2):270–273. doi: 10.1104/pp.57.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Phospholipids in the developing soybean seed. Plant Physiol. 1974 Nov;54(5):744–747. doi: 10.1104/pp.54.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]


