Abstract
Background
Hypersensitivity reactions (HSRs) can occur with any of the available biologic drugs used to treat rheumatoid arthritis (RA). We compared drug-specific risks for HSR among RA patients enrolled in the US Medicare program.
Methods
Using Medicare data, we identified new users of infused infliximab, abatacept, rituximab, tocilizumab, golimumab, and injected biologics. After identifying HSRs using validated algorithms, for each biologic, we calculated cumulative incidence over 6 months and incidence rates (IR) within 0–1, 2–14 and 15–30 days of administration. For each biologic administration, follow-up started on the infusion/injection date and ended at the earliest of HSR, subsequent biologic administration, death, coverage loss, 30-day follow-up, or 12/31/2013. Adjusted robust Poisson regression was used to compare the HSR risks across biologics. Sensitivity analysis was conducted using a nested case-crossover design.
Results
We identified 725,591 biologic administrations and 248 HSRs among 80,587 new biologic users. Of these, 26.9% were for IV abatacept, 4.6% rituximab, 5.8% IV tocilizumab, 22.9% infliximab, and 39.7% injectable anti-TNFi. The cumulative incidence of HSRs over 6 months for all biologics was low (< 1%). The IRs for HSR ranged from 2.4 (with abatacept) to 239.5 (with rituximab) per 106 per person days. After adjustment, and using injectable anti-TNF during 0–30 days as the referent, rituximab, infliximab, abatacept, and tocilizumab infusions were associated a significant higher risk of HSR. Sensitivity analysis yielded similar results.
Conclusion
Among RA patients taking biologics, rituximab and infliximab were most strongly associated with HSRs. The absolute IRs of HSR events for all biologic exposures were low.
Introduction
Drug hypersensitivity reactions (HSR) comprise a set of undesirable responses from an activated immune system (1). These reactions can range from minimally uncomfortable to severe and life threatening. Over the past decade, U.S Food and Drug Administration (FDA) has approved a number of biologic drugs for different conditions, and these agents have revolutionized treatment options for patients with rheumatoid arthritis (RA) and other auto-immune illnesses. Biologics approved for treatment of RA include five that target tumor necrosis factor alpha (e.g. etanercept [a fusion protein], infliximab, adalimumab, and golimumab [either chimeric or humanized monoclonal antibodies], and certolizumab [a Fab′ fragment linked to a large PEG molecule]), rituximab (a monoclonal antibody against CD20), tocilizumab (an anti-IL-6 receptor humanized monoclonal antibody), and abatacept (a soluble, recombinant fusion protein that inhibits co-stimulation). Given structural and functional differences between biologics, distinct safety profiles might be expected for each of them with respect to their likelihood to causing HSRs. While data are available from clinical trials in RA, the propensity of each biologic for inducing HSRs has not been well studied at a population level (2).
A set of HSR-type responses has been characterized as infusion reactions, ranging from anaphylaxis (immediate HSR) or other acute responses to more delayed HSRs and those involving immune complex formations which have a more subacute presentation. Patients with RA are at risk of HSRs both for the first administration and also subsequent administrations (3) (4). Most information about the incidence of HSRs among biologic user comes from relatively small clinical trials of homogeneous patient types or has been derived from the experience of single centers. Infliximab, a chimeric antibody with murine components, has been reported to have a higher frequency and severity of HSRs (13.8%) than other anti-TNFs (etanercept (5.3%) and adalimumab (3.5%)) in a study that examined 671 patients with autoimmune diseases (5). In contrast, HSRs for etanercept, adalimumab, certolizumab and golimumab, which are administered subcutaneously, are less common (6–9). Abatacept, tocilizumab and rituximab also have been reported to cause HSRs (10–12), including immediate HSRs (e.g. anaphylaxis) in 0.1% to 2% of patients (13, 14). A post-marketing case of fatal HSR has been reported in one older RA patient who was treated with intravenous tocilizumab, triggering a regulatory agency required notification to health care providers (15).
Given a paucity of population-based data on which to obtain estimates related to the incidence of HSRs associated with biologic use for RA in real-world settings, the objective of the current study was to evaluate the incidence of HSRs occurring in RA patients and to compare risks between biologic agents.
METHODS
Study Design and Data Sources
We conducted a retrospective cohort study using 2006–2013 fee-for-service Medicare claims data for all RA patients from the Centers for Medicare and Medicaid Services (CMS) Chronic Condition Data Warehouse (16), which provides Medicare claims and assessment data linked across the spectrum of care. We obtained patients’ demographic and insurance coverage information from the Medicare beneficiary summary file, claims for inpatient, outpatient, skilled nursing facility, non-institutional provider, home health, hospice, durable medical equipment services from inpatient (Part A) and outpatient medical care (Part B) files, and prescription drug information from the prescription drug events file (Part D).
Eligible Criteria
The study cohort consisted of eligible Medicare patients with RA who were new users of adalimumab, certolizumab, etanercept, golimumab, infliximab, abatacept, rituximab and tocilizumab during 2006–2013. We required all patients to have continuous “full coverage” by Medicare during the 12 months (baseline) prior to the initiation of biologic use and throughout follow-up. Full coverage was defined as traditional Medicare fee-for-service (Parts A and Parts B Medicare coverage, and not enrolled in a Medicare Advantage plan) and pharmacy coverage (Part D Medicare coverage). We identified RA patients using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-9-CM) diagnosis codes for RA (714.x) on two separate rheumatologist visits, with at least one visit occurring during the baseline. New biologic use was defined as no use of the specific agent in the prior 12 months.
To enable us to detect incident HSRs, we excluded patients who had a diagnosis code of HSRs during the baseline period. Prevalent HSRs was identified using ICD-9-CM diagnosis codes of 995.0, 999.4, 995.3, 995.2 and E930–E949 (descriptions provided in Appendix table). Since RA patients with cancer might have other HSR-related risk factors compared to RA patients without cancer, we further excluded patients with a claim containing a physician diagnosis of a malignant neoplasm (excluding non-melanoma skin cancer) during baseline period, and censored patients during follow-up if they had evidence of malignancy. The unit of analysis was the time interval following each biologic administration, starting on each date of drug infusion/injection and ending at the earliest date of: first occurrence of hypersensitivity; the next administration of that same biologic; switch to another biologic; 30-days follow-up, death; loss of Medicare coverage; diagnosis of malignant neoplasm other than non-melanoma skin cancer, or the end of study (Dec 31, 2013). With this design, each biologic administration contributed one observation, so one patient could contribute multiple observations for each specific biologic (Appendix figure 1). We adjusted for the clustering of observations within patients in the analysis according to the method of Huber-White “sandwich” variance estimator (17).
Appendix table 1.
ICD 9 codes description for incident and prevalent hypersensitivity reactions
| ICD9 codes | Descriptions |
|---|---|
| Incident (outcome) and prevalent (exclusionary during baseline) events | |
| 995.0 | Other anaphylactic reaction |
| 995.2 | Unspecified adverse effect of unspecified drug, medicinal and biological substance |
| 995.3 | Allergy, unspecified, not elsewhere classified |
| 999.4 | Anaphylactic shock-serum |
| Prevalent (exclusionary during baseline) events | |
| E930 | Antibiotics causing adverse effects in therapeutic use |
| E931 | Other anti-infectives causing adverse effects in therapeutic use |
| E932 | Hormones and synthetic substitutes causing adverse effects in therapeutic use |
| E933 | Primarily systemic agents causing adverse effects in therapeutic use |
| E934 | Agents primarily affecting blood constituents causing adverse effects in therapeutic use |
| E935 | Analgesics antipyretics and antirheumatics causing adverse effects in therapeutic use |
| E936 | Anticonvulsants and anti-parkinsonism drugs causing adverse effects in therapeutic use |
| E937 | Sedatives and hypnotics causing adverse effects in therapeutic use |
| E938 | Other central nervous system depressants and anesthetics causing adverse effects in therapeutic use |
| E939 | Psychotropic agents causing adverse effects in therapeutic use |
| E940 | Central nervous system stimulants causing adverse effects in therapeutic use |
| E941 | Drugs primarily affecting the autonomic nervous system causing adverse effects in therapeutic use |
| E942 | Agents primarily affecting the cardiovascular system causing adverse effects in therapeutic use |
| E943 | Agents primarily affecting gastrointestinal system causing adverse effects in therapeutic use |
| E944 | Water mineral and uric acid metabolism drugs causing adverse effects in therapeutic use |
| E945 | Agents primarily acting on the smooth and skeletal muscles and respiratory system causing adverse effects in therapeutic use |
| E946 | Agents primarily affecting skin and mucous membrane ophthalmological otorhinolaryngological and dental drugs causing adverse effects in therapeutic use |
| E947 | Other and unspecified drugs and medicinal substances causing adverse effects in therapeutic use |
| E948 | Bacterial vaccines causing adverse effects in therapeutic use |
| E949 | Other vaccines and biological substances causing adverse effects in therapeutic use |
Appendix figure.
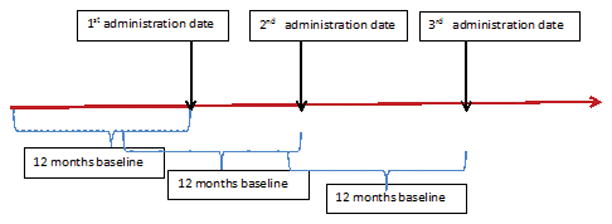
Retrospective cohort study design
Exposures
For biologics administered by infusion, such as golimumab (intravenous), infliximab, abatacept (intravenous), rituximab and tocilizumab (intravenous), we identified these agents using Part B Healthcare Common Procedures Coding System (HCPCS) codes. Each part B infusion date was the index date. We identified injected biologics, including etanercept, adalimumab, certolizumab, golimumab, abatacept (subcutaneous), and tocilizumab (subcutaneous), using part D pharmacy records, which provide national drug codes (NDC), generic drug name, and days of supply. The index date for injected biologics was the date each prescription was dispensed.
Each person day during follow-up was classified into three categories based upon the interval of time starting at each administration date of the medications under study: occurred within 0 (same day) to 1 day after the index date; occurred within 2–14 days of the index date; or occurred within 15–30 days of the index date. Although the recommended dosing frequency for infliximab (56 days) and for rituximab (180 days) was longer than 30 days, only the first 30 days after administration were included in the follow-up period to provide comparability to the time windows examined for the other biologics. Similarly, follow up for the first administration was censored if the patient received a second dose within 30 days, and the follow up for the second administration started on the day of the second administration.
Outcome
The outcome of interest was the first occurrence of a hypersensitivity reaction during follow-up. Using an algorithm for hypersensitivity reactions validated in the U.S. Food and Drug Administration (FDA) Mini-Sentinel drug surveillance program(18), we identified HSRs using three criteria: A) Inpatient or emergency department encounter (ED) for anaphylactic shock (ICD9 codes: 995.0 or 999.4); B) Outpatient encounter for anaphylactic shock plus a diagnosis or a procedure to treat bronchospasm (519.11), stridor (786.1), hypotension (458.9), epinephrine (J0170 or J0171), injection of diphenhydramine (J1200) or cardiopulmonary resuscitation (92950 or 99.60); C) Inpatient or ED encounter for unspecified allergy (995.3 or 995.2) plus a diagnosis or a procedure of bronchospasm, stridor, hypotension, epinephrine, injection of diphenhydramine or cardiopulmonary resuscitation. The positive predictive value (PPV) of the entire algorithm using any of the 3 criteria was previously demonstrated to be 63.1% (95% CI: 53.9–71.7%) (18). The PPVs for criteria A (69%; 95%CI: 58.0–78.7%) and B (65.2%; 95%CI:42.7–83.6%) were higher than the PPV for Criterion C (45.8%; 95% CI: 25.6–67.2%), but the confidence intervals for Criterion C was wide and overlapped (18).
Confounders
Based on prior literature, the most important factors that were considered for potential confounder adjustment included age, gender, Charlson comorbidity index (CCI), intravenous steroids use on the day of biologic administration, oral steroid dose, and methotrexate (MTX) use. Age was updated at each administration date. We calculated CCI using all information obtained during the 12-months baseline, whereas methotrexate and oral glucocorticoid dose were estimated using the 6 months average dose immediately before each index date. Glucocorticoid dosage use was categorized as none, ≤ 7.5mg/day, >7.5 mg/day, and methotrexate dosage was categorized as none, >0–10, >10–15, >15–20, and >20mg/week. Intravenous glucocorticoid use on the same day was evaluated as a dichotomous variable. Using all available data prior to the first administration date, we evaluated the total number of different biologics used, categorized as none, exactly one biologic, exactly two biologics, and three or more biologics. Same day glucocorticoid infusion was not controlled for given that the data does not allow it to be differentiated as to whether it was given as a pre-medication to avoid a HSR, or as treatment for a HSR that had developed.
Statistical analysis
We compared the characteristics of new intravenous biologic users during 2006–2013 by specific drug. In order to estimate a ‘background’ rate of HSRs in an RA population, the IV biologic users were compared to patients filling prescriptions for subcutaneously (SQ) injected anti-TNFs. The same 0–1, 2–14, and 15–30 day time intervals initially were used for SQ anti-TNFs, with the expectation that the prescription fill date was not truly the date the medication was necessarily taken but would serve as a reasonable proxy for the “background” rate of HSR among SQ users. After confirming that the rate was comparable between these three time intervals, they were combined into a single category for the SQ anti-TNF users. After evaluating the frequency distribution of HSRs according to number of infusions received, we calculated the incidence rate (IR) of HSRs for each biologic during 0–1, 2–14 and 15–30 days of follow-up, with the expectation that more severe, causally-related events (e.g. anaphylaxis) would occur within 0–1 days of biologic administration. Robust Poisson regression was used to compare the risks of HSRs across biologics, adjusting for potential confounders. We applied the Huber-White “sandwich” variance estimator to control for correlations among the repeated administration observations nested within the same person (17). Due to the limited sample size and events, use of intravenous golimumab, subcutaneous abatacept and subcutaneous tocilizumab were not included in the Poisson regression model.
Subgroup analysis restricting time periods of study
To examine whether the risk of hypersensitivity was elevated early after starting therapy, we conducted two subgroup analyses. The first evaluated the cumulative incidence of HSR over all administrations in the first six months after initiation, and the second limited the observations to only the initial administration of each biologic.
Sensitivity analysis using nested case crossover design
Given the potential for between-person confounding that might not be able to be adequately controlled for in a traditional cohort design, as a sensitivity analysis, we conducted a complementary nested case crossover analysis (19). In this design, only patients who experienced HSRs (‘cases’) were included. The day of the event and the preceding day prior to the HSR was defined as the hazard period, whereas the preceding 2 to 29 days were categorized into 14 control periods, each also two days wide (Figure 1). We used conditional logistic regression models to compute odds ratios (ORs) for the association between biologic administration and HSRs for each specific biologic. This evaluated the timing of HSRs in relation to preceding biologic exposure. To compare these risks between biologics, we used the OR for HSRs for each individual drug divided by the OR for subcutaneous biologics with 95% confidence interval obtained through bootstrapping (20).
Figure 1.
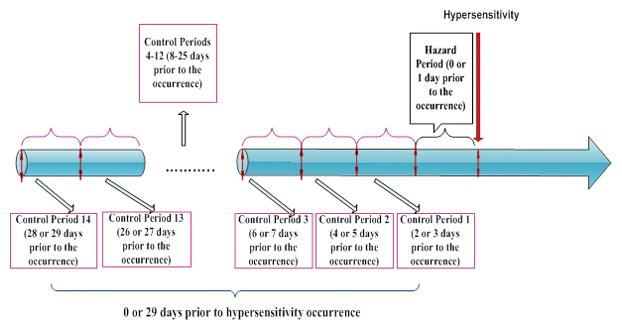
Sensitivity analysis using a nested case crossover design
This study was approved by a Data Use Agreement from the Centers for Medicare and Medicaid system (CMS) and the Institutional Review Board of the University of Alabama at Birmingham (UAB). All statistical analyses were performed in SAS 9.3 (SAS institute).
RESULTS
Among 80,587 new biologic users, 15,820 patients received intravenous abatacept, 9 intravenous golimumab, 17,613 infliximab, 9,125 rituximab, 5,466 intravenous tocilizumab, 1,843 subcutaneous abatacept, 40 subcutaneous tocilizumab and 30,671 injection anti-TNFs. Among these people, we identified 725,591 biologic administrations or prescription fills, 26.9% for intravenous abatacept, 22.9% for infliximab, 4.6% for rituximab, 5.8% intravenous tocilizumab, 39.7% for subcutaneous biologics (Table 1). Of these injection/infusion administrations, more than 80% were among women and Caucasians. The mean age of the patients was younger for SQ biologics compared to IV biologics. Among IV biologic administrations, patients with abatacept, golimumab, and infliximab infusions had similar mean age, and were older than patients with rituximab and tocilizumab. Compare to other biologics, patients who were treated with infliximab were more likely to use concurrent methotrexate, whereas patients receiving rituximab were more likely to use concurrent oral glucocorticoids. In addition, patients administered IV biologics were more likely to receive same day glucocorticoid infusion compared to SQ biologics. Among IV biologic users, rituximab users have the highest proportion of same day glucocorticoids (79.4%), followed by infliximab (11.7%), tocilizumab (8.8%), and abatacept (5.5%).
Table 1.
Patients characteristics for each administration of new biologic users among rheumatoid arthritis patients during 2006–2013
| Patient Characteristics | Biologic Agents | |||||||
|---|---|---|---|---|---|---|---|---|
| Intravenous | Subcutaneous | |||||||
| ABA | GOL | INF | RIT | TOC | ABA | TOC | Injected anti-TNFs | |
| N of new users | 15,820 | 9 | 17,613 | 9,125 | 5,466 | 1,843 | 40 | 30,671 |
| N of administrations | 195,422 | 12 | 165,943 | 33,460 | 42,233 | 10,513 | 50 | 277,958 |
| Age, Mean (SD) | 68.8 (10.9) | 68.6 (7.3) | 69.0 (10.4) | 65.6 (12.1) | 66.3 (12.2) | 60.7 (12.5) | 56.0 (13.8) | 63.2 (12.8) |
| Age category, % | ||||||||
| <50 | 6.72 | 0.00 | 6.23 | 11.69 | 9.99 | 19.55 | 40.00 | 15.57 |
| 50–59 | 10.29 | 16.67 | 8.91 | 15.13 | 14.04 | 26.29 | 26.00 | 21.35 |
| 60–64 | 7.39 | 8.33 | 6.15 | 9.16 | 8.82 | 12.44 | 6.00 | 10.92 |
| 65–69 | 20.62 | 16.67 | 22.92 | 19.97 | 22.07 | 15.75 | 6.00 | 16.94 |
| 70–74 | 25.13 | 33.33 | 27.09 | 21.36 | 20.99 | 12.95 | 14.00 | 16.24 |
| 75–79 | 16.59 | 25.00 | 17.20 | 13.80 | 13.20 | 7.59 | 2.00 | 10.61 |
| 80–84 | 9.43 | 0.00 | 8.53 | 6.41 | 7.98 | 3.79 | 12.00 | 5.82 |
| >=85 | 3.82 | 0.00 | 2.96 | 2.48 | 2.92 | 1.65 | 4.00 | 2.55 |
| Female, % | 83.86 | 75.00 | 80.58 | 82.62 | 83.33 | 86.65 | 92.00 | 81.88 |
| Charlson Score, % | ||||||||
| 0 | 41.80 | 58.33 | 42.92 | 35.76 | 42.31 | 41.19 | 52.00 | 42.01 |
| 1 | 27.44 | 8.33 | 28.37 | 28.73 | 27.86 | 28.15 | 26.00 | 27.78 |
| 2 | 14.57 | 8.33 | 13.99 | 15.80 | 13.56 | 13.92 | 14.00 | 13.72 |
| 3+ | 16.19 | 25.00 | 14.72 | 19.71 | 16.27 | 16.75 | 8.00 | 16.49 |
| Methotrexate, mg/week, % | ||||||||
| None | 53.38 | 50.00 | 36.31 | 56.61 | 56.93 | 57.64 | 48.00 | 49.48 |
| >0–<=10 | 22.17 | 41.67 | 27.29 | 22.10 | 20.04 | 19.72 | 22.00 | 22.33 |
| >10–<=15 | 12.43 | 0.00 | 17.10 | 10.17 | 10.67 | 9.34 | 12.00 | 13.17 |
| >15–<=20 | 9.41 | 8.33 | 15.15 | 8.43 | 8.98 | 8.90 | 10.00 | 11.37 |
| >20 | 2.61 | 0.00 | 4.14 | 2.69 | 3.39 | 4.41 | 8.00 | 3.64 |
| Prednisone, % | ||||||||
| None | 48.87 | 33.33 | 51.57 | 40.30 | 43.60 | 39.79 | 58.00 | 50.23 |
| 0< 7.5 | 37.78 | 50.00 | 36.20 | 39.22 | 36.43 | 33.21 | 36.00 | 35.59 |
| >7.5 | 13.35 | 16.67 | 12.23 | 20.48 | 19.97 | 27.00 | 6.00 | 14.18 |
| Same day steroid infusion for all administrations, % | ||||||||
| 5.5 | 0.0 | 11.7 | 79.4 | 8.8 | 0.6 | 0.0 | 0.9 | |
| N of prior biologic use, % | ||||||||
| 0 | 29.94 | 25.00 | 67.56 | 19.73 | 8.68 | 11.35 | 2.00 | 50.52 |
| 1 | 51.35 | 25.00 | 23.36 | 41.72 | 33.08 | 36.21 | 34.00 | 34.77 |
| 2 | 14.81 | 16.67 | 6.43 | 26.84 | 35.25 | 34.43 | 34.00 | 10.07 |
| 3 or greater | 3.89 | 33.33 | 2.65 | 11.71 | 22.99 | 18.01 | 30.00 | 4.63 |
ABA = Abatacept, INF = infliximab, RIT = Rituximab, TOC = Tocilizumab, GOL = Golimumab
Of 248 HSRs identified during follow-up, 17.3% (n=43) occurred associated with inpatient hospitalization, 78.2% (n=194) emergency department (without hospitalization) and 4.4% (n=11) outpatient (Table 2). Among 93 cases occurring within one day of biologic administration, 33.7%, 8.4%, 57.9% were identified by the HSR algorithm criterion A, B, and C respectively. Only one death, which occurred after the administration of tocilizumab, was identified in the 30 days after any infusion during the study period among patients with HSRs.
Table 2.
Number and type of hypersensitivity reactions*
| Criteria | Encounters | Biologics | ||
|---|---|---|---|---|
| Intravenous | Subcutaneous | All | ||
| A | Emergency only | 45 | 11 | 56 |
| Inpatient Hospitalization | 10 | 3 | 13 | |
| Total “A” Events | 55 | 14 | 69 | |
| B | Outpatient | 10 | 1 | 11 |
| C | Emergency only | 105 | 33 | 138 |
| Inpatient hospitalization | 22 | 8 | 30 | |
| Total “C” Events | 127 | 41 | 168 | |
| Total | 192 | 56 | 248 | |
according to classification in validated hypersensitivity algorithm (18)
The incidence rate of HSR varied by specific biologic and the timing of exposure. During 0–1 day after the administration, the IR ranged from 41.1 per 106 per person days for abatacept to 239.5 for rituximab (Table 3). The IRs ranged from 2.4 for abatacept to 12.1 for rituximab during 2–14 days of follow-up, and ranged from 4.3 for abatacept to 13.2 for rituximab during 15–30 days. Due to the limited exposure in the data, only 1 HSR event occurred among patients with SQ abatacept, and no events occurred among patients with IV golimumab or SQ tocilizumab. After adjustment using injectable anti-TNF during 0–30 days as the referent and ranked in descending order of incidence, infliximab (RR:26.9, 95% 17.4 – 41.5), tocilizumab (RR:22.2, 95% 11.6 – 42.4), rituximab (RR:18.0, 95% 8.9 – 36.2), and abatacept (RR: 7.1, 95% 3.9 – 12.8) infusions were associated a significant higher risk of HSRs during 0–1 days of administration(Table 3). MTX had a significant inverse association with HSR risk (0–10 mg/day vs none: RR: 0.7, 95% 0.5–0.9; 10–15 mg/day: RR: 0.5, 95% 0.3–0.8; 15–20 mg/day: RR: 0.4, 95% 0.2–0.7; >20mg/day: RR: 0.5, 0.2–1.3).
Table 3.
Events, absolute incidence rate and adjusted risk ratio of hypersensitivity reactions, by biologic exposure and timing of exposure
| Biologic and Timing of Exposure | Biologic | Events | Incidence rate per 106 person days (95% CI) | Adjusted Risk Ratio* (95% CI) | Adjusted Risk Ratio** (95% CI) with 1st dose | |
|---|---|---|---|---|---|---|
| 0–1 days | IV | Abatacept | 16 | 41.1(25.2–67.1) | 7.1 (3.9 – 12.8) | 26.9 (11.3 – 64.0) |
| Golimumab | 0 | 0 (0,153703) | N/A | N/A | ||
| Infliximab | 48 | 145.1 (109.3– 192.5) | 26.9 (17.4 – 41.5) | 6.1 (1.6 – 22.7) | ||
| Rituximab | 16 | 239.5 (146.7, 390.9) | 18.0 (8.9 – 36.2) | 6.0 (1.9 – 18.3) | ||
| Tocilizumab | 13 | 155.5 (90.3, 267.8) | 22.2 (11.6 – 42.4) | 10.8 (2.3 – 51.0) | ||
| SQ | Abatacept | 0 | 0 (0, 175.8) | N/A | N/A | |
| Tocilizumab | 0 | 0 (0, 38425) | N/A | N/A | ||
| 2–14 days | IV | Abatacept | 6 | 2.4 (1.1, 5.4) | 0.4 (0.2 – 1.1) | 0.4 (0.1 – 3.2) |
| Golimumab | 0 | 0 (0, 25796) | N/A | N/A | ||
| Infliximab | 18 | 8.5 (5.4, 13.5) | 1.8 (1.0 – 3.1) | 2.7 (1.0 – 7.0) | ||
| Rituximab | 5 | 12.1 (5.1, 29.2) | 1.0 (0.4 – 2.8) | 1.4 (0.1 – 1.7) | ||
| Tocilizumab | 4 | 7.5 (2.8, 20.0) | 1.1 (0.4 – 3.0) | N/A | ||
| SQ | Abatacept | 0 | 0 (0, 27.6) | N/A | N/A | |
| Tocilizumab | 0 | 0 (0, 8107) | N/A | N/A | ||
| 15–30 days | IV | Abatacept | 10 | 4.3 (2.3, 8.1) | 0.8 (0.4–1.6) | N/A |
| Golimumab | 0 | 0 (0, 22493) | N/A | N/A | ||
| Infliximab | 15 | 6.6 (4.0, 10.9) | 1.4 (0.8–2.5) | 1.0 (0.1–8.1) | ||
| Rituximab | 4 | 13.2 (4.9, 35.1) | 0.8 (0.2–2.9) | 1.2 (0.3–4.9) | ||
| Tocilizumab | 5 | 9.1 (3.8, 21.7) | 1.3 (0.5–3.3) | 0.8 (0.1–6.6) | ||
| SQ | Abatacept | 1 | 7.7(1.1, 54.4) | N/A | N/A | |
| Tocilizumab | 0 | 0 (0, 16322) | N/A | N/A | ||
| 0–30 days, any injectable anti-TNF | 44 | 5.8 (4.3, 7.8) | Ref | |||
adjusting for age, gender, Charlson comorbidity score, concomitant steroid, methotrexate use and number of previous biologics
adjusted for all factors above, and restricted to only the first dose received
N/A = not applicable
The frequency distribution of HSRs occurrences according to number of administrations that patients received is shown in Figure 2. HSRs occurring within one day of administration were more likely to happen during the first administration for abatacept (68.8%) and rituximab (43.8%). In contrast, the highest frequency of HSRs occurred among infliximab users with the second administration (41.7%) and the third (30.8%) among tocilizumab users.
Figure 2.
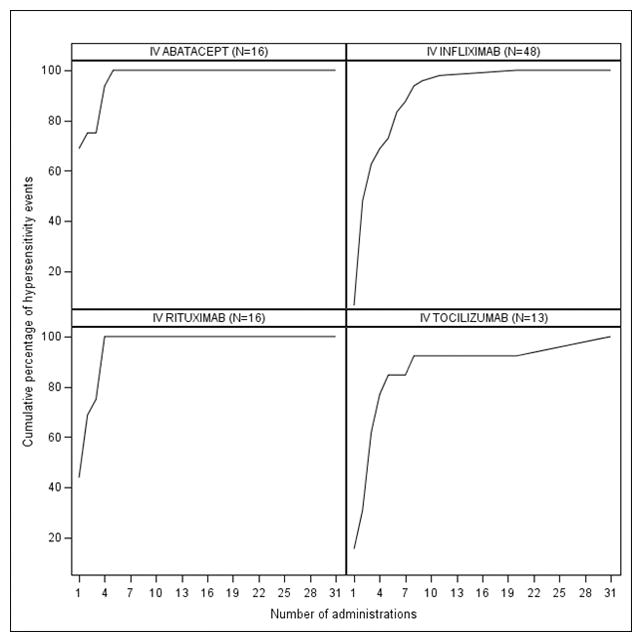
Frequency distribution of hypersensitivity reactions according to number of administrations received among patients experiencing a hypersensitivity reaction
Considering patients as the unit of analysis, the cumulative incidence of HSR in the first 6 months of treatment was 0.17% (abatacept), 0.37% (infliximab), 0.31% (rituximab), and 0.33% (tocilizumab).
The multivariable analysis that limited to first biologic administrations (Table 3) showed rituximab, tocilizumab, infliximab, and abatacept infusions were associated a significant higher risk of HSRs during 0–1 days of administration; the adjusted risk ratio for abatacept was highest (RR:26.9, 95% 11.3 – 64.0), followed by tocilizumab (RR:10.8, 95% 2.3 – 51.0), infliximab (RR:6.1, 95% 1.6 – 22.7), and rituximab (RR:6.0, 95% 1.9 – 18.3). These estimates for HSRs associated with the first infusion were based on small numbers of events and the confidence intervals were wide and overlapping.
The results of the nested case crossover analysis were qualitatively similar to those of the main analysis (Table 4). Compared to subcutaneous biologics, all infusion medications users were significantly associated with HSRs. Overall, rituximab had the strongest association with HSRs, followed by tocilizumab and infliximab.
Table 4.
Sensitivity analysis using nested case crossover design of hypersensitivity reactions associated with biologics for rheumatoid arthritis
| Administration type | Treatments | Comparison of exposure within 0–1 days of the event compared to other time periods Odds ratio (95% CI) |
Between-drug comparison, Odds Ratio (95% CI) |
|---|---|---|---|
| Intravenous | Abatacept | 13.2 (6.5 – 26.9) | 21.3 (6.0 – 168.6) |
| Infliximab | 15.4 (9.8 – 24.3) | 24.8 (9.1 – 121.7) | |
| Rituximab | 19.0 (8.3 – 73.8) | 30.6 (7.8 – 254.9) | |
| Tocilizumab | 15.8 (6.7 – 37.5) | 25.5 (6.4 – 218.8) | |
| Subcutaneous | Anti-TNF* | 0.62 (0.15– 2.56) | Ref (1.0) |
etanercept, adalimumab, certolizumab and golimumab
DISCUSSION
Healthcare providers providing care for RA patients have been alerted to the potential for drug HSRs, especially among patients receiving intravenous (IV) biologics. However, little is known about the incidence of more severe or life-threatening HSRs in a population-based setting (4). Our study found only one death occurring within 30 days associated with intravenous biologic use, as had already been reported (15). Overall, we found a low absolute incidence rate of HSRs among older RA patients. The cumulative incidence in the first 6 months of treatment was less than 1%, and events were more likely to occur with the first or second infusions. From highest to lowest, the incidence rates of HSRs associated with intravenous biologics were largest for rituximab, infliximab, tocilizumab and abatacept. Our sensitivity analysis using case-crossover design yielded similar results.
In the most commonly used classification, Gell and Coombs (21) divided HSRs into four types: 1) immediate or anaphylactic hypersensitivity, mediated by IgE, and usually occurring within 15–30 minutes from time of exposure, 2) cytotoxic hypersensitivity, primarily mediated by IgM or IgG antibodies and complement, with occurrence time from minutes to hours; 3) immune complex hypersensitivity, mediated by soluble immune complexes, mostly IgG, with occurrence time is 3–10 hours; 4) delayed hypersensitivity, mediated by T cells. Recently because some HSR cannot be explained in this classification, some researchers favor a more recent classification system proposed by Sell et al, which divides HSR into seven categories and accounts for multiple components of the immune system that could participate in various types of HSRs. Due to the limited validation studies for HSR events in administrative data, we did not distinguish the types of HSRs in current analyses. Instead, we used 30 days post biologic administration to capture all possible HSRs using validated algorithms. Our expectation was the severe HSRs would occur within 24 hours of administration, and this principle guided our selection of the relevant risk windows that we studied.
We found administration of infliximab, rituximab and tocilizumab were strongly associated with HSRs among RA patients taking biologics compared to injectable anti-TNFs. In a prior analysis that used data from 671 anti-TNF (include infliximab, adalimumab, etanercept) treated patients with auto-immune diseases, the highest reported frequency and severity of reaction occurred in patients treated with infliximab (5). Our finding that more than 60% of infliximab and rituximab related HSRs occurred during the first or second infusions, and risks decreased dramatically after the first cycle, are comparable to previous reports (22, 23). A ‘Dear Doctor’ letter reported a fatal HSR in a U.S. patient treated with tocilizumab, and the FDA recommended health providers to consider the diagnosis of hypersensitivity or anaphylaxis in any patient experiencing an infusion reaction during or following tocilizumab administration. We found that fatal HSRs were exceedingly rare, and did not observe further fatal events in this large cohort, suggesting that tocilizumab does not have a uniquely adverse safety profile (24, 25).
Prior work has evaluated and validated the claims-based definitions we used for accurately identifying drug related hypersensitivity. The FDA’s Mini-sentinel drug safety surveillance system (18) developed a claims-based algorithm and adjudicated HSR cases compared to the gold standard of medical record review. Of 122 potential cases, 93 patients experienced confirmed HSRs (including anaphylaxis and severe allergic reactions), yielding a positive predictive value (PPV) of 63.1% (95% CI, 67.7%–83.5) (18). We preferred this definition since prior studies using different algorithms to identify HSRs either did not report the PPV or had a very low PPV (26–29). We recognize that patients with milder events may not have been identified in claims data in a way that would satisfy our claims-based algorithm, and therefore this might underestimate the true incidence of less severe HSRs (18). Moreover, if “prn” (pro re nata) medications used to treat infusion reactions were not billed to insurance, under-ascertainment of HSRs was possible. However, conditional on any given severity of HSRs and given the expense of these medications, we would not expect differential misclassification between infusion biologics, such that our risk-ratios should therefore be biased toward the null. Additionally, our cumulative incidence results over the first 6 months of treatment are highly similar (between 0.1 to 1% of patients) to those reported in clinical trials (10–12, 30).
Although the multivariable adjusted model that only used the first administrations showed rituximab, tocilizumab, infliximab, and abatacept infusions all were associated a significant higher risk of HSRs during 0–1 days of administration, but the rank order and values of the adjusted risk ratios were somewhat different than the main analysis that used all administrations. Abatacept-associated HSRs occurring within one day of administration were more likely to happen during the first administration, whereas infliximab associated HSRs were more likely to happen during the second administration. Likewise, tocilizumab associated HSR were likely to happen during subsequent administrations.
Similarly, sensitivity analysis using a nested case control study design also showed that rituximab, tocilizumab, infliximab, and abatacept infusions were associated with a significantly higher risk of HSRs compared to injectable anti-TNFs. However, the magnitude of the adjusted risk ratios and the rank order were not in a complete agreement with our main analysis. In the main analysis, infliximab had the highest risk ratio, followed by tocilizumab, rituximab and abatacept, whereas in the nested case crossover analysis, rituximab had the highest and followed by tocilizumab, infliximab and abatacept. The possible reasons for the difference could be 1) the nested case-crossover study could better control between-person confounders; 2) the referent group in the case-crossover study compared exposure within 0–1 days of the event to other time periods for the same patient, whereas in the cohort study, the referent group was patients receiving injectable biologics. Thus, the background rate for the referent groups differed between the two study designs; and finally, 3) the cohort design evaluated all 30 day periods after each biologic administration, whereas the nested case crossover design restricted the analysis only to administrations after which a HSR event occurred.
The results of this study need to be considered in light of several limitations. First, patients were not randomized into different exposure groups and so has limitations common to observational analysis. For example, patients with a HSR prior to enrollment in Medicare could be preferentially channeled to receive abatacept, a drug historically associated with a lower rate of HSRs. Additionally, we used administrative data, which lacked information on disease severity and lifestyle factors, and thus residual confounding is possible. To provide some reassurance on this point, our sensitivity analysis using a case crossover design minimized between-person confounding and yielded similar results. We were not able to confirm hypersensitivity events with medical records, although we relied on a validated algorithm developed as part of the FDA Mini-Sentinel system, and any misclassification is unlikely to be differential by drug exposure. We did not distinguish the types of HSRs because administrative data did not allow for such distinction. Finally, as Medicare RA patients are older, our results may not be able to generalizable to patients who are younger or covered by commercial insurance.
In conclusion, we found that the absolute rate of HSRs for all biologics was low among RA patients taking biologics. However, compared to subcutaneous biologics, administration of intravenous biologics was associated with increased risk of HSRs. Infliximab, rituximab and IV tocilizumab were most strongly associated with immediate hypersensitivity events. Because the risk of serious HSR is the highest in the first several administrations, particular vigilance needs to be paid with early administrations, yet patients remain at risk throughout the course of therapy.
Significance and Innovation.
The absolute rate and cumulative incidence over 6 months of HSRs for all biologics was low (i.e. < 1%) among RA patients taking biologics.
However, administration of intravenous biologics was associated with an increased risk of HSRs compared to subcutaneous TNFi therapy
Hypersensitivity events were more likely to occur after the first or second infusions.
Infliximab, rituximab and tocilizumab were most strongly associated with hypersensitivity events.
Footnotes
Contributions:
Substantial contributions to study design conception and design: Huifeng Yun, Fenglong Xie, Randall N. Beyl, Lang Chen, James D. Lewis, Kenneth G Saag, Jeffrey R Curtis
Substantial contributions to acquisition of data: Huifeng Yun, Fenglong Xie, Fenglong Xie, Jeffrey R Curtis
Substantial contributions to analysis and interpretation of data: Huifeng Yun, Fenglong Xie, Lang Chen, James D. Lewis, Kenneth G Saag, Jeffrey R Curtis
Drafting the article or revising it critically for important intellectual content: Huifeng Yun, Randall N. Beyl, James D. Lewis, Kenneth G Saag, Jeffrey R Curtis
Final approval of the version of the article to be published: Huifeng Yun, Fenglong Xie, Randall N. Beyl, Lang Chen, James D. Lewis, Kenneth G Saag, Jeffrey R Curtis
Disclosures:
- Funding: Agency for Healthcare Research and Quality (AHRQ) (R01 HS018517) and the Patient Centered Outcomes Research Institute (PCORI)
- Dr. Yun was supported by grant 1 K12 HS021694 from AHRQ and has research grant from Amgen for unrelated work
- JRC receives support from the NIH P60 (AR064172) and has research grants and/or consulting for unrelated work with Amgen, BMS, CORRONA, Janssen, Pfizer, UCB
- JDL: Research grants and/or consulting for unrelated work with Shire, Takeda, Centocor, Amgen, Millennium Pharmaceuticals, Pfizer, Abbott, Prometheus, Nestle, Lilly, Gilead, Merck, Janssen
- KGS: Research grants and/or consulting for unrelated work with Roche/Genentech, Abbvie, BMS, Bayer, Merck,
References
- 1.Pichler WJ, Adam J, Daubner B, et al. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. The Medical clinics of North America. 2010;94(4):645–64. xv. doi: 10.1016/j.mcna.2010.04.003. Epub 2010/07/09. [DOI] [PubMed] [Google Scholar]
- 2.Leach MW, Rottman JB, Hock MB, et al. Immunogenicity/hypersensitivity of biologics. Toxicologic pathology. 2014;42(1):293–300. doi: 10.1177/0192623313510987. Epub 2013/11/19. [DOI] [PubMed] [Google Scholar]
- 3.Galvao VR, Castells MC. Hypersensitivity to biological agents-updated diagnosis, management, and treatment. The journal of allergy and clinical immunology In practice. 2015;3(2):175–85. doi: 10.1016/j.jaip.2014.12.006. quiz 86. Epub 2015/03/11. [DOI] [PubMed] [Google Scholar]
- 4.Steenholdt C, Svenson M, Bendtzen K, et al. Acute and delayed hypersensitivity reactions to infliximab and adalimumab in a patient with Crohn’s disease. Journal of Crohn’s & colitis. 2012;6(1):108–11. doi: 10.1016/j.crohns.2011.08.001. Epub 2012/01/21. [DOI] [PubMed] [Google Scholar]
- 5.Puxeddu I, Giori L, Rocchi V, et al. Hypersensitivity reactions during treatment with infliximab, etanercept, and adalimumab. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2012;108(2):123–4. doi: 10.1016/j.anai.2011.11.004. Epub 2012/02/01. [DOI] [PubMed] [Google Scholar]
- 6.ENBREL (etanercept), package insert. Immunex Corp; Calif, USA: 2013. [Google Scholar]
- 7.HUMIRA (adalimumab), package insert. Abbott Laboratories; III, USA: 2008. [Google Scholar]
- 8.SIMPONI (golimumab), package insert. Centocor Ortho Biotech, Inc; Pa, USA: 2009. [Google Scholar]
- 9.CIMZIA (certolizumab pegol), package insert. Inc UCB; Ga, USA: 2013. [Google Scholar]
- 10.ORENCIA (abatacept), package insert. Bristol-Myers Squibb; NJ, USA: 2005. [Google Scholar]
- 11.Rituxan (rituximab), package insert. Genentech, Inc; CA, USA: 2014. [Google Scholar]
- 12.ACTEMRA (tocilizumab), package insert. Genentech, Inc; CA, USA: 2014. [Google Scholar]
- 13.Hong DI, Bankova L, Cahill KN, et al. Allergy to monoclonal antibodies: cutting-edge desensitization methods for cutting-edge therapies. Expert review of clinical immunology. 2012;8(1):43–52. doi: 10.1586/eci.11.75. quiz 3–4. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 14.Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63(10):2854–64. doi: 10.1002/art.30463. Epub 2011/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [assessed on July 6th, 2015];ACTEMRA (tocilizumab) - risk of fatal anaphylaxis - for health professionals: Health canada endorsed important safety information on ACTEMRA (Tocilizumab) http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2010/14586a-eng.php.
- 16.Chronic Conditions Data Warehouse. [Accessed on Feb11, 2014]; https://www.ccwdata.org/web/guest/about-ccw.
- 17.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–8. [Google Scholar]
- 18.Walsh KE, Cutrona SL, Foy S, et al. Validation of anaphylaxis in the Food and Drug Administration’s Mini-Sentinel. Pharmacoepidemiol Drug Saf. 2013;22(11):1205–13. doi: 10.1002/pds.3505. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maclure M, Mittleman MA. Should we use a case-crossover design? Annual review of public health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. Epub 2000/07/08. [DOI] [PubMed] [Google Scholar]
- 20.Wang SV, Gagne JJ, Glynn RJ, et al. Case-crossover studies of therapeutics: design approaches to addressing time-varying prognosis in elderly populations. Epidemiology. 2013;24(3):375–8. doi: 10.1097/EDE.0b013e31828ac9cb. Epub 2013/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gell PGH, Coombs RRA. The classification of allergic reactions underlying diseases. In: Coombs RRR, Gells PGH, editors. Clinical Aspects of Immunology. Oxford, England: Blackwell; 1963. [Google Scholar]
- 22.Campbell P, Marcus R. Monoclonal antibody therapy for lymphoma. Blood reviews. 2003;17(3):143–52. doi: 10.1016/s0268-960x(03)00005-5. Epub 2003/06/24. [DOI] [PubMed] [Google Scholar]
- 23.Rituxan (rituximab) [Package insert] South San Francisco: Genentech, Inc; 2005. [Google Scholar]
- 24.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70(12):2148–51. doi: 10.1136/ard.2011.151092. Epub 2011/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocchi V, Puxeddu I, Cataldo G, et al. Hypersensitivity reactions to tocilizumab: role of skin tests in diagnosis. (letters to the editior) Rheumatology. 2014;53:1527–9. doi: 10.1093/rheumatology/keu181. [DOI] [PubMed] [Google Scholar]
- 26.Bohlke K, Davis RL, DeStefano F, et al. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. The Journal of allergy and clinical immunology. 2004;113(3):536–42. doi: 10.1016/j.jaci.2003.11.033. Epub 2004/03/10. [DOI] [PubMed] [Google Scholar]
- 27.Johannes CB, Ziyadeh N, Seeger JD, et al. Incidence of allergic reactions associated with antibacterial use in a large, managed care organisation. Drug safety. 2007;30(8):705–13. doi: 10.2165/00002018-200730080-00007. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 28.Miller DR, Oliveria SA, Berlowitz DR, et al. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51(6):1624–30. doi: 10.1161/HYPERTENSIONAHA.108.110270. Epub 2008/04/17. [DOI] [PubMed] [Google Scholar]
- 29.Brown NJ, Ray WA, Snowden M, et al. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clinical pharmacology and therapeutics. 1996;60(1):8–13. doi: 10.1016/S0009-9236(96)90161-7. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 30.Remicade (infliximab), package insert. Janssen Biotech, Inc; PA, USA: 2015. [Google Scholar]


