Abstract
Chronic lymphocytic leukemia (CLL) is frequently associated with autoimmune complications such as autoimmune hemolytic anemia, immune thrombocytopenia, pure red cell aplasia and autoimmune granulocytopenia. It is critical to diagnose cytopenias from these secondary complications of CLL accurately, since prognosis and therapy are substantially different from patients who have cytopenias due to extensive bone marrow infiltration by CLL. The pathogenesis of autoimmune cytopenias in CLL is complex; and involves antigen presentation by CLL cells to polyclonal B-cells resulting in production of autoantibody and alteration of the T-cell milieu tilting the balance in favor of an autoimmune response. Traditional therapy of autoimmune complications in CLL consists of immunosuppression with corticosteroids and/or anti-CD20 monoclonal antibodies. In patients who have a suboptimal response, treating the underlying CLL is generally effective in ameliorating secondary cytopenias. Although novel oral therapies such as ibrutinib, idelalisib and venetoclax have been shown to be extremely effective in the management of CLL; prospective data from larger numbers of patients with longer follow-up are needed prior to recommending their routine use in the management of autoimmune cytopenias in CLL.
Keywords: Autoimmune cytopenias, small lymphocytic lymphoma, autoimmune hemolytic anemia, immune thrombocytopenia, pure red cell aplasia
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world1, with an estimated incidence of 4.5 cases per 100,000 individuals annually in the USA.2 Patients with CLL are at an increased risk of infections and non-hematologic malignancies, due to profound defects in their immune system. Although hypogammaglobulinemia is the most commonly reported immune defect in CLL,3 abnormalities of T-cells, NK-cells, monocytes and neutrophils all contribute to these secondary events.4–8 Paradoxically, there also appears to be a significant proportion of CLL patients who are at risk for autoimmune cytopenias during the course of their disease. In this review, we will focus on the epidemiology, pathogenesis, diagnosis, treatment, and outcome of patients with autoimmune cytopenias in CLL.
EPIDEMIOLOGY
Autoimmune cytopenias occur in approximately 4–10% patients with CLL, although the exact incidence may be difficult to determine because these can precede CLL diagnosis and present at any time of disease.9–11 Autoimmune hemolytic anemia (AIHA) is the most common type of autoimmune cytopenia and involves antibody-mediated red blood cell destruction. Immune thrombocytopenia (ITP) is the second most common and involves antibody-mediated platelet destruction.12 Pure red cell aplasia (PRCA) and autoimmune granulocytopenia (AIG) are relatively rare and occur in <1% patients.13 PRCA develops due to an immune-mediated destruction of red cell precursors and manifests as reticulocytopenia and anemia. AIG involves an immune-mediated destruction of myeloid precursors, and presents as isolated granulocytopenia with an otherwise unremarkable complete blood count.
In a study of 1750 CLL patients observed over 10 years at the Mayo Clinic, autoimmune cytopenias were diagnosed in 75 (4.3%) patients. AIHA occurred most commonly (2.3%), followed by ITP (2.0%), PRCA (<0.5%), and AIG (<0.5%).14 In another large retrospective study (n=960), autoimmune cytopenias were diagnosed in 7% of patients (27% were detected at time of CLL diagnosis, 4% preceding the diagnosis of CLL, and 68% during the course of disease). In these patients, AIHA occurred in 5% of patients, ITP in 2% of patients, and Evans syndrome (concomitant AIHA and ITP) in 0.1%.9 These data indicate that ~1 out of every 16 CLL patients will have autoimmune cytopenias during the course of their disease, and practicing clinicians need to be cognizant about recognizing and managing them appropriately.
PATHOPHYSIOLOGY OF AUTOIMMUNE CYTOPENIAS IN CLL
Autoimmune cytopenias associated with CLL are believed to occur by a variety of complex mechanisms, which have recently been well-reviewed.15,16 Secondary autoimmune cytopenias in the setting of CLL are primarily due to non-malignant B-cells producing polyclonal high-affinity immunoglobulin G (IgG) antibodies that are directed against antigens on red blood cells and platelets, that lead to hemolytic anemia and immune thrombocytopenia, respectively.17 These antibodies can also be directed against maturing erythroblasts and granulocyte precursors in the bone marrow, resulting in PRCA and AIG, respectively.18 CLL B-cells are known to produce IgM autoimmune antibodies which can lead to intravascular hemolysis in a small proportion of patients. Additionally, CLL cells produce several cytokines that can result in inhibition of normal erythropoiesis or megakaryopoiesis, leading to the development of AIHA and ITP.16 The T-cell compartment is significantly altered in CLL patients; while this can increase the risk of infections, it also contributes to the development of autoimmune complications. T regulatory cells (Tregs), which play a role in suppression of immune responses, are shown to be abnormally increased in CLL patients.19 A more recent study demonstrated that T-helper 17 cells (TH17 cells) are associated with an increased incidence of autoimmune cytopenias in CLL, and that an imbalance in the ratio between TH17 cells and Tregs leads to development of autoimmune cytopenias in CLL patients.20 It is hoped that with the advent of newer technologies such as mass cytometry,21 we will be better able to define the specific types of T-cell subsets that contribute to autoimmune cytopenias in CLL.
It is well known that the immunoglobulin heavy chain variable region (IGHV) mutational status influences clinical outcome in CLL patients. CLL patients whose IGHV genes are mutated (>2% deviation from the germline sequence) have a longer progression-free survival (PFS) and longer overall survival (OS) compared to patients who have unmutated IGHV genes.22 Patients who have autoimmune cytopenias are more likely to have unmutated IGHV genes compared to those who do not have autoimmune cytopenias14; it is possible that the B-cell receptor on the surface of CLL cells with unmutated IGHV is more prone to stimulation by erythrocyte antigens causing activation and auto-antibody production. Another mechanism of autoimmune complications in CLL is a result of abnormal expression of toll-like receptors (TLRs). Reduced TLR4 activity specifically was associated with an increased risk of autoimmune complications in CLL patients. However, reduced TLR4 expression was more evident in patients with advanced disease and unmutated IGHV, and the relative contribution of reduced TLR activity in the contribution of CLL remains unclear.23
Finally, CLL therapy can induce autoimmune cytopenias, particularly AIHA. Single-agent purine analog therapy (such as fludarabine) is known to induce hemolytic anemia in CLL patients.24,25 Patients who have received multiple prior therapies appear to be at a higher risk for this complication. The exact mechanism by which fludarabine induces hemolysis is unclear, but it likely secondary to an alteration in the peripheral blood TH17/Treg ratio in these patients.26 While single-agent fludarabine therapy is contraindicated in patients with hemolytic anemia, combination chemoimmunotherapy such as FCR (fludarabine, cyclophosphamide and rituximab) may not cause a similar degree of hemolysis. In the CLL8 trial27 that randomized previously untreated CLL patients to either FCR or FC (fludarabine and cyclophosphamide), there was no difference in the incidence of hemolytic anemia in the two arms (<1%). However, it is unclear what proportion of patients had a history of autoimmune hemolytic anemia prior to starting FCR or FC therapy on the CLL8 trial. In our practice, we avoid using single-agent fludarabine and combination chemoimmunotherapy in patients who have a history of severe hemolysis in the past. In patients with history of severe hemolysis in the past, and in whom chemoimmunotherapy is contemplated, therapy with bendamustine and rituximab (BR) is well tolerated and does not induce significant hemolysis. In a series of 64 refractory CLL patients28 who were treated with low-dose alemtuzumab (defined as a total weekly dose of <45 mg or cumulative dose of <600 mg), 6 (9%) patients developed treatment-emergent ITP. This was hypothesized to be due to alemtuzumab’s role in T-cell dysregulation, leading to increased autoimmunity. Bendamustine-based therapy is also associated with autoimmune cytopenias in CLL, particularly PRCA.29
AUTOIMMUNE HEMOLYTIC ANEMIA
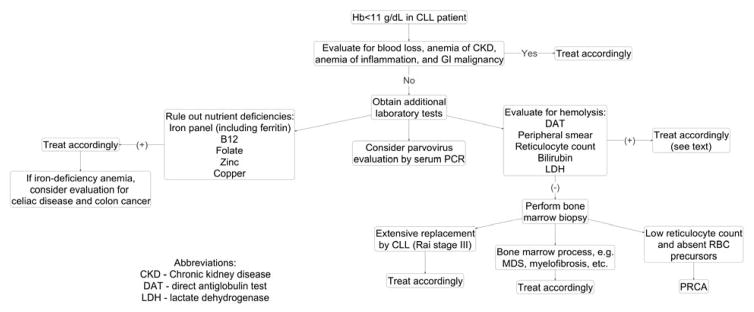
A suggested approach to a CLL patient with anemia is shown in Figure 1. Anemia in CLL may be due to various etiologies, such as bone marrow failure, bleeding, vitamin or iron deficiencies, anemia of renal disease, anemia of inflammation and autoimmune hemolytic anemia. A diagnosis of AIHA can be made if the following criteria are met:10,16,30,31
Figure 1.
Approach to anemia in patients with CLL
Hb <11 gm/dL, in the absence of any cytotoxic therapy in the preceding month or other identified etiology; AND
At least one laboratory sign of hemolysis: such as increased unconjugated bilirubin without liver disease, elevated lactate dehydrogenase (LDH) without alternative cause, reduced haptoglobin, increased absolute reticulocyte count in the absence of bleeding; AND
Direct or indirect evidence of an autoimmune process, such as positive direct antiglobulin test (DAT) for either IgG or C3d, or cold agglutinins
Unfortunately, the diagnosis of AIHA can be difficult to make in the setting of CLL because the laboratory values for hemolysis may be distorted in CLL due to disease progression or therapy. For example, LDH levels may be elevated because of disease progression; haptoglobin levels may be elevated as an acute phase response; reticulocytosis may not be present in patients who have decreased bone marrow production because of prior therapy or bone marrow infiltration.16 Although DAT can be an important diagnostic tool in the approach to AIHA, its positivity does not necessarily predict whether CLL patients will develop AIHA.32 In a study of 36 Binet Stage A CLL patients who had a positive DAT at baseline, ~1/3rd patients developed the clinical manifestations of AIHA during follow-up.32 To complicate matters in the interpretation of DAT, there are reports of DAT negative AIHA in CLL patients.16,33 These are thought to be associated with the presence of low-affinity autoantibodies or to a number of RBC-bound IgG molecules that is below the threshold of positivity for the test.16 In such cases, more sensitive testing may be required, such as using microcolumn and solid-phase tests, or other sophisticated techniques, such as mitogen-stimulated DAT. These tests are thought to detect up to 30 to 40 molecules of anti-RBC autoantibodies,16 thereby leading to identification of more cases of CLL associated AIHA (30%) compared to 4% when using standard DAT.34 Considering the many caveats of laboratory testing for anemia in CLL patients, and after exclusion of secondary causes of anemia, a bone marrow biopsy may become necessary to document the extent of CLL infiltration or to investigate other reasons for anemia (including myelodysplastic syndrome).
Patients presenting with acute anemia should initially be managed based on their hemoglobin level and symptoms; blood transfusions are required for symptomatic patients with severe anemia. For AIHA due to warm antibodies, first line therapy is prednisone at 1 mg/kg per day for 3–4 weeks, which may be slowly tapered off during the following 1–2 months. An alternative regimen is pulsed high dose dexamethasone (40 mg for 4 consecutive days), although this would need further investigation for use in secondary autoimmune cytopenias in CLL. For patients with secondary AIHA who present with significant hemolysis, intravenous immunoglobulin infusion (IVIg) at 1 gm/kg on days 1 and 2 may also be used.16,35 Patients who have secondary AIHA due to cold antibodies often have a milder presentation. The decision to treat them should be reserved for patients who have transfusion-dependent symptomatic anemia or symptoms secondary to cold agglutinin disease. Corticosteroids are generally not as effective in these patients, as opposed to those with AIHA due to warm antibodies. In these patients, rituximab is generally considered the first-line treatment option. At doses of 375 mg/m2 weekly for four weeks, rituximab has been shown to be effective and well-tolerated, with objective responses seen in approximately 70%–100% of patients.35,36,37 A similar approach can be used in steroid refractory warm antibody mediated AIHA in CLL. Use of lower doses of rituximab at 100 mg/m2 for 4 weekly infusions in conjunction with steroids has been studied only in primary AIHA, although a similar strategy could be used in CLL patients with AIHA.38 Newer generation monoclonal antibodies such as ofatumumab and obinutuzumab may also be used in secondary AIHA.39 Although alemtuzumab has been used in this circumstance,40 we do not recommend its use because of the potential to cause severe infectious complications. If there is no response to corticosteroids within 4–6 weeks, or if response is not prolonged, then alternative therapies such as mycophenolate mofetil, cyclosporine, cyclophosphamide and azathioprine could be considered.41
In many instances, treatment of the underlying CLL becomes essential in controlling the AIHA accompanying CLL (Table 1). There are several potential treatment options in this situation. Therapy with BR has been shown to be a safe and effective therapy in patients with AIHA secondary to CLL, refractory to other therapies, who also required control of their progressive CLL. In a study of 26 CLL patients treated with BR,42 the overall response rate was 81% for AIHA and 77% for CLL with a median time to next treatment of 28 months for AIHA and 26 months for CLL. In a study of 8 CLL patients who had steroid refractory AIHA,43 the combination of rituximab-cyclophosphamide-dexamethasone (RCD), was shown to have an overall response rate of 89% and a complete response rate of 83%. The median duration of response was 24 months and was dependent on the type of autoimmune cytopenia – it was shorter for patients with Evans syndrome and ITP.44 Bowen et al30 used a combination of rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) in 20 patients with CLL associated autoimmune cytopenias – 14 patients had complete remission (CR) and 5 patients had partial remission (PR) from their autoimmune cytopenias.30 Similarly, rituximab in combination with cyclophosphamide and prednisone (R-CP) has also been safe and effective in patients with CLL-associated AIHA refractory to steroids and IVIg.45 A suggested algorithm to management of AIHA in CLL is shown in Figure 2.
Table 1.
A Partial List of Studies Detailing Types of Treatment and Response Rates in CLL Patients with Autoimmune Cytopenias
| Reference | N | AIC subtypes | Management | Response (Complete response = CR, Partial response = PR)* |
|---|---|---|---|---|
| Alzaki (2014)62 | 30 | 16 – AIHA 8 – ITP 5 – Evans 1 – PRCA |
Alkylator based therapy vs. chemo-immunotherapy |
|
| Bowen (2010)30 | 20 | 17 – AIHA 8 – ITP 2 - PRCA |
R-CVP x 2–6 cycles (median 4.9 cycles) |
|
| Carli (2016)74 | 25 | 25 – Evans | Steroids +/− IVIg in 9/18 patients; chemotherapy +/− rituximab in 9/18 patients |
|
| Cortes (2001)75 | 31 | 16 – Anemia 29 – ITP |
Cyclosporin A at 300 mg/day |
|
| D’Arena (2006)37 | 14 | 14 – AIHA | Rituximab (375 mg/m2) x 4 cycles |
|
| Gomez-Almaguer (2009)76 | 11 | 5 – AIHA 6 – ITP |
Alemtuzumab, rituximab |
|
| Gourguechon (2014)77 | 11 | 11 – Evans | Steroids +/− IVIg – first line; splenectomy = 4; rituximab =4; azathioprine =3, hydroxychloroquine =1, vinkaalcaloids =1 |
|
| Gupta (2002)43 | 8 | 8 – steroid-refractory AIHA | RCD x 4 cycles |
|
| Kaufman (2009)48 | 21 | 18 – AIHA 1 – ITP 2 – Evans |
RCD x 1–9 cycles (median 2.5 cycles) |
|
| Rossignol (2011)60 | 48 | 26 – AIHA 9 – ITP 8 – Evans 5 – PRCA |
RCD x median 4 cycles |
|
| Michel (2009)78 | 68 | 68 – Evans | Steroids; 50 required second-line treatment (splenectomy =19, rituximab =11) |
|
| Sharma (2014)79 | 22 | 22 – AIHA | RCD in 20 patients or R-CVP in 2 patients x 4 median cycles |
|
Treatment response criteria for autoimmune cytopenia14
AIHA and PRCA:
CR = normal hemoglobin
PR = hemoglobin >10 g/dL
NR = hemoglobin < 10 g/dL or no improvement
ITP:
CR = normal platelet count
PR = platelet count ≥ 30 × 109/L
NR = platelet count < 30 × 109/L or no improvement
AIG:
CR = normal neutrophil count
PR = ANC ≥ 1 × 109/L
NR = ANC < 1 × 109/L or no improvement
Abbreviations of regimen:
RCD – Rituximab, cyclosphosphamide, dexamethasone
R-CVP – Rituximab, cyclophosphamide, vincristine, prednisone
Figure 2.
Approach to management of AIHA in CLL
IMMUNE THROMBOCYTOPENIA
ITP can be difficult to diagnose in CLL patients. It should be considered in patients with CLL who present with thrombocytopenia, especially if they are asymptomatic, do not have concomitant anemia, and if physical examination does not demonstrate significant splenomegaly. Prior to making a diagnosis of CLL-related ITP, it is critical to perform a bone marrow biopsy in order to exclude significant CLL in the marrow that can contribute to thrombocytopenia.
A diagnosis of secondary ITP associated to CLL can be made by the following criteria:10,16,30,31
Platelet count <100 × 109/L, that is otherwise unexplained, AND
No evidence of hypersplenism/palpable splenomegaly or bone marrow failure, AND
Normal or increased number of megakaryocytes on bone marrow examination, AND
No cytotoxic treatment in the last month, AND
Exclusion of other causes of thrombocytopenia, such as megakaryocyte dysplasia, infections, drug-induced thrombocytopenia, thrombotic thrombocytopenia purpura, heparin-induced thrombocytopenia, and disseminated intravascular coagulation.
The diagnostic work-up should include a peripheral blood smear and bone marrow evaluation in order to demonstrate that there is sufficient platelet production and in order to exclude any other etiologies. Antiplatelet antibody tests are typically not used in the diagnosis of CLL-related ITP because they are non-specific (positive test results are seen in both immune and non-immune thrombocytopenia).28
Once a diagnosis of ITP is established, treatment is indicated in patients who have platelet counts below 30 × 109/L or who have signs or symptoms of bleeding. First-line therapy with prednisone at 1 mg/kg per day for approximately 3–4 weeks, followed by a gradual taper over several weeks is the recommended treatment. Approximately half of patients will respond to steroids, which is lower than the response rate seen in patients with primary ITP. In patients who require a more rapid response because of significant bleeding or prior splenectomy, IVIg 1 gm/kg may be given on days 1 and 2. Approximately half of patients will respond to IVIg alone, compared to an approximately 80% response rate to IVIg in patients with primary ITP.16,35 Second-line therapies using monoclonal antibodies with or without the addition of chemotherapy may be considered in patients who do not respond to steroids or IVIg. In patients with fludarabine-associated ITP in CLL, for example, rituximab monotherapy (dosed at 375 mg/m2 per week for 4 weeks) was found to rapidly increase platelet counts.46,47 In addition, a course of rituximab combined with cytotoxic therapy (RCD and R-CVP) has a high rate of response lasting 20–24 months.44,48
Thrombopoietin mimetics (such as romiplostin and eltrombopag) have been shown to be useful compared to standard therapy in primary idiopathic ITP in improving platelet count.49,50 These agents have also shown durable responses in patients with refractory CLL-associated ITP, and may be considered in patients with minimal CLL disease burden and unremitting severe thrombocytopenia.51,52 Jain et al53 demonstrated in their enrolling phase II trial of eltrombopag that at varying doses, eltrombopag produced an overall response in 55% of all patients with thrombocytopenia, either due to CLL-associated ITP or CLL marrow infiltration. Overall, both groups of patients had a 45% complete response (CR) or 9% major response (MR). The response rate was 80% in the CLL-associated ITP group, with 70% CR and 10% MR of only patients with CLL-associated ITP.53 Splenectomy can also be an effective treatment option for patients who have relapsed/refractory ITP with CLL.
PURE RED CELL APLASIA
PRCA should be considered in patients with CLL who present with anemia and reticulocytopenia, albeit it is a rare diagnosis based on the limited information about the prevalence of PRCA in CLL.14 It is important to rule out other causes of anemia, such as hemolysis, and to evaluate for any vitamin and/or folic acid deficiency.16
PRCA is characterized by the following criteria:10,16,30
Normocytic and normochromic anemia, with Hb <11 gm/dL, that is otherwise unexplained, AND
Absolute reticulocytopenia, AND
Bone marrow with markedly reduced erythroid precursors with relatively intact leukocyte and megakaryocyte production
All patients suspected to have PRCA due to CLL should undergo a bone marrow examination to demonstrate absent erythroid precursors with intact granulocytic and megakaryocytic lineages.10,31 PRCA can also be associated with viral infections, such as EBV, CMV,54 and human parvovirus B19.55 In a large case series of PRCA in CLL (n=30), parvovirus testing was performed in 24 patients and was noted to be positive in 6 (25%) patients. Of those 6 patients, parvovirus B19 infection was detected by polymerase chain reaction (PCR) testing in 5 patients, and serologic testing in 1 patient.29
The goal of treatment in secondary PRCA is to recover erythropoiesis and avoid repeated packed red cell transfusions. PRCA secondary to CLL is typically poorly responsive to corticosteroids, although there are isolated case reports of patients with PRCA responding to oral prednisone at 1 mg/kg alone.56 In patients who have secondary PRCA associated with parvovirus B19, IVIg is typically considered the treatment of choice at a dose of 2 gm/kg usually divided over 5 days (400 mg/kg/day);57 it is thought to neutralize antibodies against the virus.58 In non-parvovirus associated PRCA, cyclosporine is most commonly used as either first line therapy or after corticosteroids, with a response rate greater than 75%.57 Prolonged therapy with cyclosporine (>12 months) may be required to maintain remission from PRCA in some instances. Other treatment options using either rituximab alone59 or in combination with prednisone29 have also been explored for treatment of PRCA in CLL. Combination therapy with rituximab, cyclophosphamide, and dexamethasone (RCD) had an excellent response in five patients with PRCA, with complete remission in four of five patients; the fifth patient was in second complete remission after a second RCD course.44,60
AUTOIMMUNE GRANULOCYTOPENIA
Secondary autoimmune granulocytopenia in CLL occurs very rarely and can be considered as a diagnosis of exclusion because there are no clear diagnostic criteria. It should be suspected in patients who have isolated neutropenia. It is important to exclude all other causes of granulocytopenia prior to diagnosis, such as evaluating for any cause of secondary immune neutropenia, nutritional deficiencies and long-term toxicity from prior therapy.16 The latter is particularly important to exclude; after a median follow-up of 6 years, ~4% of patients treated on the FCR arm of the CLL8 trial had persistent neutropenia more than 12 months after completing therapy.27 It is also important to exclude secondary myelodysplasia based on a careful review of the bone marrow biopsy.
Treatment options are not clearly delineated for autoimmune granulocytopenia because of its rarity. Although some cases of autoimmune granulocytopenia can resolve spontaneously without treatment, most patients require therapy with G-CSF or other immunosuppressive medications including corticosteroids and anti-CD20 monoclonal antibodies.14 Splenectomy may also be considered in refractory patients with limited success.16
THE IMPACT OF AUTOIMMUNE CYTOPENIAS ON OUTCOME IN CLL
Regardless of the cause of cytopenia, the 2 widely accepted Rai and Binet staging systems traditionally placed CLL patients with anemia or thrombocytopenia at an advanced stage of disease with a poorer prognosis.11 However, the management of CLL is not identical in patients with autoimmune cytopenias versus cytopenias secondary to bone marrow infiltration. The 1996 NCI-WG and the 2008 IWCLL guidelines acknowledge this issue and suggest treating autoimmune cytopenias in CLL as outlined in the aforementioned sections, and reserving CLL-specific therapy only in those patients who do not respond.61 Despite this clarification, however, the prognostic significance of autoimmune cytopenias secondary to CLL remains unclear.
Several studies demonstrate that autoimmune cytopenias in CLL can be associated with a more aggressive phenotype and worse OS. In a population-based study of 754 CLL patients in British Columbia, Canada, Alzaki et al62 identified 80 patients with cytopenias (50 due to extensive bone marrow infiltration and 30 due to autoimmune cytopenias). Although the time to first CLL therapy was similar between CLL patients who had autoimmune cytopenias and CLL patients without cytopenias (8.2 years vs. 8.1 years, p=not significant), median OS was significantly worse for patients with autoimmune cytopenias (8.2 years vs. not reached, p<0.005).62 Moreno et al9 presented similar results among 960 unselected CLL patients, of whom 70 (6%) were diagnosed with autoimmune cytopenias during the course of their disease. CLL patients with cytopenias due to bone marrow infiltration had a significantly worse OS compared to patients with cytopenias due to autoimmune complications (3.7 years vs. 7.4 years, p=.02). Several other studies have reported shorter OS among CLL patients with autoimmune cytopenias compared to patients with cytopenias due to bone marrow infiltration by CLL.63,64
In contrast, isolated studies demonstrate that autoimmune cytopenias do not necessarily predict a worse prognosis in patients with CLL. In an observational study of 132 patients by Kyasa et al,31 the survival of CLL patients with autoimmune cytopenias was not statistically different from CLL without autoimmune cytopenias. In a study by Zent et al,10 patients with autoimmune cytopenias (n=75) had a median survival that was not significantly different from patients who did not develop any CLL-related cytopenia, whether from autoimmune cytopenias or bone marrow failure. When compared to patients who presented with cytopenias from bone marrow failure, patients with autoimmune cytopenias had an improved median survival time from onset of cytopenia (median 9.1 years vs. 4.4 years, p < 0.001).10
Patients with autoimmune cytopenias are more likely to have advanced Rai stage, older age, high white cell count, short lymphocyte doubling time, unmutated IGVH, and poor risk cytogenetics.15 Given the contrasting results reported in the literature and the lack of systematic long-term follow-up in many of these studies, it remains unclear if the worse outcome seen in CLL related autoimmune cytopenias is due to its association with these high-risk biological markers or if it is an independent predictor of shorter OS.
THERAPY OF AUTOIMMUNE CYTOPENIAS IN THE ERA OF NOVEL AGENTS
The treatment landscape of patients with previously untreated and relapsed/refractory CLL has changed considerably. Oral tyrosine kinase inhibitors, such as ibrutinib (that targets Bruton’s tyrosine kinase) and idelalisib (that targets phosphoinositide 3-kinase), are approved for the treatment of CLL patients. Venetoclax, a novel BCL-2 antagonist, was recently approved for the treatment of relapsed CLL with del17p. In general, these agents are extremely efficacious, particularly in the high-risk group of patients (such as those with unmutated IGHV and adverse risk cytogenetics such as del17p and del11q).65–67
No clinical trials have directly studied the role of these novel signal inhibitors in the management of autoimmune cytopenias in CLL. The largest study to date of autoimmune cytopenia in CLL patients treated with ibrutinib is Vitale et al’s68 recent study of 13 CLL patients who had signs of autoimmune cytopenia at time of iburtinib treatment initiation for CLL progression. All patients had a history of autoimmune cytopenia, for which 62% of patients received prior therapy. At the time of ibrutinib initiation, 54% were well controlled without treatment, 23% had control with therapy, and 23% had active autoimmune cytopenia. The response to ibrutinib was highly variable: in all 13 patients, 46% had resolution of their autoimmune cytopenia; 23% had stabilized peripheral blood counts with persistent mild cytopenia; 15% developed transfusion-dependent PRCA that required use of convention therapy to treat the cytopenia; the remaining 15% had no autoimmune cytopenia and continued on ibrutinib. Therefore, most patients were successfully treated with use of ibrutinib, with some requiring the temporary addition of conventional therapy to treat the autoimmune process.68 In another large retrospective analysis of 301 CLL patients treated with ibrutinib at Ohio State University Cancer Center,69 6 patients developed treatment-emergent autoimmune cytopenias. These episodes of cytopenia did not appear to be temporally related with ibrutinib initiation, occurring within 0.2 – 23 months after initiation of ibrutinib. Ibrutinib treatment was suspended in four of the six patients, and all patients received appropriate therapy with either prednisone, IVIg, or both. Approximately 25% of patients treated with ibrutinib in this analysis had a past history of autoimmune cytopenias, and 22 (7%) patients were receiving active therapy for their autoimmune cytopenia at the time of ibrutinib initiation. The majority (86%) of patients were successfully able to discontinue concomitant therapy for autoimmune cytopenias after a median of 4.7 months on ibrutinib.69 In an ad hoc analysis of the phase 3 RESONATE study, which compared ibrutinib vs ofatumumab in previously treated CLL/SLL (n=386), ibrutinib therapy did not precipitate recurrent autoimmune cytopenias in patients with CLL who had a history of autoimmune complications.70 It is thought that ibrutinib inhibits interleukin-2–inducible kinase (ITK), which could decrease the response of autoreactive T cells, and hence may benefit autoimmune cytopenias in CLL. These studies demonstrate that ibrutinib may be used successfully in CLL patients who have a history of autoimmune cytopenias.
There is limited data on the use of idelalisib in the management of autoimmune cytopenias in CLL. There is a high incidence of autoimmune complications—including autoimmune hepatitis, colitis and pneumonitis—in idelalisib treated patients,71,72 and therefore until more data become available, it should be avoided in CLL patients who have autoimmune cytopenias. There is no data on the use of venetoclax in patients with autoimmune complications in CLL, although it would not be expected to worsen autoimmune cytopenias.
CONCLUSION
Our understanding of the mechanisms by which autoimmune cytopenias in CLL occur has evolved considerably since this association was first described by Wasserman et al73 more than 60 years ago. Traditional therapy for autoimmune complications in CLL consists of immunosuppression with corticosteroids, and if unsuccessful, treating the underlying CLL. Although useful, chemoimmunotherapy causes profound immunosuppression, which frequently leads to an increased risk of infections and second malignancies, such as myelodysplastic syndrome and Richter’s transformation. With the advent of novel agents such as ibrutinib, acalabrutinib, and venetoclax in the armamentarium against CLL, it is hoped that modulation of the immune system will allow for a more targeted approach to managing autoimmune complications in CLL.
Acknowledgments
Sameer A. Parikh is the recipient of the K12 National Cancer Institute grant K12 CA090628.
Footnotes
Conflicts of Interest
Sameer A. Parikh has received research funding from Pharmacyclics (no personal compensation), and has participated in advisory board meetings for Pharmacyclics (no personal compensation).
References
- 1.Hamblin T. Just how exactly common is CLL? Leukemia Research. 2009;33:1452–3. doi: 10.1016/j.leukres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; [Accessed October, 2015]. at http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 3.Parikh S, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer. 2015;121:2883–91. doi: 10.1002/cncr.29438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiorazzi N, Fu SM, Montazeri G, et al. T cell helper defect in patients with chronic lymphocytic leukemia. J Immunol. 1979;122:1087–90. [PubMed] [Google Scholar]
- 5.Kay N. Abnormal T-cell subpopulation function in CLL: excessive suppressor (T gamma) and deficient helper (T mu) activity with respect to B-cell proliferation. Blood. 1981;57:418–20. [PubMed] [Google Scholar]
- 6.Platsoucas C, Galinski M, Kempin S, et al. Abnormal T lymphocyte subpopulations in patients with B cell chronic lymphocytic leukemia: an analysis by monoclonal antibodies. J Immunol. 1982;129:2301–12. [PubMed] [Google Scholar]
- 7.Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10:1509–13. [PubMed] [Google Scholar]
- 8.Kontoyiannis D, Georgiadou SP, Wierda WG, et al. Impaired bactericidal but not fungicidal activity of polymorphonuclear neutrophils in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1730–3. doi: 10.3109/10428194.2012.750723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno C, Hodgson K, Ferrer G, et al. Autoimmune cytopenia in chronic lymphocytic leukemia: prevalence, clinical associations, and prognostic significance. Blood. 2010;116:4771–6. doi: 10.1182/blood-2010-05-286500. [DOI] [PubMed] [Google Scholar]
- 10.Zent C, Wei D, Schwager SM, et al. The prognostic significance of cytopenia in chronic lymphocytic leukemia/small lymphocytic leukemia (CLL) Br J Haematol. 2008;141:615–21. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson B, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group Guidelines for Chronic Lymphocytic Leukemia: Revised Guidelines for Diagnosis and Treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 12.Strati P, Caligaris-Cappio F. A matter of debate in chronic lymphocytic leukemia: is the occurrence of autoimmune disorders an indicator of chronic lymphocytic leukemia therapy? Current Opinion in Oncology. 2011;23:455–60. doi: 10.1097/CCO.0b013e328348c683. [DOI] [PubMed] [Google Scholar]
- 13.Zent C, Kay NE. Autoimmune complications in Chronic Lymphocytic Leukemia (CLL) Best Practice & Research Clinical Haematology. 2010;23:47–59. doi: 10.1016/j.beha.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zent C, Wei D, Reinalda MS, et al. Autoimmune cytopenia in Chronic Lymphocytic Leukemia/Small Lymphocytic Leukemia (CLL): changes in clinical presentation and prognosis. Leukemia Lymphoma. 2009;50:1261–8. doi: 10.1080/10428190903026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgson KFG, Montserrat E, Moreno C. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96:752–61. doi: 10.3324/haematol.2010.036152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visco C, Barcellini W, Maura F, et al. Autoimmune cytopenias in chronic lymphocytic leukemia. American Journal of Hematology. 2014;89:1055–62. doi: 10.1002/ajh.23785. [DOI] [PubMed] [Google Scholar]
- 17.Hall A, Vickers MA, McLeod E, Barker RN. Rh autoantigen presentation to helper T cells in chronic lymphocytic leukemia by malignant B cells. Blood. 2005;105:2007–15. doi: 10.1182/blood-2003-10-3563. [DOI] [PubMed] [Google Scholar]
- 18.Hamblin T, Oscier DG, Young BJ. Autoimmunity in chronic lymphocytic leukaemia. J Clin Pathol. 1986;39:713–6. doi: 10.1136/jcp.39.7.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riches J, Ramsay AG, Gribben JG. T-cell function in chronic lymphocytic leukemia. Seminars in Cancer Biology. 2010;20:431–8. doi: 10.1016/j.semcancer.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Lad D, Varma S, Varma N, et al. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leukemia Lymphoma. 2015;56:2424–8. doi: 10.3109/10428194.2014.986479. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer M, Gherardini PF, Fragiadakis GK, et al. An interactive reference framework for modeling a dynamic immune system. Science. 2015:349. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh S, Strati P, Tsang M, et al. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood. 2016;127:1752–60. doi: 10.1182/blood-2015-10-620864. [DOI] [PubMed] [Google Scholar]
- 23.Barcellini W, Imperiali FG, Zaninoni A, et al. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: relationship with infections, autoimmunity and disease progression. Leukemia Lymphoma. 2014;55:1768–73. doi: 10.3109/10428194.2013.856426. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R, Freiman J, Kweder SL, et al. Haemolytic anaemia after fludarabine therapy for chronic lymphocytic leukemia. J Clin Oncol. 1998;16:1885–9. doi: 10.1200/JCO.1998.16.5.1885. [DOI] [PubMed] [Google Scholar]
- 25.Gonazalez H, Leblond V, Azar N, et al. Severe autoimmune haemolytic anaemia in eight patients treated with fludarabine. Hematol Cell Ther. 1998;40:113–8. [PubMed] [Google Scholar]
- 26.Molica S, Polliack A. Autoimmune hemolytic anemia (AIHA) associated with chronic lymphocytic leukemia in the current era of targeted therapy. Leuk Res. 2016;50:31–6. doi: 10.1016/j.leukres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–15. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 28.Reda G, Maura F, Gritti G, et al. Low-dose alemtuzumab-associated immune thrombocytopenia in chronic lymphocytic leukemia. American Journal of Hematology. 2012;87:936–7. doi: 10.1002/ajh.23268. [DOI] [PubMed] [Google Scholar]
- 29.Tsang M, Chaffee KR, Call TG, et al. Pure Red Cell Aplasia (PRCA) in Chronic Lymphocytic Leukemia (CLL): Etiology, Therapy, and Outcomes. Blood. 2015;126:4169. [Google Scholar]
- 30.Bowen DACT, Shanafelt TD, et al. Treatment of autoimmune cytopenia complicating progressive chronic lymphocytic leukemia/small lymphocytic lymphoma with rituximab, cyclophosphamide, vincristine, and prednisone. Leukemia Lymphoma. 2010;51:620–7. doi: 10.3109/10428191003682767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyasa M, Parrish RS, Schichman SA, Zent CS. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. American Journal of Hematology. 2003;74:1–8. doi: 10.1002/ajh.10369. [DOI] [PubMed] [Google Scholar]
- 32.Quinquenel A, Al Nawakil C, Baran-Marszak F, et al. Old DAT and new data: Positive direct antiglobulin test identifies a subgroup with poor outcome among chronic lymphocytic leukemia stage A patients. American Journal of Hematology. 2015;90:E5–E8. doi: 10.1002/ajh.23861. [DOI] [PubMed] [Google Scholar]
- 33.Mauro F, Foa R, Cerretti R, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–92. [PubMed] [Google Scholar]
- 34.Barcellini W, Montesano R, Clerici G, et al. In vitro production of anti-RBC antibodies and cytokines in chronic lymphocytic leukemia. American Journal of Hematology. 2002;71:177–83. doi: 10.1002/ajh.10210. [DOI] [PubMed] [Google Scholar]
- 35.Rogers K, Woyach JA. Secondary autoimmune cytopenias in chronic lymphocytic leukemia. Seminars in Oncology. 2016;43:300–10. doi: 10.1053/j.seminoncol.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Pamuk G, Turgut B, Demir M, et al. The successful treatment of refractory autoimmune hemolytic anemia with rituximab in a patient with chronic lymphocytic leukemia. American Journal of Hematology. 2006;81:631–3. doi: 10.1002/ajh.20671. [DOI] [PubMed] [Google Scholar]
- 37.D’Arena G, Laurenti L, Capalbo S, et al. Rituximab therapy for chronic lymphocytic leukemia-associated autoimmune hemolytic anemia. American Journal of Hematology. 2006;81:598–602. doi: 10.1002/ajh.20665. [DOI] [PubMed] [Google Scholar]
- 38.Barcellini W, Zaja F, Zaninoni A, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119:3691–7. doi: 10.1182/blood-2011-06-363556. [DOI] [PubMed] [Google Scholar]
- 39.Church A, VanDerMeid KR, Baig NA, et al. Anti-CD20 monoclonal antibody-dependent phagocytosis of chronic lymphocytic leukaemia cells by autologous macrophages. Clin Exp Immunol. 2016;183:90–101. doi: 10.1111/cei.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson C, Hansson L, Celsing F, Lundin J. Treatment of severe refractory autoimmune hemolytic anemia in B-cell chronic lymphocytic leukemia with alemtuzumab (humanized CD52 monoclonal antibody) Leukemia. 2007;21:511–4. doi: 10.1038/sj.leu.2404512. [DOI] [PubMed] [Google Scholar]
- 41.Borthakur G, O’Brien S, Wierda WG, et al. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludrabine, cyclophosphamide and rituximab--incidence and predictors. Br J Haematol. 2007;136:800–5. doi: 10.1111/j.1365-2141.2007.06513.x. [DOI] [PubMed] [Google Scholar]
- 42.Quinquenel A, Willekens C, Dupuis J, et al. Bendamustine and rituximab combination in the management of chronic lymphocytic leukemia-associated autoimmune hemolytic anemia: a multicentric retrospective study of the French CLL intergroup (GCFLLC/MW and GOELAMS) American Journal of Hematology. 2015;90:204–7. doi: 10.1002/ajh.23909. [DOI] [PubMed] [Google Scholar]
- 43.Gupta N, Kavuru S, Patel D, et al. Rituximab-based chemotheray for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia. 2002;16:2092–5. doi: 10.1038/sj.leu.2402676. [DOI] [PubMed] [Google Scholar]
- 44.Michallet A, Rossignol J, Cazin B, et al. Rituximab–cyclophosphamide–dexamethasone combination in management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia Lymphoma. 2011;52:1401–3. doi: 10.3109/10428194.2011.591005. [DOI] [PubMed] [Google Scholar]
- 45.Gentile M, Lucia E, Iorio C, et al. Prompt and sustained response of a steroid-refractory autoimmune hemolytic anemia to a rituximab-based therapy in a chronic lymphocytic leukemia patient. Cancer Chemother Pharmacol. 2008;62:741–3. doi: 10.1007/s00280-007-0651-0. [DOI] [PubMed] [Google Scholar]
- 46.Hegde UPWW, White T, et al. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002;100:2260–2. [PubMed] [Google Scholar]
- 47.Fernandez M, Llopis I, Pastor E, et al. Immune thrombocytoepnia induced by fludarabine successfully treated with rituximab. Haematologica. 2003;88:ELT02. [PubMed] [Google Scholar]
- 48.Kaufman MLS, Driscoll N, et al. A combination of rituximab, cyclophosphamide, and dexamethasone effectively treats immune cytopenias of chronic lymphocytic leukemia. Leukemia Lymphoma. 2009;50:892–9. doi: 10.1080/10428190902887563. [DOI] [PubMed] [Google Scholar]
- 49.Bussel J, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–8. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 50.Kuter D, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–99. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 51.Jolliffe E, Romeril K. Eltrombopag for resistant immune thrombocytopenia secondary to secondary lymphocytic leukemia. Intern Med J. 2014;44:697–9. doi: 10.1111/imj.12468. [DOI] [PubMed] [Google Scholar]
- 52.Koehrer S, Keating MJ, Wierda WG. Eltrombopag, a second-generation thrombopoietin receptor agonist, for chronic lymphocytic leukemia-associated ITP. Leukemia. 2010;24:1096–8. doi: 10.1038/leu.2010.45. [DOI] [PubMed] [Google Scholar]
- 53.Jain N, Keating MJ, Burger JA, et al. A Phase II Trial Of Eltrombopag For Patients With Chronic Lymphocytic Leukemia (CLL) and Thrombocytopenia. Blood. 2013;122:4186. doi: 10.1111/bjh.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Fang JP, Weng WJ, et al. Pure red cell aplasia associated with cytomegalovirus and Epstein-Barr virus infection in seven cases of Chinese children. Hematology. 2013;18:56–9. doi: 10.1179/1607845412Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 55.Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clinical Infectious Disease. 2013;56:968–77. doi: 10.1093/cid/cis1046. [DOI] [PubMed] [Google Scholar]
- 56.Dharmshaktu P, Gupta N, Dhanwal DK. Successful treatment of acquired pure red cell aplasia with oral corticosteroids in a patient with B-cell CLL. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Means RJ. Pure red cell aplasia. Blood. 2016;128:2504–9. doi: 10.1182/blood-2016-05-717140. [DOI] [PubMed] [Google Scholar]
- 58.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142:505–14. doi: 10.1111/j.1365-2141.2008.07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narra K, Borghaei H, Al-Saleem T, et al. Pure red cell aplasia in B-cell lymphoproliferative disorder treated with rituximab: report of two cases and review of the literature. Leuk Res. 2006;30:109–14. doi: 10.1016/j.leukres.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Rossignol J, Michallet AS, Oberic L, et al. Rituximab-cyclophosphamide-dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia. 2011;25:473–8. doi: 10.1038/leu.2010.278. [DOI] [PubMed] [Google Scholar]
- 61.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alzaki AA, Gerrie AS, Gillan TL, et al. Autoimmune cytopenia in chronic lymphocytic leukemia: Effect on outcome and survival, a population based analysis in British Columbia, Canada. Blood Conference: 56th Annual Meeting of the American Society of Hematology; ASH; 2014. p. 124. [Google Scholar]
- 63.Dearden C, Wade R, Else M, et al. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: a beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood. 2008;111:1820–6. doi: 10.1182/blood-2007-07-101303. [DOI] [PubMed] [Google Scholar]
- 64.Visco C, Cortelezzi A, Moretta F, et al. Autoimmune cytopenias in chronic lymphocytic leukemia at disease presentation in the modern treatment era: is stage C always stage C? Leukemia Lymphoma. 2013;55:1261–5. doi: 10.3109/10428194.2013.834054. [DOI] [PubMed] [Google Scholar]
- 65.Byrd J, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burger J, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373:2425–37. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furman R, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370:2352–4. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitale C, Ahn IE, Sivina M, et al. Autoimmune cytopenias in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2016;102:e254–8. doi: 10.3324/haematol.2015.138289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers K, Ruppert AS, Bingman A, et al. Incidence and Description of Autoimmune Cytopenias During Treatment with Ibrutinib for Chronic Lymphocytic Leukemia Autoimmune Cytopenias During Ibrutinib Treatment. Leukemia. 2016;30:346–50. doi: 10.1038/leu.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montillo M, O’Brien S, Tedeschi A, et al. Ibrutinib in patients with autoimmune cytopenias and previously treated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) from the phase 3 RESONATETM study. Leukemia Lymphoma. 2016;56:150–1. [Google Scholar]
- 71.Lampson B, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furman R, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wasserman L, Stats D, Schwartz L, Fudenberg H. Symptomatic and hemopathic hemolytic anemia. Am J Med. 1955;18:961–89. doi: 10.1016/0002-9343(55)90176-5. [DOI] [PubMed] [Google Scholar]
- 74.Carli G, Visco C, Falisi E, et al. Evans syndrome secondary to chronic lymphocytic leukaemia: presentation, treatment, and outcome. Annals of Hematology. 2016;95:863–70. doi: 10.1007/s00277-016-2642-x. [DOI] [PubMed] [Google Scholar]
- 75.Cortes J, O’Brien S, Loscertales J, et al. Cyclosporin A for the treatment of cytopenia associated with chronic lymphocytic leukemia. Cancer. 2001;92:2016–22. doi: 10.1002/1097-0142(20011015)92:8<2016::aid-cncr1539>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 76.Gomez-Almaguer D, Solano-Genesta M, Cantu-Rodriguez O, et al. Alemtuzumab and rituximab in the treatment of refractory autoimmune cytopenias. Blood Conference: 51st Annual Meeting of the American Society of Hematology; ASH New Orleans, LA United States. 2009. p. 114. [Google Scholar]
- 77.Gourguechon C, Royer B, Marolleau JP. Evans syndrome management: A monocentric retrospective study of 11 cases. Haematologica. 2014;99:766–7. [Google Scholar]
- 78.Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: New insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167–72. doi: 10.1182/blood-2009-04-215368. [DOI] [PubMed] [Google Scholar]
- 79.Sharma A, Geirnaert M, Banerji V, et al. A population based experience on the use of RCD or RCVP in autoimmune hemolytic anemia in CLL. Haematologica. 2014;99:320. [Google Scholar]