Abstract
Background
Approximately 35% of pregnant substance users in treatment report alcohol abuse, which increases the risk of fetal alcohol spectrum disorders (FASD) in their offspring. The present study was a preliminary evaluation of the efficacy of motivational enhancement therapy (MET) in decreasing alcohol use in pregnant women attending substance use treatment.
Methods
Secondary analysis of a trial evaluating the efficacy of MET, relative to treatment as usual (TAU), in improving treatment outcomes in 200 pregnant substance users. The present study included the 41 women (n=27 MET and n=14 TAU) who reported alcohol use in the 28 days prior to randomization. Alcohol and illicit-drug use days were assessed with self-report; illicit drug use was assessed with urine drug screens. All measures were obtained weekly for the 4 week active study phase and at 1 and 3 month follow-ups.
Results
Significant treatment-by-time interaction effects were found for illicit-drug use days during the active (X2 = 6.89, df = 1, p < .01) and follow-up (X2 = 8.26, df = 1, p < .01) phases and for alcohol use during the follow-up phase (X2 = 13.07, df = 1, p < .001), all reflecting a beneficial effect for MET, relative to TAU. All other treatment effects were non-significant.
Conclusions
These findings suggest that MET may be effective in decreasing alcohol and illicit-drug use in pregnant substance users reporting alcohol use. With 2–5% of US births affected by FASD, future research to replicate these findings seems warranted.
Keywords: pregnant, fetal alcohol spectrum disorders, alcohol use, drug use, motivational enhancement therapy
1.0 Introduction
Fetal alcohol spectrum disorders (FASD) encompass a multitude of neurological, behavioral, developmental, and physical abnormalities caused by alcohol exposure during pregnancy (Floyd et al., 2005; Hoyme et al., 2005; Manning & Hoyme, 2007; Stratton et al., 1996). The most severe FASD is fetal alcohol syndrome (FAS), with its diagnosis based on distinctive facial anomalies, growth retardation, and neurological defects. FAS occurs in approximately 0.6 – 0.9 percent of children (May et al., 2014). There has been a recent increase in identified cases of FASD from 1% (May & Gossage, 2001) to 2–5 % in the US (May et al., 2009; May et al., 2014). Many times the effects of in utero alcohol exposure are irreversible, progressive, and life-long (Sood et al., 2001; Streissguth, 2007; Streissguth et al., 2004). A systematic review found a dearth of studies examining the economic impact of FAS/FASD (Popova, Stade, Bekmuradov, Lange, & Rehm, 2011), but one of the more recent studies (Lupton et al., 2004) estimated a lifetime cost of $2 million per infant affected with FAS. With overall cases of FASD occurring up to five times as often as FAS alone (May et al., 2014), this cost is a gross underestimate for all children affected by prenatal alcohol exposure. The US Surgeon General (Centers for Disease Control and Prevention: CDC, n.d.), has stated that no amount of alcohol is safe during pregnancy, nor is there a safe time to drink while pregnant. Therefore, FASD can be prevented by women not drinking alcohol during pregnancy.
Yet despite the increased identification of FASD and the US Surgeon General’s warning (CDC, n.d.), pregnant women continue to drink at levels risky to the developing fetus. Recent Behavioral Risk Factor Surveillance System (BRFSS) prevalence rates of alcohol use in the past 30 days revealed that 10.2% of pregnant women reported any alcohol use, with 3.1% reporting binge drinking (4 or more standard drinks per occasion) (Tan et al., 2015). These frequencies could be underreported due to the stigma associated with prenatal alcohol use. Also women may not have realized they were pregnant at the time of the survey. Reported rates of prenatal alcohol use are significantly higher in pregnant women seeking treatment for substance use, with 34.8% of pregnant admissions reporting alcohol abuse with or without drug abuse (27.8 % and 7.0%, respectively) (Substance Abuse and Mental Health Services Administration, 2013). With pregnant substance users consuming alcohol at much higher levels than pregnant women in the general population, the risk of alcohol-exposed pregnancies (AEPs) is increased in this high risk group. Therefore, interventions to decrease alcohol use in pregnant substance users are needed.
The goal of this study was to evaluate the efficacy of motivational enhancement therapy (MET) modified for use in pregnant substance users (MET-PS) in reducing substance use in pregnant women using alcohol: this was accomplished through a secondary analysis of a clinical trial conducted by the National Drug Abuse Treatment Clinical Trials Network (CTN; Winhusen et al., 2008). Although changes have been made in recent motivational interviewing (MI) strategies and the language used to describe them (Miller & Rollnick, 2013), this article will describe the methods in place at the time of the original study. MI as described in earlier versions (Miller & Rollnick, 1991, 2002), is a client-centered directive approach to increasing motivation to change behaviors based on four principles – establishing empathy, developing discrepancy, rolling with resistance, and supporting self-efficacy. MI has been successful in improving numerous health behaviors, including alcohol, tobacco, and marijuana use, in less time than other treatments (Lundahl et al., 2010; Lundahl & Burke, 2009; Miller & Rollnick, 2002, 2013). It has also been useful in decreasing AEPs through decreased alcohol use and more effective contraception use in the Project CHOICES clinical trial (Floyd et al., 2007; Project CHOICES Intervention Research Group, 2003) and with pregnant women who drink (Handmaker et al., 1999). Few studies have examined MI’s effect on substance use other than for alcohol, tobacco, and marijuana, but in those studies MI was found to be more effective than no treatment and equally effective to established treatments (Lundahl et al., 2010; Lundahl & Burke, 2009).
MET (Miller, 1999; Miller & Rollnick, 1991, 2002), a variation of MI (sometimes called “MI plus”) based on the four MI principles with additional feedback regarding current alcohol/substance use, has also been used to decrease alcohol and illicit-drug use with varying success. Two CTN multi-site clinical trials evaluated the effectiveness of MI/MET in individuals in outpatient substance use disorder (SUD) treatment (Carroll et al., 2006; Ball et al., 2007). MI, relative to treatment as usual (TAU), did not significantly decrease overall substance use, but did significantly decrease alcohol use in those reporting alcohol use as the primary drug of choice (Carroll et al., 2006). MET, relative to counseling as usual (CAU), did not significantly decrease substance use during the 4 week therapy phase, with both groups evidencing a significant decrease relative to baseline. However. MET, relative to CAU, was significantly more effective in sustaining the decrease in substance use compared to resumption of substance use to baseline levels by those in the CAU group over a 12 week follow-up (Ball et al., 2007). While this effect was found in the overall study sample, a subgroup analysis revealed that significant MET treatment effects were found in primary alcohol users, but not in primary drug users (Ball et al., 2007).
A CTN multisite trial comparing MET for pregnant substance users (MET-PS) to TAU did not find a significant MET-PS treatment effect in the overall sample (Winhusen et al., 2008). The present study was a secondary analysis of the CTN MET-PS trial dataset to evaluate whether, like the other two CTN MI/MET trials, there might be a significant effect of MET for alcohol users. Given the importance of addressing any alcohol use in pregnant women, the analysis evaluated the efficacy of MET-PS, relative to TAU, in decreasing alcohol and illicit-drug use in the subset of pregnant substance users who reported any alcohol use in the 28 days prior to randomization.
2.0 Methods
2.1 Participants
In the primary CTN MET-PS study, pregnant women entering treatment for substance use were recruited from four participating agencies in North Carolina, New Mexico, Indiana, and Kentucky (see Winhusen et al., 2008, for details on agency characteristics and selection). After the study was explained to the women, an informed consent form approved by the Institutional Review Boards of the participating sites was signed. Eligibility criteria for pregnant women to participate in the study included being (1)18 years of age or older, (2) pregnant (as confirmed by pregnancy testing), (3) planning to continue (not terminate) the pregnancy, and (4) identified as needing substance use disorder (SUD) treatment according to each treatment agency’s screening procedures for alcohol or illicit substances, which included marijuana at that time. Exclusion criteria for the study included (1) requiring residential or inpatient treatment (other than detoxification), (2) being more than 32 weeks pregnant, (3) planning to relocate from the area within 4 months after signing the study consent form, (4) having legal charges pending that might lead to incarceration, or (5) being of significant suicidal/homicidal risk.
2.2 Intervention
As noted earlier, the MET-PS intervention was developed using the brief motivational techniques described by Miller (1999) and Miller and Rollnick (1991), with modifications made to meet the specific needs of pregnant substance users. For pregnant substance users entering treatment for a variety of substances (including alcohol), the goal of MET-PS in this study was abstinence. This goal was consistent with the US Surgeon General’s recommendation for pregnant women to abstain from alcohol use (CDC, n.d.), although a decrease in substance use was seen as progress with this high risk population of polysubstance users. During the initial intake session, a therapeutic alliance was established with the pregnant woman through the empathic use of open-ended questions, reflective listening, affirmations, and summarizations of the woman’s feelings regarding her pregnancy by the therapist. The woman’s goals for the pregnancy were contrasted with her use of substances while pregnant, including possible adverse effects on the fetus, to identify ambivalence between goals and behaviors. The first session concluded with the clinic’s usual assessment and intake procedures. The second session involved the review of the participant’s individualized personal feedback report concerning her actual substance use behaviors and activities in which she was participating to promote a healthy pregnancy. The third session involved development of a change plan to strengthen the woman’s commitment to change. The three MET-PS sessions replaced the TAU intake session and the first two individual treatment sessions offered by 3 out of the 4 treatment programs. The fourth program which focused primarily on case management and monthly counseling visits modified its standard care to include 2 counseling sessions a week in addition to a standard intake to standardize the care across sites. Participation in other services offered by the community treatment programs (e.g., case management, group treatment, etc.) was encouraged to participants in both the MET-PS and TAU groups. Clinicians were randomly assigned to provide MET-PS or TAU to study participants. Those providing MET-PS received 20 hours of training with MET experts. They were regularly monitored for adherence and competence in MET methods by the MET-PS supervisors via ongoing review of randomly selected audiotaped sessions; 62% of the sessions were reviewed. More information on the specific MET and TAU interventions can be found in the article by Winhusen et al. (2008).
2.3 Measures
The outcome measures for the present analyses were self-report of alcohol use, illicit-drug use, and qualitative urine toxicology results. Self-report of alcohol use was measured at each research visit with the Substance Use Calendar, which assessed the woman’s use of substances for each day of the study using the Timeline Follow-Back procedure (TLFB: Fals-Stewart, 2000; Sobell and Sobell, 1992). Urine samples collected at screening, weekly during the active study phase, and at the 2 follow-up visits were tested for opiates, cocaine, methamphetamines, benzodiazepines, and marijuana using the OnTrak TesTcup®.
2.4 Procedures
In the primary MET-PS study, pregnant women entering SUD treatment were referred to the research assistant (RA) to learn more about the study. If the woman agreed to study participation, she signed the informed consent form, then completed screening and baseline assessments. Ineligible pregnant women completed the site’s standard intake assessment, then were admitted into the site’s standard treatment program. Eligible participants were randomized to MET or TAU stratified on three dichotomous variables via urn randomization: pressure to attend treatment (yes/no), self-report of drug and alcohol use during the prior 28 days (<10 or ≥10 days), and need for methadone maintenance (yes/no).
The study protocol included an active study phase which was 4 weeks in duration as well as 2-month (week 8) and 4-month (week 16) follow-ups. Participants were scheduled to meet with the RA on a weekly basis during the active phase and at each follow-up. Study participants could receive up to $225 in retail scrip or vouchers for completing research visits.
2.5 Data analysis
All analyses for the current study were completed using SAS, Version 9.3 (SAS Institute, Inc., Cary, North Carolina). Statistical tests were conducted at a 5% Type I error rate (two-sided) for all measures. The MET and TAU groups differed significantly on the days of alcohol use during the 28 days prior to randomization and so this variable was included as a covariate in all analyses. The longitudinal binary outcome variables including daily alcohol use, daily illicit-drug use, and urine drug screens were tested for a treatment effect and/or treatment-by-time interaction effect using logistic generalized mixed model regressions in the intention-to-treat (ITT) sample.
3.0 Results
3.1 Sample Characteristics
Sample characteristics by treatment group and for the sample as a whole are provided in Table 1. For the present study, pregnant women who reported drinking any alcohol in the 28 days before randomization were eligible. Of the 200 randomized pregnant women in the CTN MET-PS study (Winhusen et al., 2008), a total of 41 women (20.5%) met this criterion. Of these 41 women, 27 were randomized to MET and 14 to TAU. Women in this study were an average of 28 years old with women receiving MET being significantly older than those in TAU (29.4 years versus 24.1 years, respectively). Women were, on average, at 20.0 weeks gestation at baseline. Most women were unmarried, unemployed, and had a high school education. Diversity was noted in race with 40% of the women White, 37.5% African American and 17.5% Hispanic. The primary substance of abuse reported was cocaine (29.3%), followed by alcohol (24.4%). The MET, relative to TAU, group reported significantly more days of alcohol use in the 28 days prior to randomization (5.7 days versus 1.7 days, respectively). Yet 18.5% of the MET group reported alcohol as the primary drug of use as compared to 35.7% of the TAU group with no significant difference in primary drug of choice overall. Over 80% of women had complete TLFB alcohol data (responses for each day of each week for which alcohol use was measured) for the active phase (77.8% in MET-PS and 85.7% in TAU groups) and 63.4% had complete alcohol data at follow-up (63% in MET-PS and 64.3% in TAU groups) with no significant differences between groups. Reported illicit drug use in the 28 days pre-randomization averaged 8.9 days with no significant difference noted between groups. The number of study treatment sessions attended by women randomized to MET averaged 2.1 (SD = 1.2) as compared to 2.6 sessions (SD=0.9) in TAU, which was not a significant difference (W = 1.2, p > 0.5). No significant differences in ratio of treatment hours attended to hours scheduled in the active phase (X2 = 0.25, df = 1, p > .05), weeks attending treatment during the active (X2 = 1.56, df = 1, p > .05) or follow-up phases (X2 = 1.53, df = 1, p > .05), or days to dropping treatment (X2 = 0.04, df = 1, p > .05) were found between the MET-PS and TAU groups.
Table 1.
Participant demographic and baseline characteristics by treatment group
| MET N=27 |
TAU N=14 |
Group Analysis Statisticˆ | Total N=41 |
|
|---|---|---|---|---|
| Age (Years) | 29.4 (6.5) | 24.1 (3.7) | T=3.30** | 27.6 (6.2) |
| Race/Ethnicity (%)+ | F=0.01 | |||
| African-American | 33.3% | 46.2% | 37.5% | |
| Caucasian | 44.4% | 30.8% | 40.0% | |
| Hispanic | 22.2% | 7.7% | 17.5% | |
| Other | 0.0% | 15.4% | 5.0% | |
| Marital Status (%) | F=0.04 | |||
| Married | 11.1% | 28.6% | 17.1% | |
| Separated/Divorced | 18.5% | 7.1% | 14.6% | |
| Not Married | 70.4% | 64.3% | 68.3% | |
| Education (Years) | 11.7 (2.2) | 11.1 (1.5) | W=1.00 | 11.5 (2.0) |
| Employed Full or Part Time (%) | 22.2% | 14.3% | F=0.28 | 19.5% |
| Weeks Pregnant | 20.6 (8.9) | 18.7 (7.7) | W=0.80 | 20.0 (8.4) |
| Primary drug used (%) | F=0.00 | |||
| Alcohol | 18.5% | 35.7% | 24.4% | |
| Cocaine | 33.3% | 21.4% | 29.3% | |
| Marijuana | 11.1% | 35.7% | 19.5% | |
| Opiates | 7.4% | 0.0% | 4.9% | |
| Methamphetamine | 3.7% | 0.0% | 2.4% | |
| Other | 25.9% | 7.1% | 19.5% | |
| Days of alcohol use (past 28) | 5.7 (7.4) | 1.7 (1.1) | T=2.70* | 4.3 (6.3) |
| Days of illicit drug use (past 28) | 9.3 (10.9) | 7.9 (9.9) | W=0.2 | 8.9 (10.4) |
| Need methadone (%) | 7.4% | 0.0% | F=0.43 | 4.9% |
| Pressure to attend treatment (%) | 14.8% | 35.7% | F=0.10 | 22.0% |
Note. Where not specifically indicated, numbers represent means (standard deviations).
W = Wilcoxon, T = Student t, F = Fisher Exact
p < 0.05,
p < 0.01.
Comparisons are for Caucasian to minority participants.
3.2 Substance Use
3.2.1 Self-report of alcohol use
Analysis of alcohol use days revealed non-significant Treatment (X2 =1.49, df = 1, p > .05), Time (X2 = 2.63, df = 1, p > .05) and Treatment × Time interaction effects (X2 = 2.64, df = 1, p > .05) for the active study phase. In contrast, during the 12 week follow-up phase, significant Time (X2 = 16.76, df = 1, p < .0001) and Treatment × Time interaction (X2 = 13.07, df = 1, p < .001) effects were found, with MET-PS participants reporting lower levels of alcohol use relative to TAU. As depicted in Figure 1A, both the MET-PS and TAU groups reported a decrease in alcohol use during the active study phase. Yet women receiving MET-PS maintained lower levels of alcohol use in the follow-up period, whereas alcohol use increased to baseline levels for women receiving TAU. There was no significant Treatment effect on alcohol use days during the 12 week follow-up.
Figure 1.
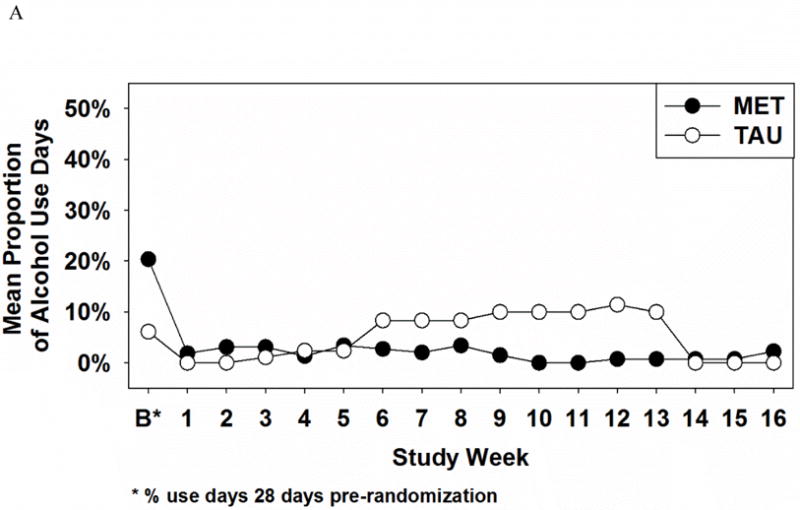
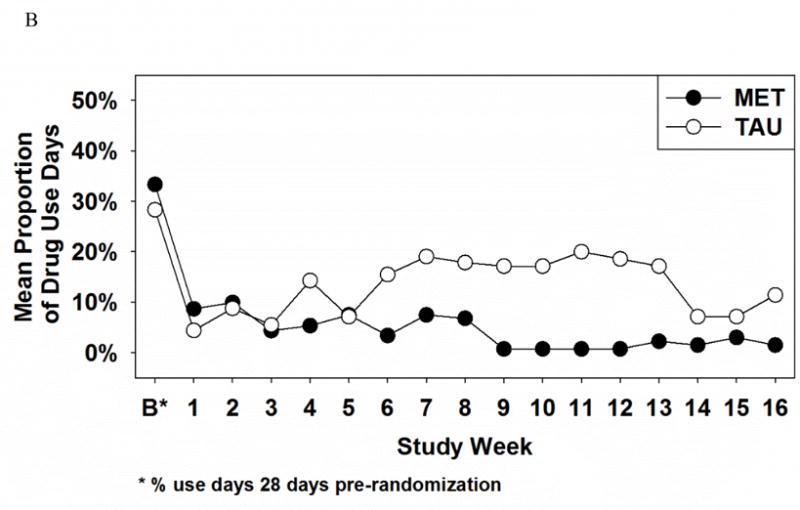
Proportion of alcohol use days (A) and illicit-drug use days (B) as a function of treatment group and time
3.2.2 Self-report of illicit-drug use
For self-reported illicit-drug use, there were significant Treatment × Time interaction effects during the active (X2 = 6.89, df = 1, p < .01) and follow-up phases (X2 = 8.26, df = 1, p < .01). The graph of this effect (see Figure 1B) reveals that both groups evidenced similar decreases in illicit-drug use by week 1 but that this decrease was sustained by the MET-PS, but not the TAU, group over the next 15 weeks. There were no significant time or treatment effects in the active or follow-up phases noted.
3.2.3 Urine Toxicology Results
For illicit-drug use assessed by urine drug screen, no significant Treatment (X2 = 0.56, df = 1, p > .05), Time (X2 = 2.11, df = 1, p > .05), or Treatment × Time (X2 = 0.40, df = 1, p > .05) effects were found for the active study phase. Similar outcomes were found during the follow-up phase of the study, with no significant Treatment (X2 = 0.09, df = 1, p > .05), Time (X2 = 0.50, df = 1, p > .05), or Treatment × Time (X2 = 0.36, df = 1, p > .05) effects.
4.0 Discussion
A CTN study found MET-PS was not effective in decreasing substance use in pregnant substance users (Winhusen et al., 2008). The present study, which analyzed the data set of the CTN MET-PS trial, found that in the subgroup who reported any alcohol use in the 28 days prior to randomization, MET-PS, relative to TAU, decreased alcohol and illicit-drug use over time. More specifically, MET-PS participants reported significantly decreased alcohol use days during the 12 week follow-up while the TAU participants increased alcohol use days, rebounding toward baseline use levels. MET-PS participants also evidenced sustained decreases in illicit-drug use compared to TAU participants who rebounded toward baseline use. This study adds to the literature on the utility of MET to decrease substance use in patients in SUD treatment, particularly when alcohol use is reported (Ball et al., 2007). More importantly, with up to 35% of pregnant women in SUD treatment abusing alcohol (SAMHSA, 2013), these findings support the potential usefulness of MET-PS to decrease prenatal alcohol use in pregnant substance users, thereby mitigating the risk of FASD.
The finding of decreased illicit-drug use in pregnant substance users reporting baseline alcohol use was an interesting outcome novel to this study. Although meta-analyses of MI found similar effect sizes for alcohol and substance use (Burke et al., 2003; Hettema et al., 2005), MET was not found to decrease substance use in primary drug users (Ball et al., 2007) or in pregnant substance users, some of whom reported alcohol use (Winhusen et al., 2008). Yet, this study suggests that MET-PS may be effective in decreasing both alcohol and illicit-drug use in pregnant substance users who use alcohol. While the CTN MET-PS study did not find significant changes in overall prenatal substance use due to MET-PS, the original study did not examine changes in alcohol and/or illicit-drug use specifically in a subgroup of alcohol users (Winhusen et al., 2008). Ball and colleagues (2007) conducted their subgroup analysis with patients reporting alcohol as the primary drug of choice as opposed to including anyone with alcohol use. Therefore, neither study (Ball et al., 2007; Winhusen et al., 2008) investigated illicit-drug use in a subgroup of substance users with alcohol use. With approximately 28% of pregnant substance users abusing alcohol in combination with others substances while 7% abuse alcohol only (SAMHSA, 2013), more studies evaluating the efficacy of MET-PS in decreasing both alcohol and illicit-drug use in pregnant substance users seem warranted.
No significant treatment differences were found for the urine drug screen (UDS) results. The finding of a significant treatment effect for MET-PS on the self-report measures but not UDS could reflect the greater sensitivity of the self-report measure, which assessed substance use for every day of the study, relative to the qualitative UDSs that were collected weekly during the active study phase and every 2 months during the follow-up phase. The difference could also be accounted for by under-reporting in the MET-PS, relative to TAU, group. The potential for under-reporting of alcohol and substance use may have been influenced by the therapeutic alliance formed through the empathic responses of the therapist to the pregnant women – a desired component of the MET-PS intervention. Yet this component of the intervention may have led to social desirability bias where the woman’s desire to not disappoint the therapist’s efforts may have led to under-reporting of substance use.
A limitation of this study is that it was a secondary analysis of a clinical trial of 200 pregnant substance users. While relatively equal randomization to treatment occurred in the main study (MET-PS = 102, TAU = 98), almost twice as many participants in the secondary analysis received MET-PS (n = 27) compared to TAU (n = 14). This nonequivalent distribution of participants may have influenced outcomes of this secondary analysis. When comparing characteristics of the MET-PS and TAU groups in this analysis (see Table 1), no statistically significant differences were found, except for age and days of alcohol use in the 28 days before baseline. Yet it is possible that the combined effect of almost twice as many women receiving MET-PS who also reported significantly more baseline alcohol use may have impacted this study’s findings. The urn randomization used by the primary MET-PS trial balanced the two groups on 3 dichotomous variables: pressure to attend treatment, self-reported alcohol and drug use, and need for methadone maintenance. A recommendation for future studies is for alcohol and illicit-drug use to be included as two separate variables in the urn randomization to create more equivalent groups for subgroup analyses.
The significant baseline difference in alcohol use between the groups with women in the MET-PS group reporting three times the number of alcohol use days in the past 28 days as compared to the TAU group (5.7 days versus 1.7 days, respectively) was a study limitation. Although included as a covariate in the statistical analysis, the difference in baseline alcohol use between groups may have influenced this study’s outcomes. Yet analysis of the active phase of this study in which baseline alcohol consumption was included found no significant main or interaction effects of treatment over time for alcohol use days. This was in contrast to the follow-up phase of the study where women who received MET-PS maintained low levels of alcohol use over time while those who received TAU resumed drinking at or above baseline levels. A meta-analysis found that MI is generally more effective with persons who are more resistant and less ready for a change (Hettema et al., 2005). Therefore, it is plausible that the women who reported drinking at higher levels at baseline were more receptive to the effects of MET-PS over time. This rationale may also hold true for why women receiving MET-PS decreased alcohol use more in the follow-up even though more women reported alcohol use as the primary drug of choice in the TAU group (18.5% versus 35.7%, respectively). This same meta-analysis also noted the effects of MI to persist and/or increase over time when provided in addition to an active treatment. With women in both the MET-PS and TAU groups encouraged to attend other treatment groups provided at the SUD treatment centers, the maintenance of decreased alcohol and drug use in pregnant substance users in this study may have been related to the additive effects of MET-PS to standard treatment. Although the additive effects of MET-PS to decrease substance use could potentially be explained by its abilities to increase treatment adherence, no significant differences in attendance were found between the MET-PS and TAU groups.
One interesting occurrence noted in Figure 1 was the decrease in alcohol and in drug use days during weeks 14–16 in the TAU group, returning or almost returning to the lower levels reported by the MET-PS group. This secondary analysis found significant time × intervention effects in favor of MET-PS over TAU to decrease both alcohol and illicit drug use. To determine whether these decreases in alcohol and drug use during weeks 14–16 in the TAU group toward the MET-PS group’s use are isolated events or clinically significant, longitudinal studies beyond 4-month follow-up are needed to understand more about the sustainability of treatment effects in pregnant substance users. Since prenatal alcohol and drug use can cause harmful effects at any stage of fetal development, decreases in prenatal substance use throughout the gestational period are essential to prevent deleterious consequences for the fetus.
Another interesting point is that both Ball et al. (2007) and Carroll et al. (2006) found significant changes in alcohol use only in patients who reported alcohol as the primary drug of choice, whereas this study found significant changes in pregnant women who reported any alcohol use at baseline. Analysis of data from the aforementioned studies to determine if patients reporting any alcohol use who received MET/MI also decreased alcohol use could potentially support this study’s findings.
The small number of women reporting alcohol use at baseline in this secondary analysis (n = 41) is a study limitation. With a small sample size, missing data could influence study results in this ITT analysis. As noted earlier, over 80% of women provided complete TLFB alcohol data in the active phase and 63.4% had complete alcohol data at follow-up with no significant differences between groups. Due to the limited sample size and power of this study, nuisance parameters, which included the significant difference in age between the MET-PS and TAU groups, were not accommodated in the regression models. Given the limitations of the present study, the findings would need to be replicated in a larger clinical trial before being given significant consideration clinically.
Lastly, the additional treatment services offered at the treatment sites could pose a limitation for this study. In addition to attendance at the intake and 2 counseling sessions required by the study, participants were encouraged to attend other treatment services offered at the treatment centers, such as case management, group treatment, etc. A pregnant woman’s attendance at these optional services provided additional interventions that might influence her alcohol/substance use, but were not controlled for by the study.
4.1 Conclusion
With FASD occurring in 2–5% of all US live births (May et al., 2014), it is imperative that women who drink any amount of alcohol that could be risky to the developing fetus receive effective interventions to assist with decreased prenatal alcohol use. With approximately 35% of pregnant substance users in treatment reporting alcohol abuse (SAMHSA, 2013), this study provides preliminary support for the use of MET-PS to decrease prenatal alcohol use in substance using women. With a large proportion of these women abusing alcohol in combination with others substances (SAMHSA, 2013), MET-PS may also be useful in decreasing illicit-drug use in this population, bettering outcomes for both mothers and children.
Highlights.
Motivational enhancement therapy (MET) was provided to pregnant substance users.
Treatment outcomes were analyzed in a subgroup of women with baseline alcohol use.
MET resulted in decreased alcohol use over time in this subgroup of women.
MET resulted in decreased illicit-drug use in this subgroup over time.
MET may decrease alcohol and drug use in pregnant substance users more than TAU.
Acknowledgments
Funding for this study was provided by the National Institute on Drug Abuse (NIDA) Clinical Trials Network: the Ohio Valley Node (Grant U10DA013732), the North Carolina Node (Grant U10DA013711), and the Southwest Node (Grant U10DA015833). The publications committee of the Clinical Trials Network (CTN) approved the manuscript. Robin Osterman and Theresa Winhusen designed this secondary analysis and contributed to the interpretation of the data. Daniel Lewis conducted the analyses and critically reviewed the manuscript. Robin Osterman wrote the first draft of the manuscript and Theresa Winhusen critically revised the manuscript for important intellectual content. All authors have approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration
ClinicalTrials.gov http://www.clinicaltrials.gov; Identifier: NCT00078143.
References
- Ball SA, Martino CN, Frankforter TL, Van Horn D, Crits-Christoph P, Woody GE, Carroll KM. Site matters: Multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J Consult Clin Psychol. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Woody GE. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug Alcohol Depend. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention: CDC. A 2005 message to women from the US Surgeon General: Advisory on alcohol use during pregnancy. n.d. Retrieved from https://www.cdc.gov/ncbddd/fasd/documents/sg-advisory.pdf.
- Fals-Stewart W, O’Farrell TJ, Frietas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug abusing patients: Psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Floyd RL, O’Connor MJ, Sokol RJ, Bertrand J, Cordero JF. Recognition and prevention of fetal alcohol syndrome. Obstet Gynecol. 2005;106:1059–1064. doi: 10.1097/01.AOG.0000181822.91205.6f. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Sobell M, Velasquez MM, Ingersoll K, Nettleman M, Sobell L, Nagaraja J. Preventing alcohol-exposed pregnancies: A randomized control trial. Am J Prev Med. 2007;32(1):1–10. doi: 10.1016/j.amepre.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handmaker NS, Miller WR, Manicke M. Findings of a pilot study of motivational interviewing with pregnant drinkers. J Stud Alcohol. 1999;60:285–287. doi: 10.15288/jsa.1999.60.285. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatr. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: A practice-friendly review of four meta-analyses. Journal of Clinical Psychology. 2009;65(11):1232–1245. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- Lundahl B, Kunz C, Brownell C, Tollefson D, Burke B. A meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Research on Social Work Practice. 2010;20(2):137–160. [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C. 2004;127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Manning MA, Hoyme HE. Fetal alcohol spectrum disorders: A practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliot AJ, Blankenship J, Kalberg WO, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134(5):855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Res Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Miller WR. Enhancing motivation for change in substance abuse treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment; 1999. (SAMHSA Treatment Improvement Protocol Series Volume 35, DHHS Publication No. (SMA) 99-3354). [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. 1st. New York: Guilford Press; 1991. [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people for change. 2nd. New York, London: The Guilford Press; 2002. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change. 3rd. New York: Guilford Press; 2013. [Google Scholar]
- Popova S, Stade B, Bekmuradov D, Lange S, Rehm J. What do we know about the economic impact of fetal alcohol spectrum disorder? A systematic literature review. Alcohol and Alcoholism. 2011;46(4):490–497. doi: 10.1093/alcalc/agr029. [DOI] [PubMed] [Google Scholar]
- Project CHOICES Intervention Research Group. Reducing the risk of alcohol-exposed pregnancies: A study of a motivational intervention in community settings. Pediatr. 2003;111(5):1131–1135. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Time line followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. New Jersey: Humana Press, Inc.; 1992. pp. 19–28. [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatr. 2001;108(2):e34. doi: 10.1542/peds.108.2.e34. Retrieved April 5, 2013 from http://www.pediatrics.org/cgi/content/full/108/2/e34. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC, editors. Fetal Alcohol Syndrome: Diagnosis epidemiology, prevention, and treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Streissguth A. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle Prospective Longitudinal Study. J Clin Psycho Med Settings. 2007;14:81–101. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (The TEDS Report).Trends in substances of abuse among pregnant women and women of childbearing age in treatment. 2013 Jul 25; Retrieved from http://www.samhsa.gov/data/spotlight/spot110-trends-pregnant-women-2013.pdf. [PubMed]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age – United States, 2011–2013. MMWR. 2015;37:1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Kropp F, Babcock D, Hague D, Erickson SJ, Renz C, Rau L, Lewis D, Leimberger J, Somoza E. Motivational enhancement therapy to improve treatment utilization and outcome in pregnant substance users. J Subst Abuse Treat. 2008;35:161–173. doi: 10.1016/j.jsat.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


