Abstract
Alcohol and nicotine intake result in neurological alterations at the circuit level. Resting state functional connectivity has shown great potential in identifying these alterations. However, current studies focus on specific seeds and leave out many brain regions where effects might exist. The present study uses a data driven technique for brain segmentation covering the whole brain. Functional magnetic-resonance-imaging (fMRI) data were collected from 188 subjects: 51 non-substance consumption controls (CTR), 36 smoking-and-drinking subjects (SAD), 28 drinkers (DRN), and 73 smokers (SMK). Data were processed using group independent component analysis to derive resting state networks (RSN). The resting state functional network connectivity (rsFNC) was then calculated through correlation between time courses. One-way ANOVA tests were used to detect rsFNC differences among the four groups. A total of 50 ANOVA tests were significant after multi-comparison correction. Results delineate a general pattern of hypo-connectivity in the substance consumers. Precuneus, postcentral gyrus, insula and visual cortex were the main brain areas with rsFNC reduction suggesting reduced interoceptive awareness in drinkers. In addition, connectivity reduction between postcentral and one RSN covering right fusiform and lingual gyri showed significant association with severity of hazardous drinking. In smokers, connectivity changes agreed with the idea of a shift towards endogenous information processing, represented by the DMN. Hypo-connectivity between thalamus and putamen was observed in smokers. In contrast, the angular gyrus showed hyper-connectivity with the precuneus linked to smoking and significantly correlated with nicotine dependence severity. In spite of the presence of common effects, our results suggest that particular effects of alcohol and nicotine can be separated and identified. Results also suggest that concurrent use of both substances affects brain connectivity in a complex manner, requiring careful consideration of interaction effects.
Keywords: Functional network connectivity, fMRI, alcohol use disorder, nicotine dependence
Graphical abstract
1 Introduction
Consumption of alcohol and nicotine results in neurological alterations that may promote continued use of both drugs (Doyon et al., 2013). These alterations are grounded in circuit level changes that can be evaluated through functional connectivity (FC) among specific brain regions (Sutherland et al., 2012). FC studies have found relationships between the intake of alcohol and cognitive, motor and coordination dysregulations (Camchong et al., 2013; Chanraud et al., 2011). Other studies suggest a link between brain FC and motor, attention and memory enhancement due to nicotine administration (Jasinska et al., 2013). In light of these advances, patterns of brain connectivity in alcohol and nicotine users have begun to be investigated as possible diagnostic biomarkers (Camchong et al., 2012; Pariyadath et al., 2014). Understanding how these drugs induce changes in the FC of the brain might be critical for the development of addiction treatment and the improvement of clinical outcomes.
Alcohol consumption causes gradual neurocognitive deficits ranging from mild deficits in social drinkers to profound memory and abstract processing impairments in chronic alcoholics (Parsons, 1998). Alcohol studies based on functional magnetic resonance imaging (fMRI) have found altered functional relationships and corresponding compensatory mechanisms among different parts of the brain linked to these deficits. For example, a finger tapping task demonstrated that alcohol dependent patients recruited areas of the parietal lobe, inferior frontal, middle temporal, pre- and post-central gyri to compensate for a dysfunctional fronto-cerebellar network used by normal controls (Parks et al., 2010). Connectivity between the cerebellum and the default mode network (DMN) is reduced in alcoholics during resting state, but increased during a spatial working memory task to compensate and achieve same performance than controls (Chanraud et al., 2011). Attenuated insula activation, observed during an emotional processing task, may be linked to reduced interoceptive awareness (Padula et al., 2011). Even after long-term alcohol abstinence, it has been found that resting state FC differs compared to controls (Camchong et al., 2013). Decreased resting state connectivity within reward, visual and executive control brain regions successfully predicts relapse in abstinent alcoholics, further evidence of the importance of examining FC (Camchong et al., 2012). A decrease of FC in executive control, sensorimotor, visual and subcortical was found in alcoholics when compared to controls (Weiland et al., 2014). Results from all these studies lead to the proposal of a “disconnection syndrome” which can be involved in the deficient behavioral control related to alcohol use disorder (Dupuy and Chanraud, 2016). Although the authors suggest that some effects could have been influenced by smoking, evidence for the existence of this disconnection pattern involving DMN, salience, subcortical and executive control networks was presented in (Müller-Oehring et al., 2014) where a whole brain analysis was performed using seed-based methods. Given the common use of alcohol and nicotine, further studies are necessary to characterize FC changes due to alcohol use not influenced by nicotine.
Nicotine is an addictive substance that is known to enhance cognitive function during acute administration whereas acute withdrawal results in cognitive impairment (Levin et al., 2006). Cole et al. (Cole et al., 2010) found that cognitive withdrawal improvement, measured using an rapid visual information-processing task (Wesnes and Warburton, 1983), observed after nicotine replacement is associated with increased anti-correlation between default mode and executive control networks. While cognitive improvement could be linked to increased connectivity effects within executive control and fronto-parietal networks, reduced connectivity has also been found within networks involving the posterior cingulate and precuneus (Pariyadath et al., 2014). In the same study, Pariyadath et al. found that both increased and decreased connectivity helps in predicting smoking status using a support-vector-machine classifier. Decreased DMN and enhanced extra-striate activity during a resting state experiment with administered nicotine have been theorized to be linked with an activity shift from internal to external information processing networks (Tanabe et al., 2011). In contrast, a model of nicotine effects during abstinence based on resting state FC results suggests a shift in network dynamics towards endogenous information processing represented by the DMN where the insula plays an important salience role (Sutherland et al., 2012). The evidence points to abnormal interactions due to nicotine addiction between brain networks of exogenous (executive control) and endogenous (DMN) attention regulated by a third salience network anchored in the insula (Fedota and Stein, 2015).
These results suggest distinctive patterns of FC change related to the use of alcohol and nicotine. One limitation of these important results is the use of predetermined regions of interest or seeds. As well described by Li, et al. (Li et al., 2009), seed-based methodology implies that detected effects in FC are sensitive to the selected seed and the knowledge used to determine the seed. Thus, it is also important to complement previous work with approaches that are less dependent on a specific seed assumption. In this work we utilize group independent component analysis (gICA) (Calhoun et al., 2001) to obtain a data driven parcellation of the brain into spatial-temporal components. Brain regions are segregated into maximally spatially independent components or networks (Erhardt et al., 2011a) in a fully data-driven manner. Using resting state fMRI data as input, the gICA algorithm provides a set of resting state networks (RSN), each composed of coherently interacting brain areas, each of which is a reflection of ‘within network’ connectivity (Joel et al., 2011). The temporal correlation between RSN time courses constitutes a measure of “among network” connectivity, called functional network connectivity (Jafri et al., 2008).
Another common limitation found in some FC studies is the lack of a clear separation between alcohol and nicotine consumption. Although it is known that the effects of alcohol and nicotine are moderately to strongly related (Istvan and Matarazzo, 1984), combined and separate influences of nicotine and alcohol use in resting state FC have not been thoroughly studied. In this work, we have carefully selected subjects that smoke nicotine and drink alcohol in a separate or combined manner. We hypothesize that different patterns of FC exist depending on drinking, smoking or combined drinking and smoking. Based on previous research we expect to see decreased connectivity associated with reward, executive control, default mode and visual brain areas in alcohol users (Camchong et al., 2012; Chanraud et al., 2011; Weiland et al., 2014). With respect to nicotine use, both increases and decreases in connectivity effects are anticipated for cingulated cortex, precuneus, and executive function areas (Pariyadath et al., 2014). Common neural pathways for drug dependence exist, but alcohol and nicotine have different mechanisms of action. While nicotine seems to be able to directly activate dopamine neurons in the ventral tegmental area, alcohol may indirectly produce a similar effect by inhibiting GABAergic receptors (Nestler, 2005). In this sense, we expect to observe both common and different brain connectivity effects in smoking and drinking subjects depending on their similar effects in the reward system, but exerted through different pathways.
The motivation for our data-driven approach is to confirm the patterns of dysfunction linked to alcohol (general disconnection) and nicotine (network dysfunction) derived from seed based analyses. Instead, we expect to observe these patterns of intrinsic connectivity using a whole brain analysis. We also expect to identify aspects of network dysfunction not previously found in the literature that can help refining future studies of substance use. Another goal is to find the main effects that discriminate between substances as well as effects that characterize their interaction. We chose to use the gICA approach because it is especially useful for capturing both specific and unique aspects of the connectivity.
2 Materials and Methods
2.1 Subjects
Data were collected from 188 subjects: 71 females and 117 males between the ages of 18 and 54 (33.3 ± 9.3) years. Subjects were excluded if there was injury to the brain, brain-related medical problems, bipolar or psychotic disorders. In addition, the exclusion criterion includes use of illicit drugs confirmed or rejected by urinalysis.
Four groups were defined according to different levels of substance dependence: control (CTR), drinker (DRN), smoker (SMK), and smoking and drinking (SAD). The DRN group is composed of 28 subjects that scored 8 or greater on the Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al., 1993). The maximum Fagerstrom Test for Nicotine Dependence (FTND) score for the DRN group is 4. The SMK group includes 73 smokers with a FTND score greater than 7 (Fagerstrom et al., 1990). The SAD group consists of 36 subjects that scored greater than 8 on the AUDIT and greater than 7 on the FTND. There is no significant difference in AUDIT score between SAD and DRN groups and there is no significant difference of FTND score between SAD and SMK groups. Detailed information about the four groups can be found in Table 1. The threshold used to select subjects for the DRN have been used before to detect hazardous drinking (Vergara et al., 2016; Weiland et al., 2014) and corresponds to the minimum criteria for at-risk drinking (Babor et al., 2001). The SMK group was selected with a more conservative threshold than previously utilized for training of machine learning algorithms (Pariyadath et al., 2014). Other relevant data collected included the Beck Depression Inventory (BDI) (Beck et al., 1988), the Impulsive Sensation Seeking Scale (ImpSS) (Zuckerman, 1996) and income as socio-economic status variable. Income was categorized in 7 levels: 1-$0-$9,999/year, 2-$10,000-$19,999/year, 3-$20,000-$29,999/year, 4-$30,000-$39,999/year, 5-$40,000-$49,999/year, 6-$50,000-$59,999/year, and 7-Over $60,000/year.
Table 1.
Subject Demographics by Groups.
| CTR | SMK | DRN | SAD | |||||
|---|---|---|---|---|---|---|---|---|
| Size | 51 | 73 | 28 | 36 | ||||
| Females | 18(35.3%) | 29(39.7%) | 12(42.9%) | 12(33.3%) | ||||
| µ | σ | µ | σ | µ | σ | µ | σ | |
| Age | 34.6 | 11.3 | 33.9 | 9.2 | 29.8 | 7.6 | 33.0 | 6.7 |
| *FTND | 0 | 0 | 9.0 | 1.4 | 2.96 | 0.9 | 9.0 | 1.5 |
| *AUDIT | N/A | N/A | 2.8 | 2.15 | 15.3 | 5.3 | 14.8 | 6.5 |
| *BDI | N/A | N/A | 5.1 | 5.7 | 8.7 | 6.5 | 8.0 | 7.16 |
| *ImpSS | N/A | N/A | 8.0 | 4.6 | 9.4 | 4.3 | 11.0 | 5.9 |
| Income | N/A | N/A | 2.8 | 1.8 | 2.4 | 1.6 | 2.9 | 1.8 |
The 188 subjects were grouped according to substance use into controls (CTR) that do not drink or smoke, smoking and drinking subjects (SAD) that use both substances, pure drinkers (DRN) and pure smokers (SMK) comprised of subjects that regularly use one of the two substances. Group differences were assessed using one way ANOVA. Significant ANOVA tests (p < 0.05) are indicated with an asterisk *. Because of missing data for the CTR group, ANOVAs for AUDIT, BDI, ImpSS and Income included SMK, DRN and SAD groups only.
The CTR group included 51 healthy individuals with no history of substance abuse/dependence assessed using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) (First et al., 2002). Control subjects with current abuse or dependence of alcohol were excluded. All control subjects reported themselves as non-smokers and scored zero for the FTND. None of the control subjects reported use of marijuana. AUDIT, BDI, ImpSS and income measures were not collected for this group.
2.2 MRI Data
Resting state functional MRI data were collected on a 3T Siemens TIM Trio (Erlangen, Germany) scanner. Participants kept their eyes open during the 5-minute resting scan. Echo-planar EPI sequence images (TR = 2,000 ms, TE = 29 ms, flip angle = 75°) were acquired with an 8-channel head coil. Each volume consisted of 33 axial slices (64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap). Resting state fMRI data were preprocessed using the statistical parametric mapping software (SPM; http://www.fil.ion.ucl.ac.uk/spm) (Friston, 2003) including slice-timing correction, realignment, co-registration and spatial normalization. Images were transformed to the Montreal Neurological Institute (MNI) standard space. The first five scans were discarded to allow for T1 equilibration. The DVARS method (Power et al., 2012) was used to find spike regressors where the root mean square (RMS) head movement exceeded 3 standard deviations. Time courses, with a size of 145 time steps, were orthogonalized with respect to i) linear, quadratic and cubic trends; ii) the 6 realignment parameters and iii) realignment parameters derivatives. The decision to pre-process these nuisances at this point is based on recent recommendations in the field (Vergara et al., 2016). The fMRI data were smoothed using a FWHM Gaussian kernel of size 6 mm. The data were then analyzed with Infomax based gICA (Calhoun et al., 2001; Calhoun and Adali, 2012) with 120 and 100 components for the first and second decomposition levels respectively (Erhardt et al., 2011b). The number of components was determined using ICASSO (Himberg et al., 2004) such that its R-index is close to the minimum and the quality index for any given component is above 0.7. A total of 39 components out of the 100 estimated components were selected based on frequency content and visual inspection in order to include components that were low noise and free of major artifacts (Allen et al., 2011). These 39 components were considered the RSNs of interest. Spatial maps of the 39 components were z-transformed in order to identify the main brain areas included in each RSN. Time courses were then filtered using a band-pass filter 0.01 to 0.15 Hz. Finally, resting state functional network connectivity (rsFNC) matrices were calculated for each subject based on the correlation coefficients between the time courses of all possible pairs formed with the 39 chosen components. Spike time courses were censored from the calculation of correlation coefficients.
2.3 Functional Connectivity Analysis
The Fisher transformation (Fisher, 1915) was applied to all rsFNC values prior to statistical testing. Age and sex differences were regressed out for each rsFNC along the subject dimension. The rsFNC matrices were then organized into the four groups: CTR, DRN, SMK and SAD. Group differences were evaluated for each rsFNC pair using one-way ANOVA. Statistical significance for each ANOVA was assessed at the p < 0.05 level and corrected using false discovery rate (FDR) multiple comparison correction. Post hoc analyses based on the Fisher's least significant difference procedure (Hayter, 1986) were applied to those ANOVA results surviving FDR correction.
The rsFNCs with significant group differences, tested using one-way ANOVA, were further analyzed for significant relationship with AUDIT and FTND variables. A regression model was implemented separately for different sample groups, where the original Fisher transformed rsFNC data was utilized as the dependent variable for each of the regression models, and confounding factors sex, age, BDI, ImpSS and income were included as independent variables. FTND as an additional independent variable was included for subjects in the SMK group. AUDIT was similarly tested for the DRN group. In the case of the SAD group, the interaction between AUDIT and FTND was added.
3 Results
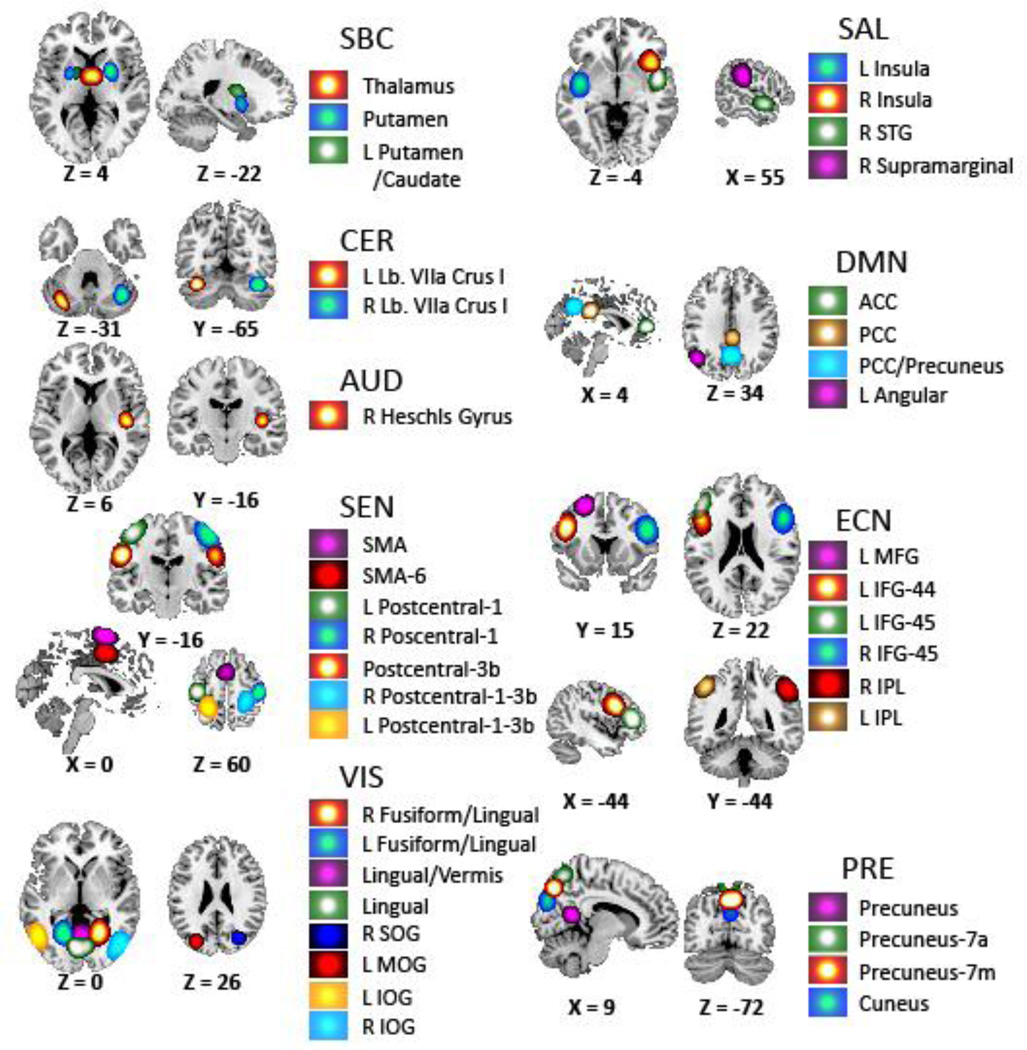
Figure 1 shows spatial maps for the set of 39 selected RSNs and Table 2 displays their peak activation coordinates. The RSN groups reflect RSN functions and anatomies following the strategy presented in previous rsFNC studies (Allen et al., 2011). Considered RSN groups are subcortical (SBC), cerebellum (CER), auditory (AUD), sensorimotor (SEN), visual (VIS), salience (SAL), default mode network (DMN), executive control network (ECN) and precuneus (PRE). Spatial overlap with functional brain areas were confirmed by visual comparison with the 90 spatial maps defined by Shirer (Shirer et al., 2012) and by running peak activation coordinates through the meta-analysis software publicly available at http://www.neurosynth.org/. RSNs with peak activations in thalamus and putamen constitute the SBC group. The CER group comprises RSNs with activations on left and right lobule VIIa. The auditory RSN is located at the Heschl’s gyrus. The SEN group covers part of the supplementary motor area (SMA) and large portions of the postcentral gyrus. Fusiform, lingual, superior, middle and inferior occipital gyri are included in the VIS group. One VIS RSN labeled Lingual/Vermis is a complex network that embraces the lingual gyrus as well as the cerebellar vermis. Areas on the left and right insula, superior temporal and supramarginal gyri are included in the SAL group. The DMN group is comprised of anterior cingulate cortex (ACC), angular gyrus and posterior cingulate cortex (PCC) with some precuneus overlap. The ECN group is composed of fronto-parietal regions including inferior parietal lobule (IPL), inferior frontal gyrus (IFG) and middle frontal gyrus (MFG). RSNs in the PRE group cover regions of the precuneus including overlap with the cuneus.
Figure 1.
Set of 39 RSNs considered in this work. The figure was obtained using the spatial t-maps of each RSN. This set has been selected from a gICA decomposition with 100 components. A total of 61 gICA components were discarded based on frequency spectrum, peak activation located outside grey matter areas and visual inspection. Peak activation coordinates can be found in Table.
Table 2.
List of the 39 RSNs and peak activations.
| RSN Group | Brain Region | MNI-X | MNI-Y | MNI-Z |
|---|---|---|---|---|
| SBC | Thalamus | 0 | −7 | 8 |
| SBC | Putamen | 24 | 6 | −4 |
| SBC | L Putamen/Caudate | −18 | 0 | 9 |
| CER | L Lb. VIIa Crus I | −32 | −73 | −38 |
| CER | R Lb. VIIa Crus I | 40 | −64 | −37 |
| AUD | R Heschl’s Gyrus | 42 | −19 | 6 |
| SEN | Postcentral-3b | 55 | −12 | 32 |
| SEN | SMA-6 | 0 | 0 | 52 |
| SEN | L Postcentral-1 | −42 | −28 | 59 |
| SEN | SMA | 0 | −1 | 74 |
| SEN | R Postcentral-1 | 46 | −27 | 57 |
| SEN | L Postcentral-1-3b | −24 | −47 | 69 |
| SEN | R Postcentral-1-3b | 28 | −38 | 70 |
| VIS | R Fusiform/Lingual | 22 | −59 | −11 |
| VIS | L Fusiform/Lingual | −20 | −59 | −11 |
| VIS | Lingual | 0 | −79 | 5 |
| VIS | L MOG | −30 | −85 | 15 |
| VIS | Lingual/Vermis | 0 | −66 | −11 |
| VIS | R SOG | 28 | −74 | 42 |
| VIS | R IOG | 48 | −68 | −11 |
| VIS | L IOG | −48 | −65 | −16 |
| SAL | R Insula | 40 | 19 | −6 |
| SAL | R Supramarginal | 61 | −32 | 27 |
| SAL | L Insula | −44 | −10 | 2 |
| SAL | R STG | 48 | 3 | −12 |
| DMN | ACC | 0 | 45 | 5 |
| DMN | L Angular | −44 | −68 | 36 |
| DMN | PCC | 0 | −38 | 26 |
| DMN | PCC/Precuneus | 0 | −63 | 31 |
| ECN | L IFG-44 | −44 | 13 | 33 |
| ECN | L IPL | −48 | −44 | 50 |
| ECN | R IFG-45 | 48 | 17 | 29 |
| ECN | L IFG-45 | −44 | 39 | 15 |
| ECN | L MFG | −24 | 3 | 61 |
| ECN | R IPL | 48 | −52 | 45 |
| PRE | Precuneus-7m | 0 | −76 | 44 |
| PRE | Cuneus | 0 | −86 | 28 |
| PRE | Precuneus-7a | 0 | −69 | 62 |
| PRE | Precuneus | 0 | −56 | 10 |
RSNs were categorized in the following functional groups: SBC (subcortical), CER (cerebellum), AUD (auditory), SEN (sensorimotor), VIS (visual), SAL (salience), DMN (default mode network), ECN (executive control network) and PRE (precuneus).
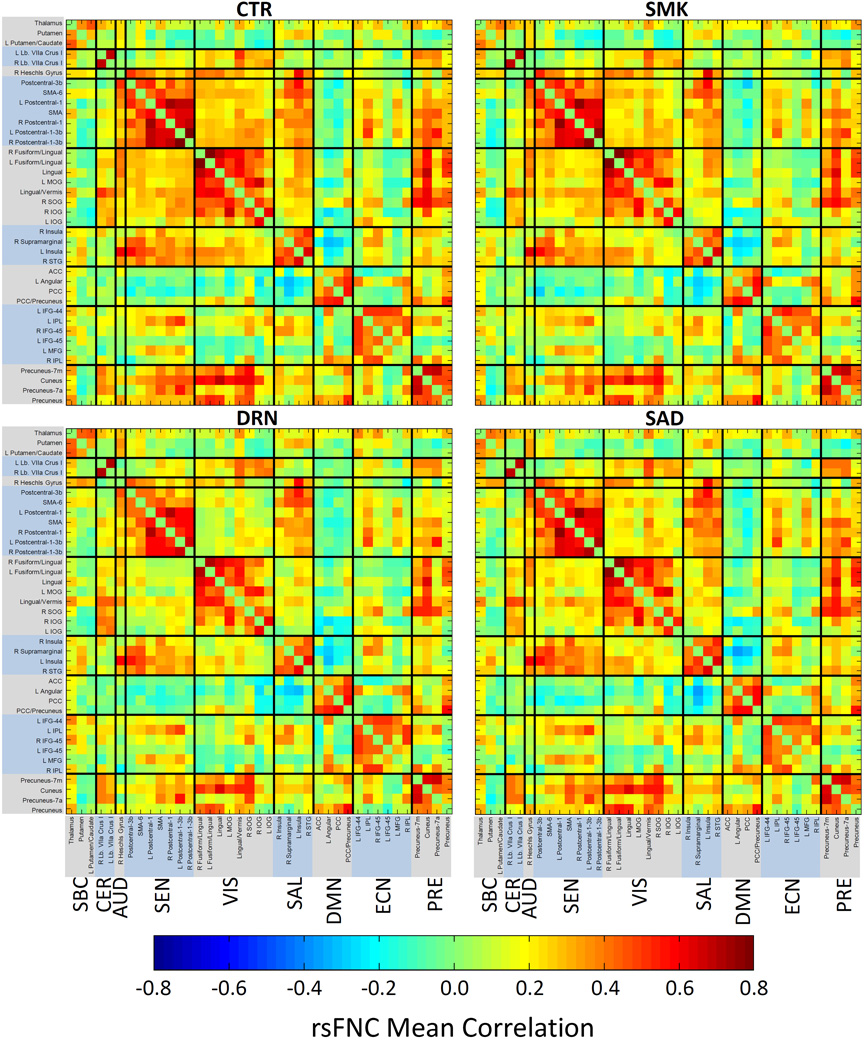
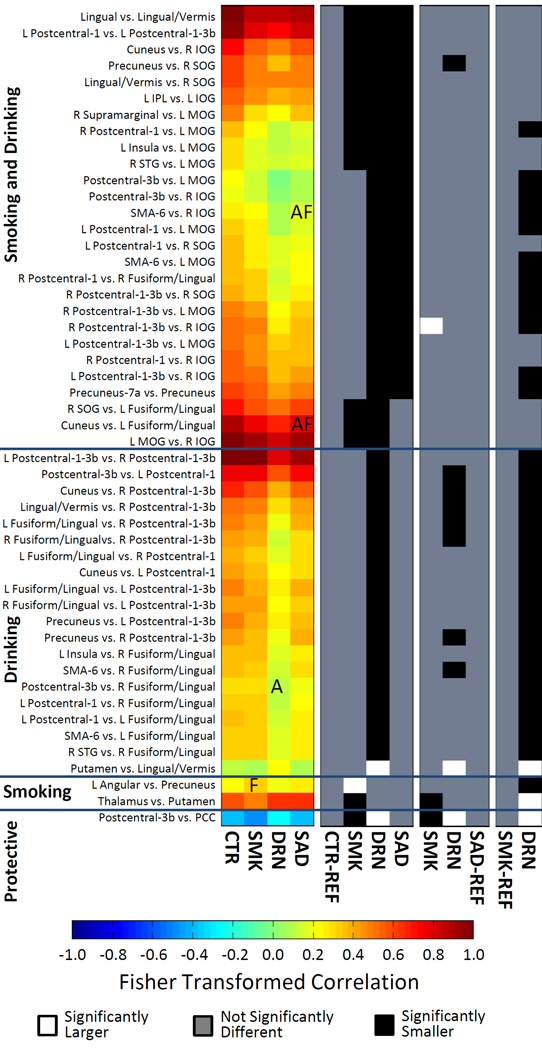
Figure 2 displays the mean rsFNC matrices for CTR, SMK, DRN, and SAD groups. Compared to the CTR matrix, DRN and SAD matrices show a clear pattern of decreased rsFNC between VIS-SEN, VIS-SAL, and SEN-PRE RSN groups. The differences between rsFNC matrices of CTR and SMK groups are few and difficult to spot. The most notable difference is an anti-correlation increase between PCC and Precentral-3b RSNs in the SMK group. The ANOVA test revealed 50 rsFNCs with significant group differences after FDR correction (p < 0.05). Supplementary Figure 1 shows ANOVA tables for the aforementioned results. The histogram presented in Figure 3 summarizes the relevant information contained in the post-hoc outcomes for a cleaner presentation of the observed effects. The significant rsFNC differences were catalogued as driven by smoking and drinking, drinking, or smoking. rsFNC differences where the SMK group was different from the CTR group were considered as driven by smoking. rsFNC differences were considered as driven by drinking when the DRN group was different from the CTR group. If the SAD group was different from the CTR group, or both SMK and DRN groups were different from the CTR group, the rsFNC difference was designated as driven by smoking and drinking. The rsFNC between Postcentral-3b and PCC was a special case because the SMK group showed increased anti-correlation, the DRN group decreased anti-correlation, but the SAD group exhibited no difference when compared to the CTR group. We labeled this case as protective in a separate group with one rsFNC.
Figure 2.
Mean rsFNC matrices for each sample group. CTR and SMK matrices are very similar with very few differences difficult to identify. The DRN and SAD matrices exhibit stronger differences compared to CTR between VIS-SEN, VISSAL and SEN-PRE groups.
Figure 3.
Histogram summarizing significant rsFNC differences. The Fisher r-to-z transformation was applied to all correlation values and the color scale was restricted to the range [−1 1]. Results were classified according to the significance found on the sample groups. In those driven by “Smoking and Drinking” either the SAD group was different from CTR or both DRN and SMK groups were different. Driven by Drinking: only DRN was different from CTR. Driven by Smoking: only SMK was different from CTR. Significance is presented in the black and white histograms on the right. In those, one of the groups is set as baseline or reference and labeled as (REF). Significant relationship with AUDIT is labeled as “A”, with FTND as “F”, and the interaction AUDIT-FTND as “AF”.
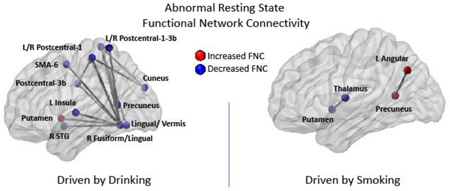
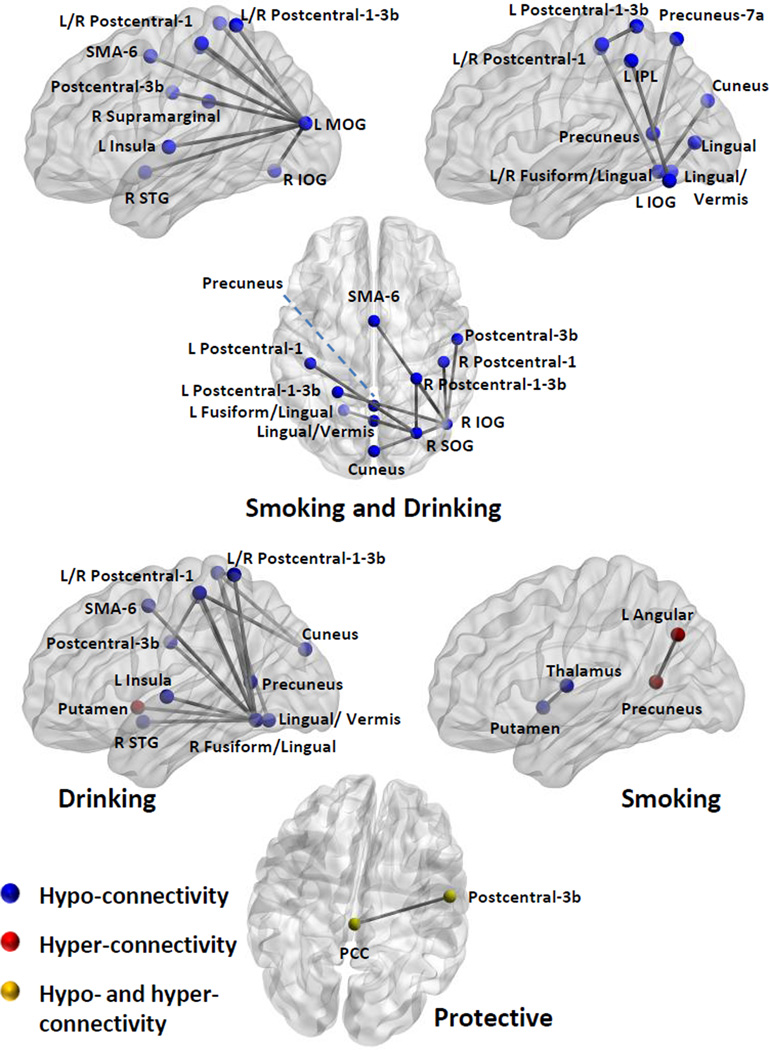
Where possible, results in the histogram of Figure 3 were organized around rsFNCs with repeated RSNs. This organization allowed us to identify RSN patterns of interest and perform an organized interpretation of the results. Figure 4 displays the spatial location of RSNs appearing in Figure 3 with their rsFNCs represented by edges. In the ANOVAs driven by smoking and drinking, the left middle occipital gyrus (L MOG) RSN exhibit hypo-connectivity with many other SEN, VIS and SAL RSNs. In the right side, right inferior occipital gyrus (R IOG) and right superior occipital gyrus (R SOG) shows similar hypo-connectivity pattern with SEN, VIS and PRE RSNs. In relation to the ECN, one hypoconnectivity was found between left inferior parietal lobule (L IPL) and a VIS RSN in the left inferior occipital gyrus (L IOG). Other connectivity reductions compared to controls involve the Lingual/Vermis and other RSNs of the VIS and PRE groups. No hyper-connectivity was observed among the ANOVA results driven by smoking and drinking.
Figure 4.
Networks with significant rsFNC differences. In this figure, changes in rsFNC are compared with respect to controls. Blue dots indicate the position of RSNs where hypo-connectivity was observed. Hypo-connectivity is the most common observation among rsFNCs driven by “Smoking and Drinking”. Red dots designate RSNs with hyper-connectivity. Hyper-connected RSNs were found in rsFNC driven by “Drinking” alone and “Smoking” alone. The yellow dots were use for the RSN pair where smokers had increased negative connectivity (anti-correlation), but the drinkers exhibited decreased negative connectivity.
The rsFNC differences driven by drinking followed a similar pattern as those in the smoking and drinking category. The most prominent result is hypo-connectivity among SAL, PRE, SEN and VIS RSNs. A distinctive exception to the spread hypo-connectivity pattern is the increased connectivity in the DRN group compared to the CTR group between the reward system, represented by the putamen, and areas of visual processing characterized by the Lingual/Vermis RSN. Only two rsFNC results were found to be driven by smoking including a hypo-connectivity between thalamus and putamen plus a hyperconnectivity between precuneus and left angular gyrus.
Significant relationship with AUDIT and FTND were found in four rsFNCs as described in Table 3. No significant relationship with the other considered confounding factors was found in any of the four results displayed in Table 3. In smokers, the connectivity between L Angular and Precuneus RSNs increases with the FTND measure. No relationship with FTND was found for thalamus vs. putamen, which is the only other rsFNC with group difference between CTR and SMK groups. The rsFNC between Postcentral-3b and R Fusiform Gyrus/Lingual RSNs decreased as the AUDIT score increases for the DRN samples. This relationship coincides with the general hypo-connectivity found in alcohol consumers between sensorimotor and visual areas. Two rsFNCs were related to the interaction of FTND with AUDIT. The SMA-6 vs. R IOG and the L Fusiform/Lingual vs. Cuneus rsFNCs decreased as the interaction term increased.
Table 3.
List of rsFNCs significantly related to FTND and AUDIT.
| RSN Pair | Samples | Variable Tested | beta | p-value |
|---|---|---|---|---|
| SMA-6 vs. R IOG | SAD | FTND × AUDIT | −0.0015 | 0.037 |
| L Fusiform/Lingual vs. Cuneus | SAD | FTND × AUDIT | −0.0019 | 0.020 |
| Postcentral-3b vs. R Fusiform/Lingual | DRN | AUDIT | −0.0162 | 0.028 |
| L Angular vs. Precuneus | SMK | FTND | 0.0408 | 0.015 |
4 Discussion
The objective of this work was to use a data driven analysis technique to find different patterns of rsFNC characterizing groups of subjects who smoke, drink or both. The data driven nature of our approach reduced possible bias present in seed-based analyses. Obtained results do not invalidate some of the previously reported observations using a-priori regions of interests (Camchong et al., 2012, 2013; Claus et al., 2011; Hong et al., 2009), but instead reveal the strongest effects found in the population under study. The observed general pattern describes a hypo-connectivity in substance dependence subjects compared to controls. The hypo-connectivity was prominent between visual RSNs connected to sensorimotor, salience and precuneus RSNs. On the contrary, hyper-connectivity observations were few, but pointing to important brain regions including the DMN, precuneus and putamen. Results suggest that nicotine and alcohol produce both similar and distinctive effects in brain connectivity indicating the possibility of disentangling rsFNC dysfunctions related to alcohol use disorder from those associated to nicotine dependence. An increase in the connectivity between DMN and precuneus was found to be an important feature that identified smokers with a low probability of alcohol use disorders. This distinction may provide an important clue for future biomarkers research.
Our analysis distinguished visual and sensorimotor regions as principal areas where the rsFNC might be reduced due to alcohol or nicotine use. The significant hypo-connectivity of SMK, SAD and DRN subjects when compared to the CTR subjects revolved around these RSN groups. Other RSN groups affected by both smoking and drinking were ECN, SAL and PRE indicating the existence of common effects across the brain. These results agree with the detriment of network global efficiency previously observed in both smokers (Fedota and Stein, 2015; Lin et al., 2015) and drinkers (Sjoerds et al., 2015). Few differences were detected when the post-hoc analysis in our data compared rsFNC differences of SAD vs. DRN and SAD vs. SMK groups indicating that common effects cannot differentiate between the substances. A different study found an opposite outcome among visual, sensorimotor, cuneus and insula areas in subjects using cocaine, marijuana and alcohol, but this hyper-connectivity was observed after abstinence periods longer than 4 days (Wang et al., 2015). These rsFNC dysfunctions likely specify effects that may be commonly linked to multiple substances of abuse where hyper-connectivity during withdrawal follows an initial hypo-connectivity during consumption. Despite using DSM-IV-TR Axis I to exclude subjects with alcohol problems in controls, the absence of an AUDIT score for the CTR subjects limits our capacity to perform a more thorough interpretation of these results.
We observed hyper-connectivity in the set driven by drinking associated with the rsFNC between lingual/cerebellar vermis and putamen. For this pair, the rsFNC was higher for the DRN group than for any of the other three groups. This observation points to an abnormal influence over the brain reward system known to be affected by alcohol (Di Chiara, 1997). In particular, the putamen is an area where an increase of dendritic spine density and an enhancement of glutamatergic transmission was linked to alcohol in primates (Carlson et al., 2011). This neuroadaptation could in part explain an increase of connectivity related to the putamen. A different result was obtained for the insula where hypo-connectivity was observed. Since the insula is an area known to be involved in interoception of bodily functions (Craig, 2003), insula hypo-connectivity observed in our results is consistent with reduced interoceptive awareness previously linked to alcohol addiction (Çöl et al., 2016). Hypoconnectivity within the insula has been previously reported and hypothesized as playing a role in alcohol relapse because of a diminished awareness of substance use (Camchong et al., 2012). Another possible explanation for the observed hypo-connectivity is the known damage to white matter caused by alcohol (Pfefferbaum et al., 2000) which disrupts the anatomical connection between left insula and lingual gyrus (Ghaziri et al., 2015).The insula and the putamen are regions that has been previously associated with the brain reward system and with different directions of effects including loss of connectivity (Kuceyeski et al., 2013) and increased connectivity (Zhu et al., 2015). However, we could not confirm a link between putamen or insula connectivity changes and the AUDIT score, which might be due to small sample size and small AUDIT range in the DRN population. Our data suggests that connectivity of the reward system may go in different directions of effects where putamen connectivity increases due to a neuroplasticity mechanism, but insular connectivity decreases due to microstructural disruption of white matter.
Reduced connectivity among insula, precuneus, SMA, postcentral, lingual/vermis and fusiform gyri characterized the rsFNCs driven by drinking. These results are consistent with previous hypoconnectivity observations in visual and sensorimotor areas (Weiland et al., 2014). Our analysis also pointed to the precuneus as an area associated with alcohol use disorders as it has been previously found (Claus et al., 2011). Abnormal activation in the precuneus of subjects with alcohol use disorder has been thought to be associated with craving and the processing of visual memories (Park et al., 2007). In our results, sensorimotor, precuneus and visual dysfunctions seem ubiquitous in both smokers and drinkers after looking at the rsFNCs driven by smoking and drinking. However, we point out that visual dysfunction associated to drinking in our data were located around fusiform and lingual gyri. In contrast, rsFNC differences driven by both smoking and drinking were more frequently located about superior, middle and inferior occipital gyri. These results agree with an alcohol study by Camchong et al. (Camchong et al., 2012) where reduced connectivity within insula and within occipital gyrus was reported, but smoking was allowed among the subjects. Our observations add a reduced connectivity between sensorimotor, precuneus, occipital and insula areas to the picture. Furthermore, the magnitude of connectivity reduction between sensorimotor and visual areas was significantly associated with AUDIT score and the interaction between AUDIT and FTND. These reduced connectivity results support the idea delineated by Camchong (Camchong et al., 2012) of a link between alcohol and dysfunction in a brain network in charge of sensory awareness and attention that provides important information necessary for decision-making and assessment. Another important observation in our data is the protective effect that smoking exerted in visual memory and visual processing regions. Previous reports indicate that nicotine produce enhanced activation in the fusiform gyrus (Ghatan et al., 1998; Lawrence et al., 2002), but in our resting state results the effect might have prevented observing a difference between SAD and CTR groups. This might explain why drinkers span a larger set of visual rsFNC differences including those not present in smokers. These observations provide an important distinction between nicotine and alcohol effects.
Previous studies on nicotine use have focused in areas such as the insula (Droutman et al., 2015; Maria et al., 2015), known to reduce nicotine withdrawal when injured (Abdolahi et al., 2015), the default mode and executive control networks (Fedota and Stein, 2015; Sutherland et al., 2012). In our resting state analysis, the insula was involved with decreased connectivity with other regions in all three groups DRN, SAD and SMK, but a link with continuous measures of AUDIT and FTND could not be established. The involvement of the ECN was also very scarce and only comprised one parietal region. In contrast, the DMN connectivity (angular gyrus vs. precuneus) was found to be strongly affected by nicotine as group difference and FTND association both indicated a significant connectivity increment. The RSN pair PCC and postcentral gyrus also show connectivity increment linked to the DMN as smokers exhibited stronger anti-correlation. These results and the fact that smokers were abstinent for at least 3 hours before scanning seem concordant with the hypothesis that nicotine withdrawal might promote an enhancement in DMN functioning and shift network dynamics away from task positive areas in order to maintain homeostasis in a brain suffering from withdrawal effects (Sutherland et al., 2012). In contrast, hypo-connectivity in smokers was found within the reward system including the brain areas of thalamus and putamen. The thalamus is a main hub of information in the brain important in the study of nicotine addiction. It has been found that smoking reduces glutamate (one of the major neurotransmitters in the mammalian brain) in the thalamus (Durazzo et al., 2015; O’Neill et al., 2014), thus affecting its information relay function. Thalamic activity has been previously found to be correlated with FTND scores (Rose et al., 2007) and has been mentioned in many studies as part of affected brain networks (Hahn et al., 2007; Hong et al., 2009; Janes et al., 2012). Both putamen and thalamus have been found to be abnormally active during smoking cues presentation to abstinent smokers (McClernon et al., 2009). Lack of connectivity between putamen and thalamus can be an indication of reward dysregulation that enhances nicotine-seeking behavior in smokers.
One of the limitations of our data is the unequal behavioral scores collected to measure alcohol use. Subjects in the CTR group were excluded after screening for alcohol dependence or alcohol abuse, but no information regarding number of drinks or AUDIT score was collected. However, several studies indicate that AUDIT and DSM-IV have similar specificity (Dawson et al., 2012; Foxcroft et al., 2015) providing evidence that subjects in the CTR group were likely assigned to the correct group. A second limitation of the current study is the lack of inclusion of other measures that could have been useful to distinguish among the four sample groups. For example, prior studies (Ahn and Vassileva, 2016; Pariyadath et al., 2014) have used machine learning algorithms to distinguish controls from various substance dependent samples using features from neurocognitive, personality, and psychiatric measures, whereas the current study only used neuroimaging features. Because we relied on a convenience sample combined from existing studies, few measures overlapped, thus preventing us from conducting our analyses with behavioral assessments. Future studies that combine neuroimaging and behavioral assessments will be valuable for determining the utility of neuroimaging measures in predicting group membership. Another limitation originates from the strict multiple comparison correction utilized to reduce the number of false positives. Reported findings likely reflect only the strongest effects of drinking or smoking, while other possible effects like the ones observed by hypotheses-driven (Chanraud et al., 2011; Janes et al., 2012) region-of-interest may be missed. This could explain the relatively small number of ECN effects observed in this work compared to previous studies (Cole et al., 2010; Weiland et al., 2014). In this study, participants were not actively using other drugs (including methamphetamine, cocaine, ecstasy, prescription pain medication, prescription sedatives and stimulants). As has been done here between alcohol and nicotine, future studies should be conducted to evaluate the potential cross effect with other common substances such as marijuana. In spite of the multiple group differences observed, the results show few relationships with AUDIT and FTND, which could be indication of pre-existing brain states unrelated to drug use. It could also indicate that observed differences might be better captured by other covariates not considered in this study. Another possible explanation is the stationary behavior assumed for the time courses in the applied method. Methods that analyze dynamic connectivity, e.g. (Allen et al., 2012), can identify and separate brain states which may exhibit closer relationship with behavioral measures. Finally, this study was designed as a resting state analysis free of important cognitive measures that can be obtained using different task paradigms. Our observations are then limited to task-free outcomes and leave a cognitive evaluation for future work and experimentation.
5 Conclusion
Our data provide evidence for interoceptive and sensorial dysfunctions as reduced connectivity in insula, precentral and visual areas might be associated to reduced awareness of input signals observed in alcohol (Çöl et al., 2016). Affected brain areas also span motor (SMA) and cognitive (precuneus) functions suggesting that top-down body control pathways might also be impaired. Nicotine induced a protective effect in drinkers who also smoked for particular brain areas in the default network (PCC) and higher visual processing network (lingual gyrus and fusiform girus), but did not improve the reduced connectivity of the primary visual region. These different effects in visual areas may be useful in separating drinkers and drinkers that smoke. In addition to PCC, nicotine also increased connectivity in the angular gyrus, which is another important part of the DMN. This DMN hyper-connectivity supports the idea of a salience disruption that shifts network dynamic towards the DMN in order to resolve withdrawal effects in the brain (Sutherland et al., 2012). Our observations seem to lack the ECN emphasis previously found by alcohol studies (Weiland et al., 2014). Only one ECN RSN embracing the left IPL showed significant hypo-connectivity with the left IOG. In our data, reduced ECN-visual connectivity resembles the sensorimotor-visual hypo-connectivity pattern in smoking and drinking subjects. The similar hypo-connectivity effect in ECN and sensorimotor areas may be related to the hypothesis of a network dynamic that shifts away from exogenous information processing (Fedota and Stein, 2015). In addition, results support the existence of a putamen-thalamus dysfunction that might be involved in nicotine craving behavior as previously observed in (McClernon et al., 2009). The different effects observed in our study indicate that alcohol, nicotine and their interaction affect the brain in particular ways that may help understand the specific or interactive mechanisms of substance use on brain function. These results also show that concurrent use of substances must be carefully controlled when studying substance specific effects on the brain.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health grant numbers R33DA027626 to JL and NIH 2R01EB005846 and P20GM103472 to VC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author(s) declare that there was no other financial support or compensation that could be perceived as constituting a potential conflict of interest.
References
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, Block RC, Wijngaarden E. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction. 2015;110:1994–2003. doi: 10.1111/add.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn W-Y, Vassileva J. Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug and alcohol dependence. 2016;161:247–257. doi: 10.1016/j.drugalcdep.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cerebral cortex. 2012:bhs352. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. Audit. The Alcohol Use Disorders Identification Test (AUDIT): Guidelines for use in primary care. 2001 [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8:77–100. [Google Scholar]
- Calhoun V, Adali T, Pearlson G, Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. Biomedical Engineering, IEEE Reviews in. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cerebral cortex. 2012:bhs190. doi: 10.1093/cercor/bhs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 2013;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson VCC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel A-L, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çöl IA, Sönmez MB, Vardar ME, Köşesi H. Evaluation of Interoceptive Awareness in Alcohol-Addicted Patients. Evaluation. 2016;53 doi: 10.5152/npa.2015.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Craig A. Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Smith SM, Saha TD, Rubinsky AD, Grant BF. Comparative performance of the AUDIT-C in screening for DSM-IV and DSM-5 alcohol use disorders. Drug and alcohol dependence. 2012;126:384–388. doi: 10.1016/j.drugalcdep.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Alcohol and dopamine. Alcohol Research and Health. 1997;21:108. [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochemical pharmacology. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends in cognitive sciences. 2015;19:414–420. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy M, Chanraud S. Imaging the Addicted Brain: Alcohol. International Review of Neurobiology. 2016 doi: 10.1016/bs.irn.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abé C, Gazdzinski S, Murray DE. Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-acetylaspartate and glutamate levels. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Allen EA, Damaraju E, Calhoun VD. On network derivation, classification, and visualization: a response to Habeck and Moeller. Brain connectivity. 2011a;1:105–110. [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human brain mapping. 2011b;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski L. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69:763–765. [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Annals of the New York Academy of Sciences. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute. 2002 [Google Scholar]
- Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- Foxcroft DR, Smith L, Thomas H, Howcutt S. Accuracy of Alcohol Use Disorders Identification Test (AUDIT) for detecting problem drinking in 18–35 year-olds in England. Viitattu. 2015;21:2015. doi: 10.1093/alcalc/agu095. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Neuroscience Databases. Springer; 2003. Statistical parametric mapping; pp. 237–250. [Google Scholar]
- Ghatan P, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde J-C, Boucher O, Gilbert G, Descoteaux M, Lippé S, Rainville P, Nguyen DK. The corticocortical structural connectivity of the human insula. Cerebral cortex. 2015:bhv308. doi: 10.1093/cercor/bhv308. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. The Journal of neuroscience. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter AJ. The maximum familywise error rate of Fisher's least significant difference test. Journal of the American Statistical Association. 1986;81:1000–1004. [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Archives of general psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychological bulletin. 1984;95:301. [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and alcohol dependence. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel SE, Caffo BS, van Zijl P, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. Magnetic Resonance in Medicine. 2011;66:644–657. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A, Meyerhoff DJ, Durazzo TC, Raj A. Loss in connectivity among regions of the brain reward system in alcohol dependence. Human brain mapping. 2013;34:3129–3142. doi: 10.1002/hbm.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Li K, Guo L, Nie J, Li G, Liu T. Review of methods for functional brain connectivity detection using fMRI. Computerized Medical Imaging and Graphics. 2009;33:131–139. doi: 10.1016/j.compmedimag.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Wu G, Zhu L, Lei H. Altered brain functional networks in heavy smokers. Addiction biology. 2015;20:809–819. doi: 10.1111/adb.12155. [DOI] [PubMed] [Google Scholar]
- Maria MS, Megan M, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addiction biology. 2015;20:407–414. doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung Y-C, Pfefferbaum A, Sullivan EV, Schulte T. The resting brain of alcoholics. Cerebral cortex. 2014:bhu134. doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, Oh EY, Hellemann GS, Nurmi EL, London ED. Thalamic glutamate decreases with cigarette smoking. Psychopharmacology. 2014;231:2717–2724. doi: 10.1007/s00213-014-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Simmons AN, Matthews SC, Robinson SK, Tapert SF, Schuckit MA, Paulus MP. Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: a pilot study. Alcohol and alcoholism. 2011;46:547–552. doi: 10.1093/alcalc/agr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Frontiers in human neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M-S, Sohn J-H, Suk J-A, Kim S-H, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and alcoholism. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Parks MH, Greenberg DS, Nickel MK, Dietrich MS, Rogers BP, Martin PR. Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcoholism: Clinical and Experimental Research. 2010;34:1098–1109. doi: 10.1111/j.1530-0277.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcoholism: Clinical and Experimental Research. 1998;22:954–961. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Stufflebeam SM, Veltman DJ, Van den Brink W, Penninx BW, Douw L. Loss of brain graph network efficiency in alcohol dependence. Addiction biology. 2015 doi: 10.1111/adb.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR. Nicotine effects on default mode network during resting state. Psychopharmacology. 2011;216:287–295. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara VM, Mayer AR, Damaraju E, Hutchison K, Calhoun VD. The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Suh J, Li Z, Li Y, Franklin T, O’Brien C, Childress AR. A hyper-connected but less efficient small-world network in the substance-dependent brain. Drug and alcohol dependence. 2015;152:102–108. doi: 10.1016/j.drugalcdep.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, Mayer AR, Hutchison KE. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2014;38:2445–2453. doi: 10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes K, Warburton D. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addiction biology. 2015 doi: 10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Item revisions in the sensation seeking scale form V (SSS-V) Personality and Individual Differences. 1996;20:515. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.