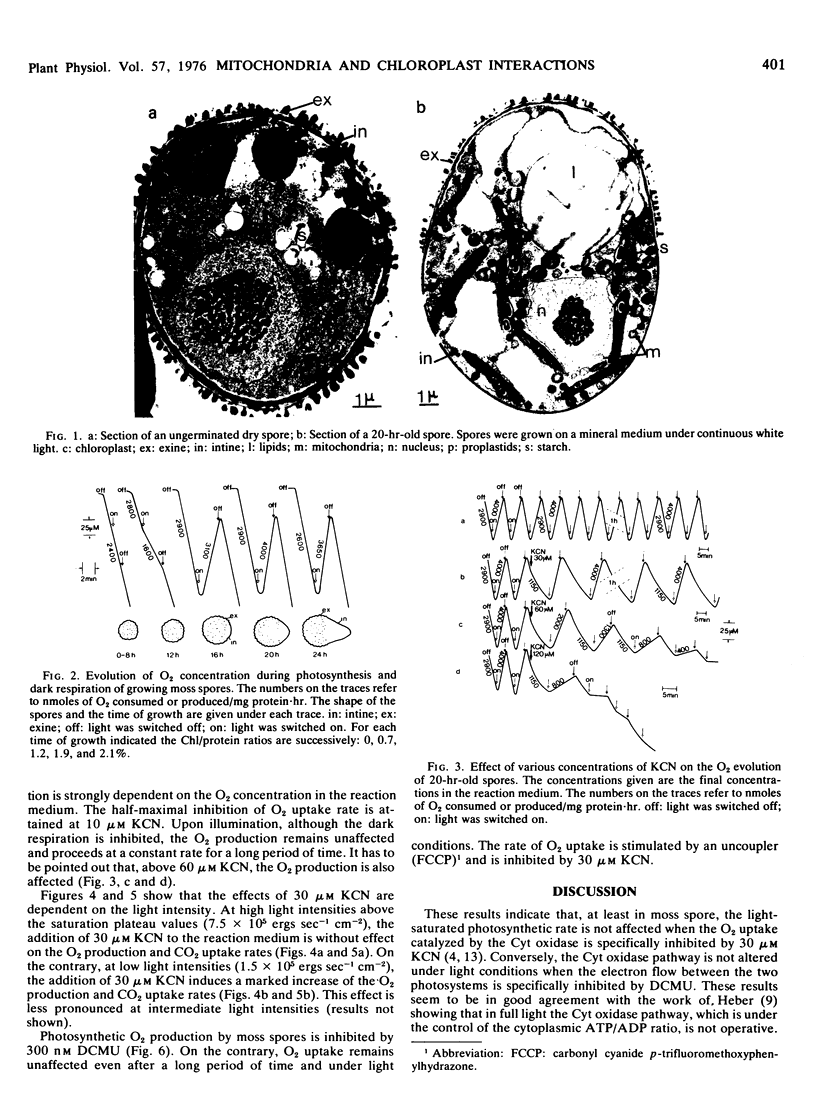
Abstract
The effects of cyanide and 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) on photosynthesis and respiration of intact chlorophyllic moss (Funaria hygrometrica) spore was investigated. Thirty micromolar cyanide strongly inhibited dark respiration, was without effect on photosynthesis at high light intensities (above the saturation plateau values), and stimulated photosynthesis at low light intensities (below the saturation plateau values). Three hundred nanomolar DCMU inhibited the photosynthesis and was without effect, even under light conditions, on the dark respiration. It seems likely, therefore, that in the chlorophyllic moss spore the cytochrome oxidase pathway is not functioning under high light intensities unless the photosynthesis is inhibited by DCMU.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM D., WALKER D. A. Some effects of light on the interconversion of metabolites in green leaves. Biochem J. 1962 Mar;82:554–560. doi: 10.1042/bj0820554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Passam H. C., Souverijn J. H., Kemp A., Jr Adenine nucleotide translocation in Jerusalem-artichoke mitochondria. Biochim Biophys Acta. 1973 Apr 27;305(1):88–94. doi: 10.1016/0005-2728(73)90234-x. [DOI] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]




