Abstract
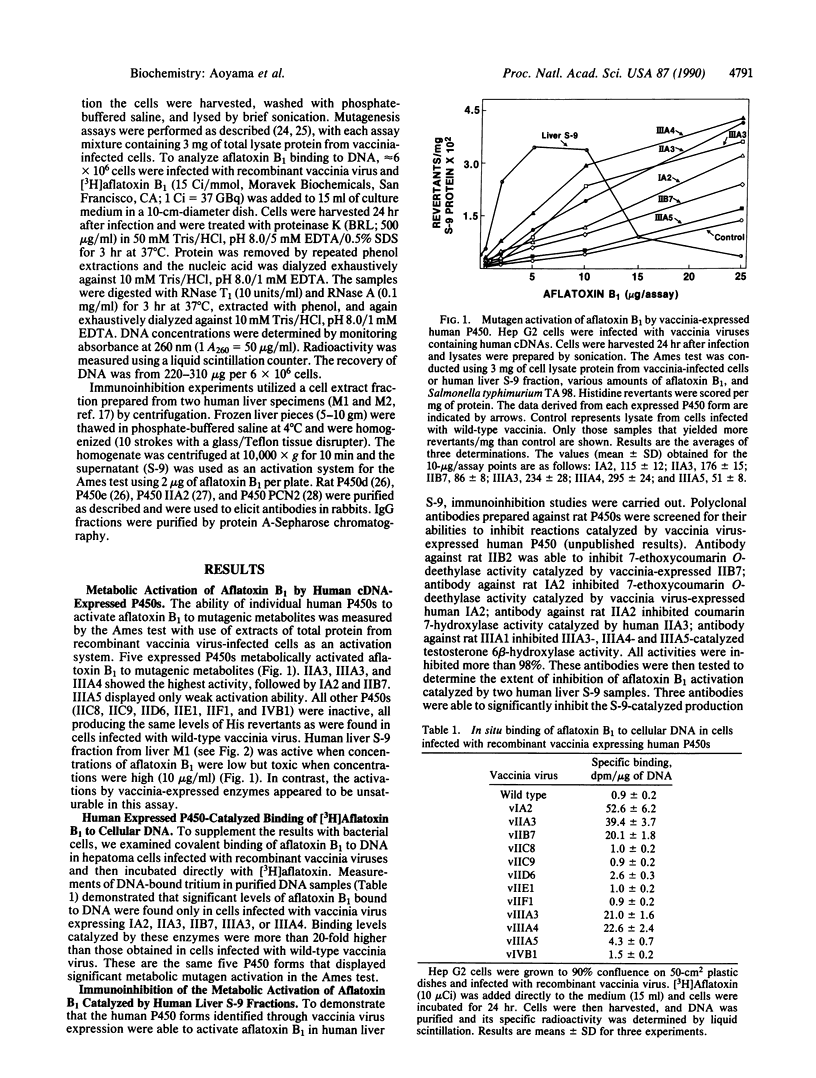
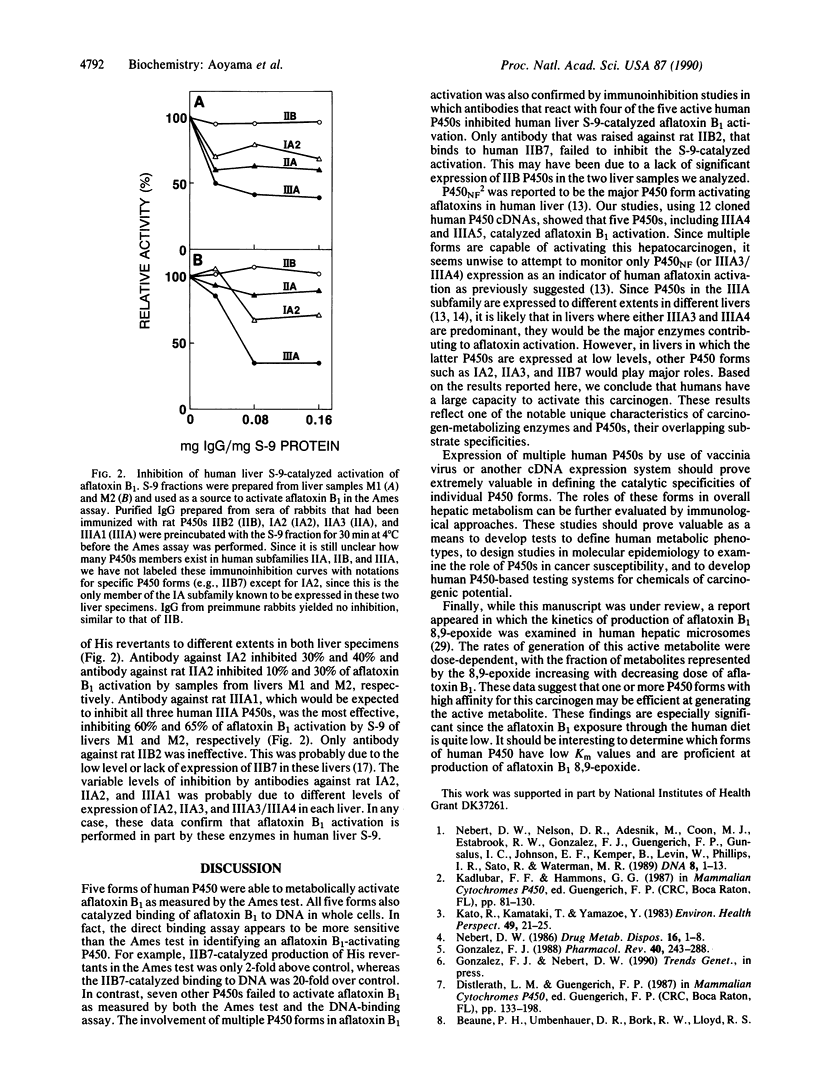
Twelve forms of human hepatic cytochrome P450 were expressed in hepatoma cells by means of recombinant vaccinia viruses. The expressed P450s were analyzed for their abilities to activate the potent hepatocarcinogen aflatoxin B1 to metabolites having mutagenic or DNA-binding properties. Five forms, P450s IA2, IIA3, IIB7, IIIA3, and IIIA4, activated aflatoxin B1 to mutagenic metabolites as assessed by the production of His revertants of Salmonella typhimurium in the Ames test. The same P450s catalyzed conversion of aflatoxin B1 to DNA-bound derivatives as judged by an in situ assay in which the radiolabeled carcinogen was incubated with cells expressing the individual P450 forms. Seven other human P450s, IIC8, IIC9, IID6, IIE1, IIF1, IIIA5, and IVB1, did not significantly activate aflatoxin B1 as measured by both the Ames test and the DNA-binding assay. Moreover, polyclonal anti-rat liver P450 antibodies that crossreact with individual human P450s IA2, IIA3, IIIA3, and IIIA4 each inhibited aflatoxin B1 activation catalyzed by human liver S-9 extracts. Inhibition ranged from as low as 10% with antibody against IIA3 to as high as 65% with antibody against IIIA3 and IIIA4. These results establish that metabolic activation of aflatoxin B1 in human liver involves the contribution of multiple forms of P450.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Yamano S., Waxman D. J., Lapenson D. P., Meyer U. A., Fischer V., Tyndale R., Inaba T., Kalow W., Gelboin H. V. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989 Jun 25;264(18):10388–10395. [PubMed] [Google Scholar]
- Beaune P. H., Umbenhauer D. R., Bork R. W., Lloyd R. S., Guengerich F. P. Isolation and sequence determination of a cDNA clone related to human cytochrome P-450 nifedipine oxidase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8064–8068. doi: 10.1073/pnas.83.21.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Guzelian P. S. Separation, purification, and characterization of a novel form of hepatic cytochrome P-450 from rats treated with pregnenolone-16 alpha-carbonitrile. J Biol Chem. 1980 Feb 25;255(4):1279–1285. [PubMed] [Google Scholar]
- Gonzalez F. J., Schmid B. J., Umeno M., Mcbride O. W., Hardwick J. P., Meyer U. A., Gelboin H. V., Idle J. R. Human P450PCN1: sequence, chromosome localization, and direct evidence through cDNA expression that P450PCN1 is nifedipine oxidase. DNA. 1988 Mar;7(2):79–86. doi: 10.1089/dna.1988.7.79. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Gonzalez F. J., Vilbois F., Hardwick J. P., McBride O. W., Nebert D. W., Gelboin H. V., Meyer U. A. Human debrisoquine 4-hydroxylase (P450IID1): cDNA and deduced amino acid sequence and assignment of the CYP2D locus to chromosome 22. Genomics. 1988 Feb;2(2):174–179. doi: 10.1016/0888-7543(88)90100-0. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Martin M. V., Beaune P. H., Kremers P., Wolff T., Waxman D. J. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986 Apr 15;261(11):5051–5060. [PubMed] [Google Scholar]
- Kato R., Kamataki T., Yamazoe Y. N-hydroxylation of carcinogenic and mutagenic aromatic amines. Environ Health Perspect. 1983 Mar;49:21–25. doi: 10.1289/ehp.834921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T., Nagata K., Holsztynska E. J., Lapenson D. P., Smith A., Kato R., Gelboin H. V., Waxman D. J., Gonzalez F. J. Gene conversion and differential regulation in the rat P-450 IIA gene subfamily. Purification, catalytic activity, cDNA and deduced amino acid sequence, and regulation of an adult male-specific hepatic testosterone 15 alpha-hydroxylase. J Biol Chem. 1988 Dec 5;263(34):17995–18002. [PubMed] [Google Scholar]
- Molowa D. T., Schuetz E. G., Wrighton S. A., Watkins P. B., Kremers P., Mendez-Picon G., Parker G. A., Guzelian P. S. Complete cDNA sequence of a cytochrome P-450 inducible by glucocorticoids in human liver. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5311–5315. doi: 10.1073/pnas.83.14.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Buppodom P., Matsunaga T., Ishimatsu M., Yamato H., Yoshihara S., Yoshimura H. Purification and characterization of seven distinct forms of liver microsomal cytochrome P-450 from untreated and inducer-treated male Wistar rats. J Biochem. 1985 Jun;97(6):1755–1766. doi: 10.1093/oxfordjournals.jbchem.a135234. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. The 1986 Bernard B. Brodie award lecture. The genetic regulation of drug-metabolizing enzymes. Drug Metab Dispos. 1988 Jan-Feb;16(1):1–8. [PubMed] [Google Scholar]
- Nhamburo P. T., Gonzalez F. J., McBride O. W., Gelboin H. V., Kimura S. Identification of a new P450 expressed in human lung: complete cDNA sequence, cDNA-directed expression, and chromosome mapping. Biochemistry. 1989 Oct 3;28(20):8060–8066. doi: 10.1021/bi00446a014. [DOI] [PubMed] [Google Scholar]
- Raineri R., Poiley J. A., Pienta R. J., Andrews A. W. Metabolic activation of carcinogens in the Salmonella mutagenicity assay by hamster and rat liver S-9 preparations. Environ Mutagen. 1981;3(1):71–84. doi: 10.1002/em.2860030108. [DOI] [PubMed] [Google Scholar]
- Ramsdell H. S., Eaton D. L. Species susceptibility to aflatoxin B1 carcinogenesis: comparative kinetics of microsomal biotransformation. Cancer Res. 1990 Feb 1;50(3):615–620. [PubMed] [Google Scholar]
- Relling M. V., Aoyama T., Gonzalez F. J., Meyer U. A. Tolbutamide and mephenytoin hydroxylation by human cytochrome P450s in the CYP2C subfamily. J Pharmacol Exp Ther. 1990 Jan;252(1):442–447. [PubMed] [Google Scholar]
- Shimada T., Guengerich F. P. Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A. 1989 Jan;86(2):462–465. doi: 10.1073/pnas.86.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Iwasaki M., Martin M. V., Guengerich F. P. Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. Cancer Res. 1989 Jun 15;49(12):3218–3228. [PubMed] [Google Scholar]
- Umeno M., McBride O. W., Yang C. S., Gelboin H. V., Gonzalez F. J. Human ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression. Biochemistry. 1988 Dec 13;27(25):9006–9013. doi: 10.1021/bi00425a019. [DOI] [PubMed] [Google Scholar]
- Yamano S., Aoyama T., McBride O. W., Hardwick J. P., Gelboin H. V., Gonzalez F. J. Human NADPH-P450 oxidoreductase: complementary DNA cloning, sequence and vaccinia virus-mediated expression and localization of the CYPOR gene to chromosome 7. Mol Pharmacol. 1989 Jul;36(1):83–88. [PubMed] [Google Scholar]
- Yamano S., Nhamburo P. T., Aoyama T., Meyer U. A., Inaba T., Kalow W., Gelboin H. V., McBride O. W., Gonzalez F. J. cDNA cloning and sequence and cDNA-directed expression of human P450 IIB1: identification of a normal and two variant cDNAs derived from the CYP2B locus on chromosome 19 and differential expression of the IIB mRNAs in human liver. Biochemistry. 1989 Sep 5;28(18):7340–7348. doi: 10.1021/bi00444a029. [DOI] [PubMed] [Google Scholar]
- Yamano S., Tatsuno J., Gonzalez F. J. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990 Feb 6;29(5):1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]



