Abstract
Store-operated calcium channels provide calcium signals to the cytoplasm of a wide variety of cell types. The basic components of this signaling mechanism include a mechanism for discharging Ca2+ stores (commonly but not exclusively phospholipase C and inositol 1,4,5-trisphosphate), a sensor in the endoplasmic reticulum that also serves as an activator of the plasma membrane channel (STIM1 and STIM2), and the store-operated channel (Orai1, 2 or 3). The advent of mice genetically altered to reduce store-operated calcium entry globally or in specific cell types has provided important tools to understand the functions of these widely encountered channels in specific and clinically important physiological systems. This review briefly discusses the history and cellular properties of store-operated calcium channels, and summarizes selected studies of their physiological functions in specific physiological or pathological contexts.
Keywords: Store-operated calcium channels, calcium signaling, mouse models, exocrine glands, neutrophils, keratinocytes
1. Introduction: What are Store-operated Channels?
Store-operated channels are plasma membrane ion (usually Ca2+) channels that are regulated by the content of Ca2+ in intracellular stores, generally the endoplasmic reticulum. Thus, store-operated channels are most commonly encountered as a component of a biphasic Ca2+ signaling mechanism, involving both release of intracellular Ca2+ and Ca2+ entry through plasma membrane channels. The history of the idea of store-operated channels has been reviewed [1]. The concept originated from observations of the kinetics of refilling intracellular stores following their release [2–5]. In 1983, Berridge and collaborators, following on the general theory developed by Michell [6], demonstrated that the most common mechanism for receptor-activated release of Ca2+ involved the second messenger, inositol 1,4,5-trisphospate (IP3) [7,8]. A key early event in the story of store-operated channels was the discovery that a plant toxin, thapsigargin [9], passively released intracellular stores independently of receptor activation or IP3 production [10], and also activated the same plasma membrane Ca2+ channels as a receptor agonist, and to the same extent [11]. In 1992, Hoth and Penner [12] published the first characterization of a store-operated Ca2+ current which they called Icrac, for calcium-release activated calcium current. This current arose from channels with high Ca2+ selectivity resulting in strong inward rectification. Single channel conductance of these CRAC channels was very low, and could only be estimated from indirect noise analysis of the current [13,14]. Subsequently, research in a number of laboratories focused on two basic issues: the identity of the store-operated channels, and the nature of the signal linking intracellular Ca2+ store content to channel activation (reviewed in [15].
For some time, the leading candidate for the channel was one or more members of the TRPC ion channel family [16]. The seven mammalian TRPC channels are homologs of the Drosophila photoreceptor channel, TRP, and like the Drosophila channel, they appear to be activated by receptors coupled to phospholipase C and the production of IP3 [17]. This is an expected property of store-operated channels, but store-operated channels are thought not to require phospholipase C or IP3 formation, as illustrated by the action of thapsigargin. This distinction was controversial, with some laboratories demonstrating that store-depletion could activate TRPCs [18–26], while others found that store-depletion was neither necessary nor sufficient for their activation [27–31] (reviewed in [15]). A general finding however was that TRPC channels clearly did not have the channel properties expected of Icrac. Unlike CRAC channels, TRPC channels have only modest Ca2+ selectivity and thus the current-voltage relationships have significant outward components at positive voltages. Nonetheless, in certain experimental conditions, TRPC channels do appear to behave as if they respond to store depletion. In these instances, their activation depends on the molecular components of the classical CRAC channels, Orai and STIM, discussed below [32–34]. Thus it is possible, although not proven, that in these instances TRPC channels are not directly activated by store-depletion, but in a pathway downstream of store-operated CRAC channels.
Attempts to identify the signaling mechanism linking store depletion to store-operated channels were similarly frustrating and controversial. Some proposed candidates were cyclic GMP [35] (but see [36]), a product of cytochrome P450 [37,38], the IP3 receptor [23,39] (but see [40]), a tyrosine kinase [41–43] (but see [44]), a small G-protein [45] and an as yet unidentified substance extracted from store-depleted cells termed “CIF” for calcium influx factor [46–48].
The first major breakthrough came in 2005 with the discovery of the signaling mechanism. By use of a limited siRNA screen, Roos et al. [49] demonstrated the essential role of a previously known Ca2+-binding protein, STIM1. Their discovery was confirmed shortly thereafter and independently by Liou et al. [50] who also provided the first evidence that STIM1 functioned as a Ca2+ sensor in the endoplasmic reticulum. Just one year later, Feske et al. [51], by use of a whole genome siRNA screen, identified Orai1 as an obligate component of the store-operated Ca2+ entry (SOCE) pathway. Very shortly thereafter, two other laboratories confirmed this finding [52,53]. Subsequently, multiple laboratories demonstrated that co-transfection of cells with STIM1 and Orai1 resulted in manifold amplification of store-operated Ca2+ entry and of Icrac [53–56], establishing these two proteins as the minimal constituents of store-operated Ca2+ signal generation. That Orai1 was indeed a pore-forming subunit of the CRAC channel was demonstrated by single amino acid mutations that altered the ion selectivity of the store-operated currents [57–59].
There are multiple forms of STIM proteins, two gene products, STIM1 and STIM2 [50], as well as a long splice variant of STIM1, STIM1L [60]. There are three Orai gene products, Orai1, 2 and 3, and two versions of Orai1, Orai1α and Orai1β, arising from alternative translation initiation [61,62]. Much less is known about the functions of Orai2 and 3. Orai1α and β both appear capable of supporting SOCE. However, in addition to its ability to form SOC channels, Orai1α, but not Orai1β, is an essential component of a distinct Ca2+ channel giving rise to a non-store-operated Ca2+ current termed Iarc [62,63]. The signal for the ARC channels is believed to be arachidonic acid, released from membrane lipids by the action of phospholipase A2 [64] or by a metabolite of arachidonic acid, leukotriene C4 [65]. ARC channels also require STIM1, but apparently not functioning as an endoplasmic reticulum Ca2+ sensor [66]. The pore of ARC channels is composed of a combination of Orai1 and 3 subunits in a pentameric structure [67]. As of this writing, little is known of the physiological function of ARC channels, as no publications on specific mouse models have appeared. Nonetheless, it should be remembered that mice lacking STIM1 or Orai1 will likely have lost Iarc as well as Icrac.
2. Cellular Function of Store-operated Ca2+ Entry
Early studies of store-operated Ca2+ entry involved observations of mechanisms and pathways by which intracellular stores are refilled after their discharge. The earliest models implicated a function for Ca2+ entry of recharging stores such that Ca2+ signals could persist from intracellular release mechanisms [2,4,5]. However, a number of more recent findings have cast doubt on a major role of store-operated channels in maintaining intracellular stores.
Calcium is required in the endoplasmic reticulum for correct protein synthesis and folding [68,69]. The loss of the ability of the endoplasmic reticulum to maintain its intracellular Ca2+ store content would thus be catastrophic to cell function and viability.
However, humans lacking functional Orai1, the major store-operated channel subunit, develop to birth but die young due to a severe combined immunodeficiency [70,71]. Mice lacking Orai1 also develop to birth [72,73]. Specific organs (lacrimal glands, mammary glands) from mice lacking Orai1, shown to completely lack store-operated entry develop normally but lack specific signaling functions [74,75]. However, other cell types show clear developmental deficiencies (skeletal muscle [72,76], sperm [77]).
In several cell biological studies, a clear and specific requirement for Ca2+ entry through store-operated channels, independent of global Ca2+ changes, has been demonstrated [78–81]. Dissociation of the signaling function of store-operated channels from global Ca2+ concentration was nicely demonstrated in studies of gene regulation in mast cells by Di Capite et al. [82]. These authors activated sustained Ca2+ oscillations in mast cells through the leukotriene receptor, which is known to activate phospholipase C and subsequently store-operated Ca2+ entry. This resulted in an increase in expression of the early gene, c-fos. In the absence of external Ca2+, the oscillations ran down rapidly and there was no increase in c-fos. In the absence of external Ca2+, oscillations could be maintained by blocking the plasma membrane with a relatively high concentration of lanthanum [83]. However, despite a pattern of global cytoplasmic oscillations indistinguishable from those in the presence of external Ca2+, no increase in c-fos expression occurred. This result strongly suggests that for this particular pathway, it is specifically the Ca2+ entering through the store-operated channels that drives gene expression, not the global increase from the IP3-induced Ca2+ oscillations.
3. Physiological Functions of Store-operated Calcium Entry
Prior to the discovery of the roles of STIM and Orai proteins in SOCE, patients were identified with loss of SOCE in lymphocytes, and were shown to have severe combined immunodeficiency [70,71,84]. The discoveries of STIM and Orai revealed additional patients with immunodeficiency associated with mutations in both STIM1 and Orai1 genes [85]. These patients do not survive very long unless they receive a bone marrow transplant. Following this, other deficiencies are observed, including diminished skeletal muscle development and ectodermal dysplasia (reviewed in [85,86]). Also following the discoveries of STIM and Orai, a number of laboratories began developing mouse models to investigate the roles of these proteins, and by inference SOCE, in a variety of physiological contexts. Not surprisingly, the first studies focused on the immune system [72,73,86]. Also, initial studies utilized whole-animal knockouts of Orai1 and STIM1, but these animals do not survive well and analysis of their various phenotypes may be complicated by interactions among different organ systems and cell types. Thus more recent studies have sometimes utilized animals with floxed genes crossed to transgenic animals expressing Cre recombinase driven by organ- or cell type-specific promoters (when appropriate transgenic mice are available). Below I summarize some studies, mostly from our laboratory, that illustrate the utility of these mouse models.
4. Mouse Models for Store-operated Calcium Entry
Mice lacking Orai1 tend to die perinatally, but can survive for prolonged periods if crossed into a mixed genetic background [72,73]. Similar to humans with null mutations in Orai1, these mice are deficient in both innate and acquired immunity [72,73,86]. The mice are generally small in stature, which may result in part from impaired skeletal muscle development [87], but also in impaired development of bone [88,89]. In Orai1 knockout mice, precursor cells for both osteoblasts (bone forming cells) and osteoclasts (bone resorbing cells) have impaired SOCE, the differentiation of the precursor cells to mature bone cells is impaired, the functions of the differentiated cells is reduced, and the mice are osteopenic, i.e., they have significantly decreased bone density [88,89].
SOCE has long been known to play a major signaling role in acquired immunity [90]. Until recently, less is known regarding the role of SOCE in innate immunity, although Icrac was first discovered as a store-operated current in mast cells [12]. Mast cells from Orai1 knockout mice showed significantly diminished SOCE, loss of Icrac, and grossly defective degranulation and cytokine secretion [72]. Similar findings were reported by Baba et al [91] with a STIM1 knockout mouse. Allergic reactions elicited in Orai1 knockout mice were significantly inhibited [72]. Interestingly, SOCE in T-cells from these mice was minimally affected, and expression of Orai1 (as determined by gene-trap) was low. This may indicate that mice show a milder phenotype with regard to acquired immunity as compared to humans.
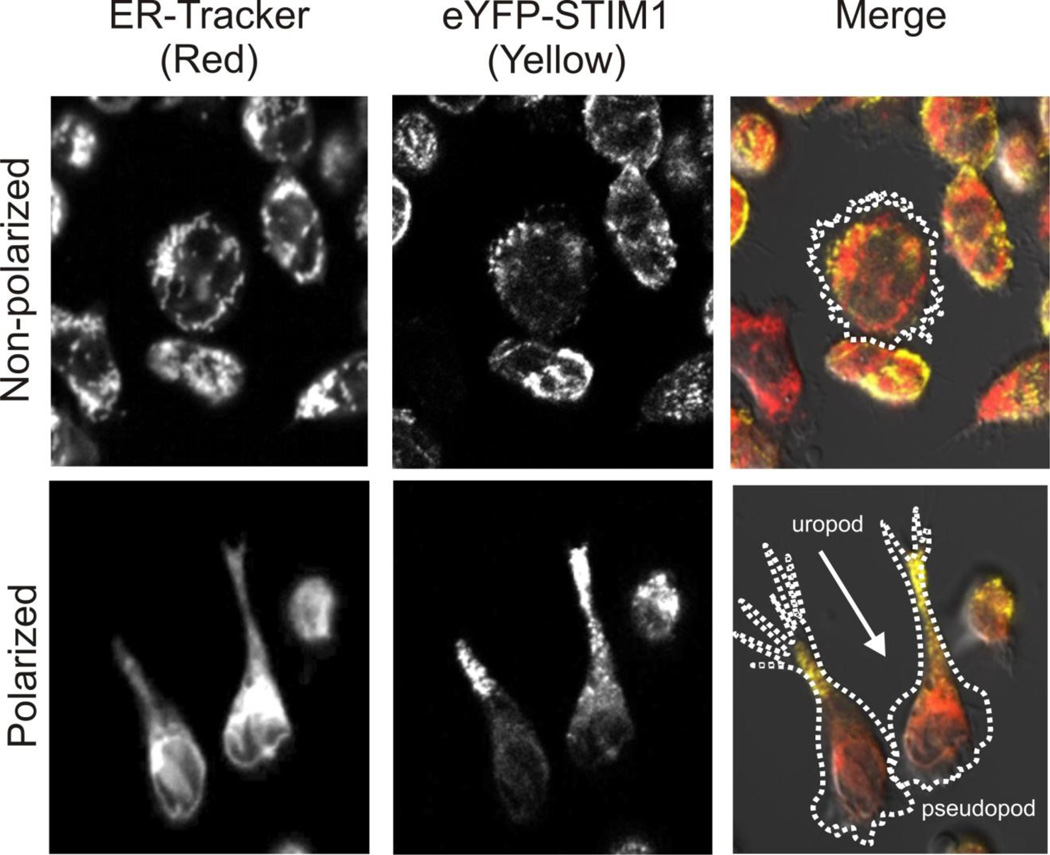
Another major player in innate immunity is the neutrophil [92]. Neutrophils sense gradients of small molecules that indicate the location of sites of infection or inflammation and migrate to those sites by the process of chemotaxis [93]. Calcium has long been known to play a central role in neutrophil chemotaxis, largely from studies of the action of the chemoattractant fMLF. fMLF is a tripeptide that activates a G-protein-coupled pathway leading to phospholipase C activation and the generation of IP3 [94,95]. A role for Ca2+ entry has also been documented [96], and the major entry mechanism appears to be SOCE [97,98]. Knockdown of either Orai1 or STIM1 in the neutrophil cell line, HL-60 significantly reduced fMLF induced Ca2+ signaling and significantly reduced fMLF induced chemotaxis [99]. If SOCE were to play a role in the directed movement of chemotaxis, one would expect that SOCE signaling would occur in an asymmetric fashion when the cells become polarized. Figure 1 shows that this is indeed the case; surprisingly STIM1 distributes to the rear of polarized HL-60 neutrophils. This is due in part because endoplasmic reticulum distributes to the real of the cell, but as shown in Figure 1, STIM1 concentrates toward the rear to a greater extent than endoplasmic reticulum.
Figure 1. STIM1 distributes to the rear of polarized HL-60 neutrophils.
Neutrophil-like HL-60 cells stably expressing eYFP-STIM1 [99] were stained with the endoplasmic reticulum labeling reagent, ER-Tracker Red (Molecular Probes). Cells were plated on coverslips covered with fibronectin (polarized cells) or fibronectin plus BSA (nonpolarized cells). In polarized cells, STIM1 clearly distributes to the rear (uropod), and to a significantly greater extent than the endoplasmic reticulum.
Psoriasis is a debilitating disease of the skin, thought to result from an autoimmunity involving several components of the immune system, including neutrophils [100]. In a mouse model of psoriasis, targeted knockout of STIM1 in myeloid lineage cells (including neutrophils) hastened the reversal of psoriasis plaques following removal of a chemical activator of psoriasis [99]. This indicates that components of SOCE might serve as useful pharmacological targets for treatment of psoriasis or other autoimmunity-involved disorders of the skin.
Calcium signaling plays a major role in the development and maintenance of the epidermis, the major pathway being initiated by a calcium sensing receptor [101]. An outward gradient of calcium in the extracellular matrix orchestrates the differentiation of keratinocytes from an undifferentiated to a fully differentiated state. In the keratinocyte cell line, HaCaT, raising Ca2+ from 0.03 mM to 1.8 mM induces expression of the keratinocyte-associated gene, KRT1, and slows growth as cells become terminally differentiated [101]. This response involves the calcium sensing receptor, known to act through activation of phospholipase C [102]. Knockdown of either STIM1 or Orai1 reduced SOCE, Icrac and completely abrogated the [Ca2+]i signal arising in response to a switch from 0.03 mM to 1.8 mM Ca2+. Cell proliferation in low Ca2+, growth suppression in high Ca2+ and expression of KRT1 were also substantially diminished [103].
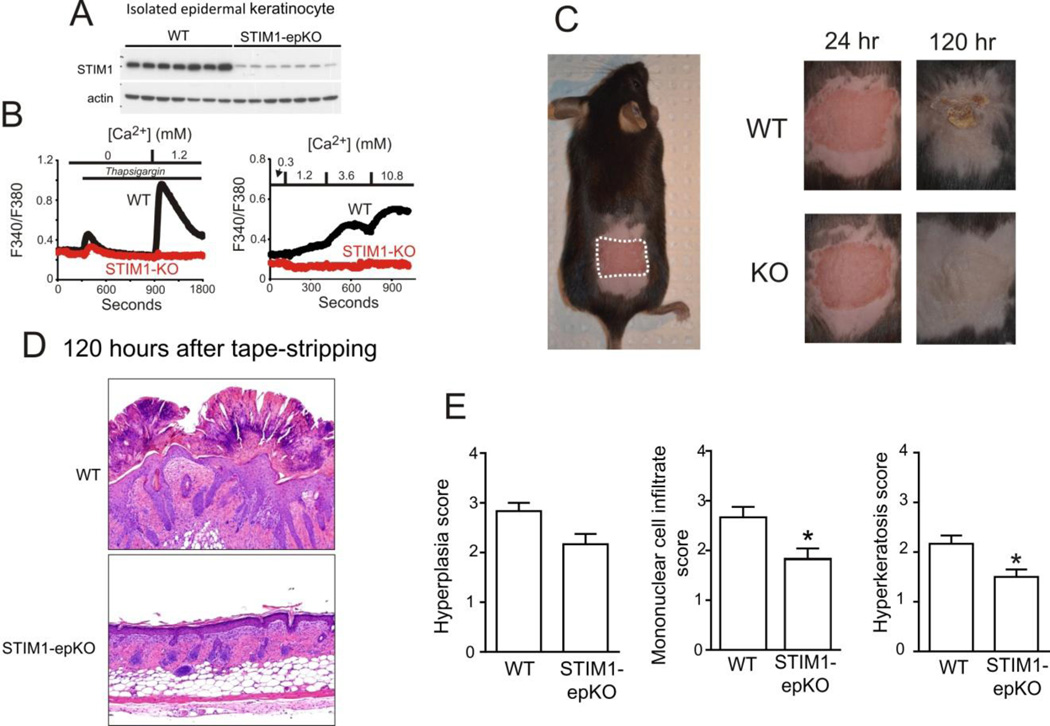
Keratinocytes play an essential role in the process of healing of superficial wounds. On the basis of in vitro studies in the HaCaT cell line described above, impaired Ca2+ signaling in keratinocytes would be expected to impair the process of wound healing in skin. Figure 2 shows that in keratinocytes from mice with STIM1 knocked down specifically in epidermal cells, Ca2+ signals in response to either thapsigargin or raised Ca2+ were almost completely abrogated. Surprisingly however, loss of STIM1 in epidermal cells resulted in an improved outcome following skin injury, particularly at longer times. (Figure 2). The reason for this unexpected finding is not yet clear, but it may be due to diminished release of chemokines from keratinocytes which are known to be pro-inflammatory [104]. In addition to their obvious role as building blocks for restoring epidermal integrity, keratinocytes also release chemokines that signal recruitment of neutrophils [104]. Neutrophils provide an initial barrier against infection and mediate clearing of necrotic cells from the damaged area [104]. A general conclusion from this result is that one cannot always predict the consequences of genetic alterations in vivo based on findings in cell lines or primary cells in vitro. Additional studies will be needed to more fully understand the role of SOCE in keratinocytes during wound healing.
Figure 2. Wound healing in wild-type and in mice with epidermal-specific knockout of STIM1.
Methods: Mice were crossed with KRT14-cre mice (Jackson laboratories). Isolation of keratinocytes was carried out as previously demonstrated [117]. Keratinocytes were cultured in CnT-07 (Zen-bio) according to manufacturer’s instruction. Calcium imaging of keratinocytes were carried out as previously reported [103]. For superficial wounding of the epidermis, mice were subjected to a tape-stripping assay. 7–9 week old female mice were anesthetized with isoflurane. Mice backs were shaved and depilated by Nair cream. Depilated back skins were tape-stripped 20 times with Scotch tape (18 mm width). Lesions were rubbed with Vaseline to be moisturized. At the end of time-courses, lesioned skin was harvested for histological analysis. A: Epidermal specific knockout of STIM1 (STIM1-epKO) results in almost complete disappearance of STIM1 protein. B: Keratinocytes from STIM1-KO mice lack Ca2+ entry in response to thapsigargin or to elevated Ca2+. C: Left panel shows an example of the wound area produced by tape stripping. Right panel shows examples of injured area in a wild type (WT) and knock-out (KO) mouse at 24 hours and 120 hours. D: Histological examination of skin at 120 hours shows extensive hyperkeratinization of skin of wild type mouse not the knockout (STIM1-epKO) mouse. E. Summary data show that skin from knockout (STIM1-epKO) mice has a slightly decreased hyperplasia score and a significantly diminished mononuclear cell infiltrate and hyperkeratosis score (P<0.05).
5. Store-operated Calcium Entry in Exocrine Glands
The Orai1 knockout mouse has been utilized to study the role of SOCE in two distinct types of exocrine glands, lacrimal glands and mammary glands. Much of the original evidence for the concept of SOCE was obtained from studies of Ca2+ signaling in exocrine glands, including lacrimal acinar cells [105,106]. Acinar cells isolated from the lacrimal glands of Orai1 knockout mice have no detectable SOCE in response to either thapsigargin or muscarinic receptor activation, and no detectable Icrac [74]. Pilocarpine-activated lacrimal secretion in vivo is substantially reduced in the knockout animals. Histological examination of the lacrimal glands shows that gland development and structure are normal with the exception, not unexpectedly, that secretory granule content following pilocarpine treatment in the knockout glands is increased. Methacholine stimulated secretion of peroxidase from wild-type lacrimal gland fragments in the presence of extracellular Ca2+ and to a lesser extent in the absence of Ca2+. With fragments from knockout mice, the secretion in the presence was reduced to that seen in the absence of Ca2+. However, the secretion in the absence of Ca2+, presumably resulting from intracellular Ca2+ release, as well as the basal rate of secretion, were unchanged in the knockout preparation [74]. This study, perhaps as clearly as any, illustrates the highly specific role of Orai1 in signaling Ca2+ entry. Thus, lacrimal glands of Orai1 knockout mice lack SOCE, Icrac, and Ca2+-dependent secretion. However, gland size, morphology and Ca2+-independent secretion are unaffected. This indicates that the basic mechanism of synthesis and storage of the secretory product, the basic mechanism of Ca2+-regulated exocytosis, and the signaling pathway through the formation of IP3 and release of intracellular Ca2+ stores are quantitatively unaffected.
Calcium signaling is known to be essential for mammalian oocyte fertilization [107–109]. Indirect evidence has implicated Orai1 and SOCE as playing a role in mammalian oocyte fertilization [110]. However, surprisingly, female Orai1 knockout mice are fertile, and bear litters of normal size when mated to a wild type male. However, pups do not gain weight and die after about four days, unless fostered to a wild type dam [75]. This indicates that Orai1 knockout female mice fail to lactate adequately. The exocrine function of mammary glands differs in some respects from other exocrine glands. Hormonal actions associated with birthing activate a constitutive secretion of milk containing calcium and other nutrients into alveolar structures. Secretion is initiated on demand by suckling through a reflex mechanism involving activation of oxytocin receptors on basket myoepithelial cells that surround the alveoli [111,112]. Analysis of milk from Orai1 knockout female mice showed a decrease in Ca2+ concentration of about 50% [75]. This likely reflects an alternative function of Orai1 in transporting Ca2+ in epithelial cells [113]. However, protein concentration in milk was normal and it is unlikely that this alteration is sufficient to account for the lactation phenotype. Mammary glands of Orai1 knockout female mice were engorged suggesting that the constitutive formation of milk proceeded normally but the discharge of stored milk was impaired. Consistent with this interpretation, oxytocin induced Ca2+ oscillations in isolated myoepithelial cells were substantially reduced in cells from Orai1 knockout mice. Visualization of alveolar contractions demonstrated that alveolar contractions in response to oxytocin were substantially diminished as well [75]. Thus, the failure of lactation in Orai1 deficient female mice likely results primarily from a failure of Ca2+ signaling in myoepithelial cells.
6. Conclusions
Our understanding of the physiological functions of store-operated calcium channels has been greatly augmented through studies of mouse models with altered genetic expression of the basic components, STIM and Orai. Hopefully this information will eventually allow exploitation of this pathway to alleviate any of a large number of diseases known to involve aberrant calcium signaling [114–116].
Highlights.
Store-operated calcium entry is a widely distributed signaling mechanism involving plasma membrane calcium channels activated when endoplasmic reticulum calcium stores are reduced.
The major molecular players in store-operated calcium entry are the endoplasmic reticulum calcium sensors, STIM1 and STIM2, and the plasma membrane channel pore forming subunits, Orai1, 2 and 3.
Use of genetically modified mice has revealed multiple physiological roles for store-operated channels, including roles in both acquired and innate immunity, in keratinocyte differentiation, and in exocrine gland function.
Acknowledgments
Work from the authors’ laboratory was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare there are no conflicts of interest.
References
- 1.Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Putney JW. Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J. Physiol. (Lond.) 1977;268:139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parod RJ, Putney JW. The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J. Physiol. (Lond. ) 1978;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells of rabbit ear artery. J. Physiol. (Lond. ) 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial store in pancreatic cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen U, Christensen SB, Sandberg F. Thapsigargin and thapsigargicin, two new histamine liberators from thapsia garganica. Acta Pharmaceut. Suec. 1978;15:133–140. [PubMed] [Google Scholar]
- 10.Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem. J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takemura H, Hughes AR, Thastrup O, Putney JW. Activation of calcium entry by the tumor promoter, thapsigargin, in parotid acinar cells. Evidence that an intracellular calcium pool, and not an inositol phosphate, regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 12.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 13.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Nat. Acad. Sci. USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakriya M, Lewis RS. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J. Gen. Physiol. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc. Nat. Acad. Sci. USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putney JW. The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol. 2004;14:282–286. doi: 10.1016/j.tcb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J. Biol. Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. J. Biol. Chem. 2003;278:11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- 20.Rosado JA, Sage SO. Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-trisphosphate receptor type II and human transient receptor potential (hTrp1) channels in human platelets. Biochem. J. 2001;356:191–198. doi: 10.1042/0264-6021:3560191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J. Biol. Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- 22.Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marguart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- 23.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 24.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 25.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 2009;3:221–225. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 26.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3 and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 2005 doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 27.Trebak M, Bird GSt.J, McKay RR, Birnbaumer L, Putney JW. Signaling mechanism for receptor-activated TRPC3 channels. J. Biol. Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–262. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 30.Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457:757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW. TRPC channels function independently of STIM1 and Orai1. The Journal of Physiology. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J. Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional Requirement for Orai1 in Store-operated TRPC1-STIM1 Channels. J. Biol. Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS. Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Star RA, Tortorici G, Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J. Biol. Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]
- 36.Gilon P, Obie J, Bian X, Bird GSt.J, Putney JW. On the role of cyclic GMP in the control of capacitative calcium entry in rat pancreatic acinar cells. Biochem. J. 1995;311:649–656. doi: 10.1042/bj3110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez J, Montero M, García-Sancho J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB J. 1992;6:786–792. doi: 10.1096/fasebj.6.2.1537469. [DOI] [PubMed] [Google Scholar]
- 38.Graier WF, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J. Physiol. (Lond. ) 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosado JA, Sage SO. Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem. J. 2000;350:631–635. [PMC free article] [PubMed] [Google Scholar]
- 40.Broad LM, Braun F-J, Lièvremont J-P, Bird GSt.J, Kurosaki T, Putney JW. Role of the phospholipase C - inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current (I crac) and capacitative calcium entry. J. Biol. Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 41.Yule DI, Kim ET, Williams JA. Tyrosine kinase inhibitors attenuate “capacitative” Ca2+ influx in rat pancreatic acinar cells. Biochem. Biophys. Res. Comm. 1994;202:1697–1704. doi: 10.1006/bbrc.1994.2130. [DOI] [PubMed] [Google Scholar]
- 42.Rosado JA, Graves D, Sage SO. Tyrosine kinases activate store-mediated Ca2+ entry in human platelets through the reorganization of the actin cytoskeleton. Biochem. J. 2000;351:429–437. [PMC free article] [PubMed] [Google Scholar]
- 43.Babnigg G, Bowersox SR, Villereal ML. The role of pp60c-src in the regulation of calcium entry via store-operated calcium channels. J. Biol. Chem. 1997;272:29434–29437. doi: 10.1074/jbc.272.47.29434. [DOI] [PubMed] [Google Scholar]
- 44.Vostal JG, Shafer B. Thapsigargin-induced calcium influx in the absence of detectable tyrosine phosphorylation in human platelets. J. Biol. Chem. 1996;271:19524–19529. doi: 10.1074/jbc.271.32.19524. [DOI] [PubMed] [Google Scholar]
- 45.Fasolato C, Hoth M, Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium entry. J. Biol. Chem. 1993;268:20737–20740. [PubMed] [Google Scholar]
- 46.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 47.Kim HY, Thomas D, Hanley MR. Chromatographic resolution of an intracellular calcium influx factor from thapsigargin-activated Jurkat cells. J. Biol. Chem. 1995;270:9706–9708. doi: 10.1074/jbc.270.17.9706. [DOI] [PubMed] [Google Scholar]
- 48.Csutora P, Su Z, Kim HY, Bugrim A, Cunningham KW, Nuccitelli R, Keizer JE, Hanley MR, Blalock JE, Marchase RB. Calcium influx factor is synthesized by yeast and mammalian cells depleted of organellar calcium stores. Proc. Nat. Acad. Sci. USA. 1999;96:121–126. doi: 10.1073/pnas.96.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 52.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci U. S. A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calcium-selected currents due to co-expression of orai1 or orai2 with the intracellular calcium sensor, stim1. J. Biol. Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM Reconstitute Store-operated Calcium Channel Function. J. Biol. Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 56.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 58.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. The Journal of Cell Biology. 2011;194:335. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of orai1 with distinct plasma membrane mobilities. J. Cell Sci. 2012 doi: 10.1242/jcs.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci. Signal. 2015;8 doi: 10.1126/scisignal.aaa8323. ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson JL, Shuttleworth TJ, Exploring the unique features of the ARC channel. a store-independent Orai channel. Channels (Austin. ) 2013;7:364–373. doi: 10.4161/chan.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology. (Bethesda. ) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia. Circ. Res. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shuttleworth TJ, Thompson JL, Mignen O. STIM1 and the noncapacitative ARC channels. Cell Calcium. 2007;42:183–191. doi: 10.1016/j.ceca.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mignen O, Thompson JL, Shuttleworth TJ. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. The Journal of Physiology. 2009;587:4181–4197. doi: 10.1113/jphysiol.2009.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 69.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J. Biol. Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 71.Feske S, Draeger R, Peter HH, Rao A. Impaired NFAT regulation and its role in a severe combined immunodeficiency. Immunobiology. 2000;202:134–150. doi: 10.1016/S0171-2985(00)80060-1. [DOI] [PubMed] [Google Scholar]
- 72.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xing J, Petranka JG, Davis FM, Desai PN, Putney JW, Bird GS. Role of orai1 and store-operated calcium entry in mouse lacrimal gland signaling and function. J. Physiol. (Lond. ) 2013;592:927–939. doi: 10.1113/jphysiol.2013.267740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW., Jr Essential role of Orai1 store-operated calcium channels in lactation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis FM, Goulding EH, D’Agostin DM, Janardhan KS, Cummings CA, Bird GS, Eddy EM, Putney JW. Male infertility in mice lacking the store-operated Ca(2+) channel Orai1. Cell Calcium. 2016;59:189–197. doi: 10.1016/j.ceca.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooper DMF, Yoshimura M, Zhang Y, Chiono M, Mahey R. Capacitative Ca2+ entry regulates Ca2+-sensitive adenylyl cyclases. Biochem. J. 1994;297:437–440. doi: 10.1042/bj2970437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene C4 secretion. J. Biol. Chem. 2004;279:29994–29999. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 80.Ng SW, Di Capite J, Singaravelu K, Parekh AB. Sustained Activation of the Tyrosine Kinase Syk by Antigen in Mast Cells Requires Local Ca2+ Influx through Ca2+ Release-activated Ca2+ Channels. J. Biol. Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 81.Ng SW, Nelson C, Parekh AB. Coupling of Ca2+ Microdomains to Spatially and Temporally Distinct Cellular Responses by the Tyrosine Kinase Syk. J. Biol. Chem. 2009;284:24767–24772. doi: 10.1074/jbc.M109.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 83.Van Breemen C. Blockade of membrane calcium fluxes by lanthanum in relation to vascular smooth muscle contractility. Arch Int Physiol Biochim. 1969;77:710–716. doi: 10.3109/13813456909059783. [DOI] [PubMed] [Google Scholar]
- 84.Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, Oleastro M, Belohradsky B, Choquet D, Fischer A. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–1062. [PubMed] [Google Scholar]
- 85.Feske S. CRAC channelopathies. Pflugers Arch. 2010 doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol (Lond) 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robinson LJ, Mancarella S, Songsawad D, Tourkova IL, Barnett JB, Gill DL, Soboloff J, Blair HC. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 2012 doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hwang SY, Foley J, Numaga-Tomita T, Petranka JG, Bird GS, Putney JW., Jr Deletion of Orai1 alters expression of multiple genes during osteoclast and osteoblast maturation. Cell Calcium. 2012;52:488–500. doi: 10.1016/j.ceca.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem. Biophys. Res Commun. 2003;311:1117–1132. doi: 10.1016/j.bbrc.2003.09.174. [DOI] [PubMed] [Google Scholar]
- 91.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 92.McDonald B, Kubes P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. (Berl) 2011;89:1079–1088. doi: 10.1007/s00109-011-0784-9. [DOI] [PubMed] [Google Scholar]
- 93.Maxfield FR. Regulation of leukocyte locomotion by Ca2+ Trends Cell Biol. 1993;3:386–391. doi: 10.1016/0962-8924(93)90088-i. [DOI] [PubMed] [Google Scholar]
- 94.Dougherty RW, Godfrey PP, Hoyle PC, Putney JW, Freer RJ. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem. J. 1984;222:307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaconi MEE, Rives RW, Schlegel W, Wollheim CB, Pittet D, Lew PD. Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J. Biol. Chem. 1988;263:10557–10560. [PubMed] [Google Scholar]
- 96.Andersson T, Dahlgren C, Pozzan T, Stendahl O, Lew PD. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol. Pharmacol. 1986;30:437–443. [PubMed] [Google Scholar]
- 97.Demaurex N, Lew DP, Krause K-H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J. Biol. Chem. 1992;267:2318–2324. [PubMed] [Google Scholar]
- 98.Alvarez J, Montero M, García-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is not mediated by production of inositol polyphosphates but by emptying of the intracellular Ca2+ stores. Biochem. Soc. Trans. 1994;22:809–813. doi: 10.1042/bst0220809. [DOI] [PubMed] [Google Scholar]
- 99.Steinckwich N, Myers P, Janardhan KS, Flagler ND, King D, Petranka JG, Putney JW. Role of the store-operated calcium entry protein, STIM1, in neutrophil chemotaxis and infiltration into a murine model of psoriasis-inflamed skin. FASEB J. 2015;29:3003–3013. doi: 10.1096/fj.14-265215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toichi E, Tachibana T, Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J. Am. Acad. Dermatol. 2000;43:391–395. doi: 10.1067/mjd.2000.103264. [DOI] [PubMed] [Google Scholar]
- 101.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 102.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 103.Numaga-Tomita T, Putney JW. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J Cell Sci. 2013;126:605–612. doi: 10.1242/jcs.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su Y, Richmond A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv Wound Care (New Rochelle) 2015;4:631–640. doi: 10.1089/wound.2014.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Putney JW, Huang Y, Bird GSt.J. Calcium signalling in lacrimal acinar cells. Adv. Exp. Med. Biol. 1998;438:123–128. doi: 10.1007/978-1-4615-5359-5_16. [DOI] [PubMed] [Google Scholar]
- 106.Putney JW, Bird GS. Calcium signaling in lacrimal glands. Cell Calcium. 2014;55:290–296. doi: 10.1016/j.ceca.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves amd Ca2+ oscillations at fertilization in mammalian eggs. Dev. Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 108.Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: The influence of subcellular localization. Mol. Reprod. Dev. 2012;79:742–756. doi: 10.1002/mrd.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 110.Gómez-Fernández C, López-Guerrero AM, Pozo-Guisado E, Álvarez IS, Martín-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Molecular Human Reproduction. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- 111.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr. Rev. 1992;13:33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 112.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 113.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mooren FC, Kinne RKH. Cellular calcium in health and disease. Biochim. Biophys. Acta. 1998;1406:127–151. doi: 10.1016/s0925-4439(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 115.Missiaen L, Robberecht W, Van Den Bosch L, Callewaert G, Parys JB, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H. Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium. 2000;28:1–21. doi: 10.1054/ceca.2000.0131. [DOI] [PubMed] [Google Scholar]
- 116.Targos B, Baranska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim. Pol. 2005 doi: 10.18388/abp.2005_3452. [DOI] [PubMed] [Google Scholar]
- 117.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]