Abstract
Introduction: The purpose of the present study was to evaluate the different concentrations of sodium hypochlorite activated with laser in removing of the smear layer in the apical, middle, and coronal segments of root canal walls by scanning electron microscopy analysis.
Methods: Sixty single-rooted human mandibular teeth were decoronated to a standardized length. The samples were prepared by using Race rotary system to size 40, 0.04 taper and divided into 4 equal groups (n = 15). Group 1, irrigated with EDTA 17% and 5.25% NaOCl, groups 2, 3 and 4, 1%, 2.5%, and 5% NaOCl activated with Nd:YAG laser, respectively. Teeth were split longitudinally and subjected to scanning electron microscope (SEM). Data were analyzed by Kruskal-Wallis, Mann-Whitney tests. P value of <0.05 was considered statistically significant.
Results: Five percent NaOCl LAI (laser-activated irrigation) showed best smear layer removal in test groups and the difference was statistically significant (P < 0.001). Control group (EDTA 17% and 5.25% NaOCl irrigation) showed significantly better outcomes in comparative with test groups (P < 0.001). In the apical third, compared to coronal and middle third, the canal walls were often contaminated by inorganic debris and smear layer.
Conclusion: All different concentrations of sodium hypochlorite activated with laser have a positive effect on removing of smear layer. Sodium hypochlorite activated with laser removed smear layer more effectively at the coronal and middle third compared to the apical third.
Keywords: Nd YAG Lasers, Hypochlorite, Sodium, Layer, Smear
Introduction
The success of root canal treatment depends on cleaning and disinfection of the canal to perform adequate obturation. The smear layer is an amorphous, irregular layer containing inorganic debris, as well as organic materials like pulp tissue, odontoblastic process, necrotic debris, microorganisms and their metabolic products.1,2
McComb and Smith were the initial investigators who found the smear layer on the instrumented root canal walls. They found it irregular, amorphous, and granular when viewed under the SEM.3 Some investigators believe that the smear layer feeds microorganisms and helps them colonize.4,5 Some researchers have reported that the smear layer prevents, or delays the action of canal irrigation solutions for disinfection of bacteria and microorganisms in dentine.6,7
The smear layer has been shown to impede the penetration of both intracanal disinfectants and sealer into the dentinal tubules, and can potentially compromise the seal of the root canal filling. The smear layer can be packed into the dentinal tubules to a depth of up to 40 µm.8 Other investigators showed that root canal sealers have a better adhesion to the root canal wall after smear layer removal.9-11
Different methods have been used to remove the smear layer. Ostby was the first investigator who used ethylene diamine tetra-acetic (EDTA) to clean and shape the canals12. Decalcifying solutions, such as phosphoric acid, citric acid, EDTA acid, and maleic acid have been reported to be suitable in removing the smear layer.9,13-17
Sodium hypochlorite (NaOCl) is the most common endodontic irrigant, and is used in concentrations ranging from 0.5% to 6%.13 It has bactericidal properties, and the ability to dissolve organic tissues, but this solution has not the ability to remove smear layer alone.14-17 Some researchers have reported that alternating the use of EDTA and NaOCl can remove the smear layer in an ideal way.11,17,18
Recently, laser has shown promising results in endodontic treatment: Weichman and Johnson were the first researchers who used laser in endodontic treatments.19 The previous studies have shown that laser could vaporize canal soft tissue and remove the smear layer.20,21 Some investigators have reported that usage of Nd:YAG laser, when followed by manual filing, can clean root canal walls and remove the smear layer and the soft tissue from the root canal. They used an Nd:YAG laser to irradiate the dentin of the root canal wall, and showed disruption of the smear layer to actual melting and recrystallization of the dentine.22,23 Recently, laser-activated irrigation (LAI) has been introduced as an activation method of irrigation solution by the transfer of pulsed energy.24 Most researchers have suggested using mid-infrared erbium lasers to activate the irrigation of root canal.24,25 Previous studies have shown that positive effect of LAI on smear layer removal.8,20,24,25 Although some studies have shown the diode laser to be ineffective in removing the smear layer from the canal,26 few published articles are available about Nd:YAG laser on the LAI.27
The purpose of this study was to compare the effect of different concentrations of sodium hypochlorite activated with Nd:YAG laser on removal of the smear layer by scanning electron microscopy.
Methods
In this experimental in vitro study, 60 recently extracted mature human mandibular teeth with straight single root canal were selected. The teeth had been recently extracted from patients for periodontal diseases and orthodontic reasons. The teeth were radiographed to confirm they had a single canal and no internal calcifications, irregularities, and other anomalies. The teeth were decoronated and prepared: longitudinally deep groove were created into buccal and lingual parts using diamond disk with a nonstop machine (Teezkavan.co, Tehran, Iran) to obtain approximately 15 mm uniform root lengths. Root canal perforated specimens were ruled out of this study. Working lengths (WL) were determined by inserting K file #15 (Dentsply, Maillefer, Ballaigues, Switzerland) until visualized at the apical foramen and subtracting 1 mm from this measurement. All root apexes were covered with melted wax to obtain closed canal system.29 Root canals were instrumented using the Race rotary system (Dentsply, Maillefer, Ballaigues, Switzerland) until the final instrument size reached size 35, with 0.04 taper and 5 mL; 5.25% NaOCl were used between each file and after the preparation. After canal preparation, teeth were stored in distilled water. The specimens were randomly divided into four groups (n = 15). One person did the entire preparation process.
Group 1 (control group): 15 teeth canals were irrigated for 1 minute with 2 mL of 17% EDTA (ApadanaTak Co., Tehran, Iran), then the canals were irrigated with 2 mL of 5.25% NaOCl, and finally irrigation was performed with 10 mL of normal saline (Emad Pars Co., Saveh, Iran) for 1 minute to remove any effects of irrigants. All irrigations were done with needle gauge No. 30 to penetrate up to the apical third of the canals.
Group 2(1% NaOCl LAI): 2 mL 1% NaOCl irrigation for 20 seconds irradiated with Nd:YAG laser (Fotona Fidelis Plus, Ljubljana, Slovenia, wave length 1.064 nm). The parameters of the Nd:YAG laser beam were selected based on previous studies.26 The setting parameters were output power 1 W, pulse energy 50 mJ/pulse, pulse frequency 20 Hz and pulse duration 100 μs (MSP: Micro Short Pulse) with 300 μm optic fiber with hand circular motion from apical foramen to coronal part of canal in a time duration of 20 seconds (4 times, 5 seconds each). After laser irradiation, the canals were irrigated with 10 mLof normal saline for 1 minute.
In groups 3 and 4, the canals were prepared in the same way as in group 2 (1% NaOCl LAI): The difference was the use of 2/5% NaOCl in group 3, and 5% NaOCl in group 4, respectively.
Teeth were longitudinally bisected into buccal and lingual parts by wedging process with the help of a spatula. Then, one half of each root was selected for processing as follows: fixation with 5% glutaraldehyde (6 hours), dehydration by ethylic alcohol 30% for 10 minutes, 50% for 20 minutes, 70% for 20 minutes, 90% for 30 minutes and 100% for 30 minutes, respectively.
After the drying process, samples were coated with gold palladium by (JFC-1100E ION SPUTTER, JEOL Co., Japan) sputter coater Bio-rade, placed into the SEM device (JEOL, ISM-5500, Tokyo, Japan) and scanned in 3 coronal, middle, and apical parts. Finally, photomicrographs were taken in ×1500 magnification, and were observed by two independent researchers. They observed the photographs and scored them using an 8-scale score that had been designed by Peeters and Suardita.8
Score 1: No detectable smear layer and clean root canal walls with very little to no debris; all dentinal tubules were clean and open.
Score 2: Clean surfaces containing small agglomerations of debris and/or a thin homogenous smear layer; most of the dentinal tubules were open.
Score 3: Many agglomerations of debris and a homogenous smear layer covering <50% of the canal wall; only a few dentinal tubules were open.
Score 4: Mostly contaminated surfaces with a heavy homogenous smear layer and a large amount of debris covering >50% of root canal walls; no dentinal tubules were open.
Score 5: Contaminated root canal walls entirely covered by a heavy and inhomogeneous smear layer and debris.
Statistical Analysis
Two observers independently evaluated the SEM images three times with 1-week interval without knowledge of the previous results. To validate the subjective findings, weighted coefficient kappa (Kw) was used to measure inter-observer and intra-observer reproducibility in separate time periods and for each observer. The differences between irrigation techniques were compared statistically by using the Kruskal–Wallis nonparametric analysis of variance. Mann–Whitney U-test was used for post hoc comparisons. The significance level for all statistical analyses was set at P < 0.05. Statistical analysis was performed with SPSS for Windows 16.0 software package (SPSS Inc., Chicago, IL).
Results
The mean value score and comparisons of smear layer removal effects in each group are presented in Tables 1-3 and SEM photomicrographs (Figures 1-4).
Table 1. Comparison of the Means Score Values and Standard Deviations of Different Root Canal Areas in Each Group .
| Groups | Areas | Mean Score | SD | P Value a |
| Control | Coronal | 1.37 | 0.49 | 0.02 |
| Middle | 1.67 | 0.66 | ||
| Apical | 1.87 | 0.82 | ||
| 1% NaOCl LAI | Coronal | 2.87 | 0.9 | 0.001 |
| Middle | 3.50 | 0.82 | ||
| Apical | 3.70 | 0.84 | ||
| 2.5% NaOCl LAI | Coronal | 2.27 | 0.98 | <0.001 |
| Middle | 3.73 | 1.17 | ||
| Apical | 4.23 | 0.57 | ||
| 5% NaOCl LAI | Coronal | 1.93 | 0.78 | 0.001 |
| Middle | 2.70 | 0.99 | ||
| Apical | 2.83 | 1.09 |
Abbreviation: SD, standard deviation.
aKruskal–Wallis tests.
Table 3. Two by Two Mann–Whitney U Tests of Means Values of Smear Layer Scores in Different 3 Regions of Root Canal In Study Groups .
| Groups | Comparing Areas | P Value |
| Control | Coronal-Middle | 0.199 |
| Coronal-Apical | 0.17 | |
| Middle-Apical | 0.483 | |
| 1% NaOCl LAI | Coronal-Middle | 0.014 |
| Coronal-Apical | 0.001 | |
| Middle-Apical | 0.637 | |
| 2/5% NaOCl LAI | Coronal-Middle | <0.001 |
| Coronal-Apical | <0.001 | |
| Middle-Apical | 0.105 | |
| 5% NaOCl LAI | Coronal-Middle | 0.001 |
| Coronal-Apical | 0.008 | |
| Middle-Apical | 0.853 |
Figure 1.
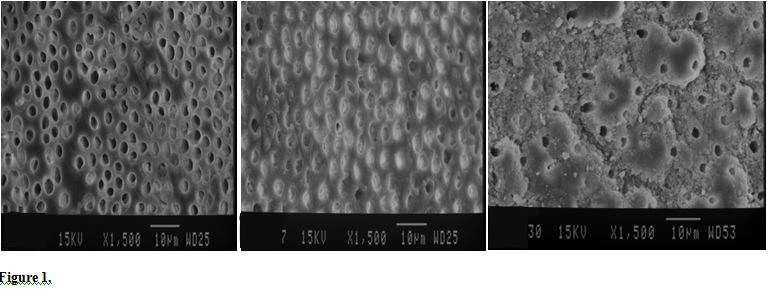
Scanning Electron Micrographs of the Root Canal Regions in the Control Group (×1500) (A: Coronal, B: Middle, C: Apical).
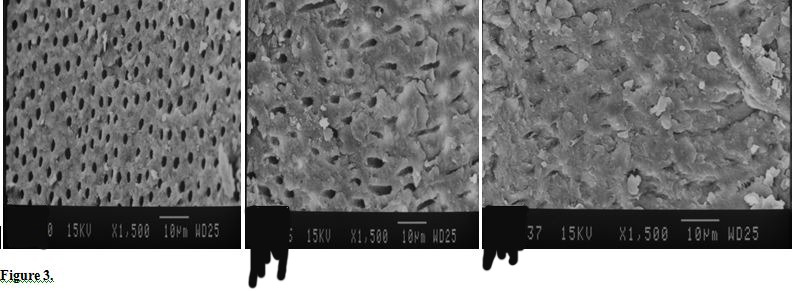
Figure 4.
SEM Photographs of Coronal (A), Middle (B) and Apical (C) Regions of Root Canal Treated by 5% NaOCL LAI (×1500).
Krusal-Wallis and Mann-Whitney tests showed no significant difference between 1% NaOCl LAI and 2.5% NaOCl LAI (P = 0.98) (Figures 2 & 3; Table 2). However, a significant difference was found between 1% NaOCl LAI and 5% NaOCl LAI (P < 0.001) (Figures 2 & 4; Table 2), as well as 2.5% NaOCl LAI and 5% NaOCl LAI (P < 0.001) (Figuress 3 & 4; Table 2) in smear layer removal. 5% NaOCl LAI significantly showed more efficiency in smear layer removal than 1% NaOCl LAI and 2.5% NaOCl LAI. The control group (EDTA 17% and 5.25% NaOCl irrigation) showed significantly better outcomes compared to 1% NaOCl LAI (P < 0.001), 2.5% NaOCl LAI (P < 0.001) and 5% NaOCl LAI (P = 0.001). Statistical analysis showed a significant difference between different areas of the canal in each group. The control group showed the same behavior in all portions of canal for smear layer removal (Coronal = Middle = Apical).
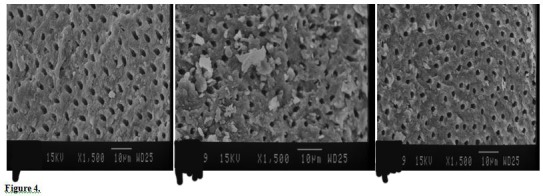
Figure 2.
SEM Photographs of Coronal (A), Middle (B) and Apical (C) Regions of Root Canal Treated by 1% NaOCL LAI (×1500).
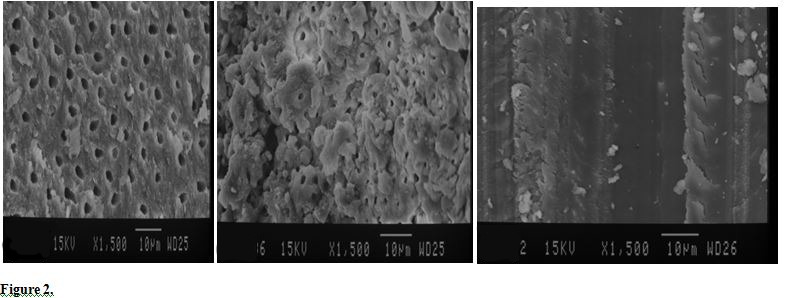
Figure 3.
SEM Photographs of Coronal (A), Middle (B) and Apical (C) Regions of Root Canal Treated by 2.5% NaOCL LAI (×1500).
Table 2. Comparison Between the Groups in the Apical, Middle and Coronal Third Regions of Specimens .
| Groups | Groups | P Valuesa | |||
| Overall | Coronal | Middle | Apical | ||
| Control | 1% NaOCl LAI | <0.001 | <0.001 | <0.001 | <0.001 |
| Control | 2.5% NaOCl LAI | <0.001 | <0.001 | <0.001 | <0.001 |
| Control | 5% NaOCl LAI | <0.001 | 0.038 | <0.001 | 0.001 |
| 1% NaOCl LAI | 2.5% NaOCl LAI | 0.98 | 0.025 | 0.781 | 0.061 |
| 1% NaOCl LAI | 5% NaOCl LAI | <0.001 | <0.001 | 0.039 | <0.001 |
| 2.5% NaOCl LAI | 5% NaOCl LAI | <0.001 | 0.387 | 0.002 | <0.001 |
Also, when different concentrations of sodium hypochlorite activated with laser were compared, in all of them the coronal area was cleaner than the middle and apical, and middle area and apical showed an equal effect on removing smear layer (smear layer removal: coronal >middle = apical).
Discussion
With the aim of increasing disinfection and smear layer removal in root canals, additional use of irrigation activation is the current approach in endodontics.25,28
Previous studies27,29,30 have reported that the use of several activation systems/techniques showed improvement in cleaning efficacy of irrigation solutions. SEM is commonly used for the identification of organic/inorganic debris and smear layer on the root canal walls after endodontic preparation, allowing to obtain detailed pictures with higher magnification imaging of the dentinal tubules.31
The results of this study showed that hypochlorite activated by laser at concentrations of 1%, 2.5% and 5%, significantly removed the smear layer from the intra-canal walls (P = 0.001, <0.001, 0.001 respectively). Previous studies are consistent with the present study.26,27,32,33 However, the conventional method with EDTA 17% and sodium hypochlorite were more efficient in removing smear layer from the intracanal compared to the use of laser activated hypochlorite 1% NaOCl LAI (P < 0.001), 2.5% NaOCl LAI (P < 0.001) and 5% NaOCl LAI (P = 0.001).
Ekim and Erdemir evaluated the effect of different irrigations activated by the laser in removing the smear layer. They showed that different irrigations activated by laser had a positive effect on the teeth.26 Minamisako et al demonstrated the efficiency of Nd:YAG laser to remove debris, smear layer and pulp tissue.34 Moreover, it was reported that the diode and Nd:YAG lasers can be effective as disinfecting and sterilizing methods for root canal. Therefore, laser can increase the success rate of endodontic treatment.35 Laser can increase the temperature of root canal. So, the possibility of damage to periapical had increased, especially when the roots were close to the mental foramen and inferior alveolar nerve or maxillary sinus.34 Laser parameters in this study were selected due to previous studies that showed that LAI with Nd:YAG laser were efficient to remove intra-canal smear layer.26,34 Canal wall temperature was not monitored during the laser operation; however, when the selected parameters and time are compared with the previous literature, they are found to be within safe limits.36 The use of lasers with irrigation solutions generates less heat and is safer.34
Currently, the LAI method has been introduced for more activation by irrigation. In this technique, the laser effect is explained by cavitation. In this method, the laser acts as a pump, in an environment, such as sodium hypochlorite activating the laser in the form of ablative and can lead to formation of a big oval-shaped vapor bubble in front of the laser tip that will expand and implode.37 The expansion of the bubble creates high pressure and the irrigating liquid moves through the canal. When the bubble explods after 100-200 ms, a negative pressure is created and the irrigation solution is pulled back into the canal, this mechanism causes secondary effects of cavitation.38 When irradiation pulse stops, the vapor bubble starts shrinking. Water surrounding the bubble sharply flows inside the decompressed vaporgap. During bubble collapse, a high-speed liquid jet is formed and results in a large shear stress acting on the root canal wall that removes debris and smear layer.37
In this study, when sodium hypochlorite at concentrations of 1%, 2.5% and 5% was activated with the laser, it could succeed in removing the smear layer (P = 0.001, <0.001, and 0.001, respectively). Peeters and Suardita in 2011 reported that LAI of NaOCl had an intra-canal antibacterial effect.8 Hasheminia et al in 2012 compared the effect of 17% EDTA, 5% maleic acid and Nd:YAG laser in removing the smear layer. Their results showed that Nd:YAG laser was less effective in smear layer removal compared to 17% EDTA and 5% maleic acid.39 Sodium hypochlorite can remove the organic material of the smear layer, but Garberglio et al reported that sodium hypochlorite 1% and 5% were not able to remove the smear layer.40
In addition, in the present study, the efficiency of laser-activated hypochlorite at a concentration of 5% compared to lower concentrations (2.5, and 1%) was more successful in removing the smear layer (P ≤ 0.001). Probably, higher concentration of sodium hypochlorite in the same conditions of temperature, time, taper of the canal and type and size of the irrigating needle produces better efficiency in removing organic material of the smear layer. Further studies are recommended. This study concluded that the conventional method equally removed the smear layer in all areas of the canal. According to Table 3 the coronal third of the root canal was cleaner than the middle and apical third. This failure to complete removal of the smear layer in this area can be related to the transfer of energy by fiber optics to the apical region. Probably, using side firing tips pulses to transmit laser energy to the apical parts of the area could be helpful in removing the smear layer. Insufficient access of the irrigators to the apical part can also be another reason for thereduction of smear layer removal in the apical part. The results of the present study are also in accordance with previous studies.26,41 Although hypochlorite activated by laser with different concentrations was successful in removing the smear layer, the control group was considered as the gold standard in removing the smear layer, and has been more successful. In the control group, mineral material was removed by EDTA, followed by the use of sodium hypochlorite which removed the organic part of the smear layer.
Because the apical third is an important area for a successful root canal treatment, and if the smear layer is removed, the chances of success of root canal therapy increase, further studies are recommended to study a combination of both laser and standard protocols to remove the smear layer.
Conclusion
Within the limitations of this study, different concentrations of sodium hypochlorite activated with laser have been demonstrated to be effective in removing the smear layer, although the standard protocol smear layer removal is more effective.
Acknowledgments
This research, as part of the post-graduate thesis of Dr. Hossein Davanloo was supported by the Endodontic Department of the Dental School of Hamadan University of Medical Sciences, Hamadan, Iran.
Ethical Considerations
The research protocol was approved by the Institutional Ethic Committee of the Vice Chancellor of Research, Hamadan University of Medical Sciences ( No. 2015-16p/308).
Conflict of Interests
The authors declare that they have no conflict of interests.
Please cite this article as follows: Shahriari S, Kasraei S, Roshanaei G, Karkeabadi H, Davanloo H. Efficacy of sodium hypochlorite activated with laser in intracanal smear layer removal: an SEM study. J Lasers Med Sci. 2017;8(1):36-41. doi:10.15171/jlms.2017.07.
References
- 1.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(6):658–666. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 2.Pashley DH. Smear layer: physiological considerations. Oper Dent Suppl. 1984;3:13–29. [PubMed] [Google Scholar]
- 3.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1(7):238–242. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 4.Brännström M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984;3:35–42. [PubMed] [Google Scholar]
- 5.Pérez-Heredia M, Ferrer-Luque CM, González-Rodríguez MP, Martín-Peinado FJ, González-López S. Decalcifying effect of 15% EDTA, 15% citric acid, 5% phosphoric acid and 25% sodium hypochlorite on root canal dentine. Int Endod J. 2008;41(5):418–423. doi: 10.1111/j.1365-2591.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- 6.Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6(4):142–149. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 7.Byström A, Sunvqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18(1):35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Peeters HH, Suardita K. Efficacy of smear layer removal at the root tip by using ethylenediaminetetraacetic acid and erbium, chromium: yttrium, scandium, gallium garnet laser. J Endod. 2011;37(11):1585–1589. doi: 10.1016/j.joen.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Tidmarsh BG. Acid-cleansed and resin-sealed root canals. J Endod. 1978;4(4):117–121. doi: 10.1016/S0099-2399(78)80201-5. [DOI] [PubMed] [Google Scholar]
- 10.Abramovich A, Goldbhrg F. The relationship of the root canal sealer to the dentine wall. Int Endod J. 1976;9(2):81–86. doi: 10.1111/j.1365-2591.1976.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 11.White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J Endod. 1984;10(12):558–562. doi: 10.1016/S0099-2399(84)80100-4. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard-Ostby B. Chelation in root canal therapy: ethylenediaminetetraacetic acid for cleansing and widening of root canals. Odontol Tidskr. 1957;65:3–11. [Google Scholar]
- 13.Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005;10(1):77–102. [Google Scholar]
- 14.Rubin LM, Skobe Z, Krakow AA, Gron P. The effect of instrumentation and flushing of freshly extracted teeth in endodontic therapy: a scanning electron microscope study. J Endod. 1979;5(11):328–335. doi: 10.1016/S0099-2399(79)80088-6. [DOI] [PubMed] [Google Scholar]
- 15.Moorer W, Wesselink P. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15(4):187–196. doi: 10.1111/j.1365-2591.1982.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 16.Wayman BE, Kopp WM, Pinero GJ, Lazzari E. Citric and lactic acids as root canal irrigants in vitro. J Endod. 1979;5(9):258–65. doi: 10.1016/S0099-2399(79)80171-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigating solutions for endodontics: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1981;52(2):197–204. doi: 10.1016/0030-4220(81)90319-4. [DOI] [PubMed] [Google Scholar]
- 18.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9(4):137–142. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 19.Weichman JA, Johnson FM. Laser use in endodontics: a preliminary investigation. Oral Surg Oral Med Oral Pathol. 1971;31(3):416–420. doi: 10.1016/0030-4220(71)90164-2. [DOI] [PubMed] [Google Scholar]
- 20.Takeda F, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999;32(1):32–39. doi: 10.1046/j.1365-2591.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 21.Ayad MF. Effects of rotary instrumentation and different etchants on removal of smear layer on human dentin. J Prosthet Dent. 2001;85(1):67–72. doi: 10.1067/mpr.2001.112792. [DOI] [PubMed] [Google Scholar]
- 22.Levy G. Cleaning and shaping the root canal with a Nd: YAG laser beam: a comparative study. J Endod. 1992;18(3):123–127. doi: 10.1016/S0099-2399(06)81312-9. [DOI] [PubMed] [Google Scholar]
- 23.Goodis HE, White JM, Marshall SJ, Marshall GW Jr. Scanning electron microscopic examination of intracanal wall dentin: hand versus laser treatment. Scanning Microsc. 1993;7(3):979–987. [PubMed] [Google Scholar]
- 24.DiVito E, Lloyd A. ER: YAG laser for 3-dimensional debridement of canal systems: use of photon-induced photoacoustic streaming. Dent Today. 2012;31(11):122, 4–7. [PubMed] [Google Scholar]
- 25.George R, Meyers IA, Walsh LJ. Laser activation of endodontic irrigants with improved conical laser fiber tips for removing smear layer in the apical third of the root canal. J Endod. 2008;34(12):1524–1527. doi: 10.1016/j.joen.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Akyuz Ekim SN, Erdemir A. Comparison of different irrigation activation techniques on smear layer removal: an in vitro study. Microsc Res Tech. 2015;78(3):230–239. doi: 10.1002/jemt.22466. [DOI] [PubMed] [Google Scholar]
- 27.Moon Y-M, Kim H-C, Bae K-S, Baek S-H, Shon W-J, Lee W. Effect of laser-activated irrigation of 1320-nanometer Nd: YAG laser on sealer penetration in curved root canals. J Endod. 2012;38(4):531–535. doi: 10.1016/j.joen.2011. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn K, Rudolph H, Luthardt RG, Stock K, Diebolder R, Hibst R. Er: YAG laser activation of sodium hypochlorite for root canal soft tissue dissolution. Lasers Surg Med. 2013;45(5):339–344. doi: 10.1002/lsm.22143. [DOI] [PubMed] [Google Scholar]
- 29.Arslan H, Ayrancı LB, Karatas E, Topçuoğlu HS, Yavuz MS, Kesim B. Effect of agitation of EDTA with 808-nanometer diode laser on removal of smear layer. J Endod. 2013;39(12):1589–1592. doi: 10.1016/j.joen.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Mancini M, Cerroni L, Iorio L, Armellin E, Conte G, Cianconi L. Smear layer removal and canal cleanliness using different irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): field emission scanning electron microscopic evaluation in an in vitro study. J Endod. 2013;39(11):1456–1460. doi: 10.1016/j.joen.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Olivi G. Laser use in endodontics: evolution from direct laser irradiation to laser-activated irrigation. J Laser Dent. 2013;21:58–71. [Google Scholar]
- 32.Ribeiro EM, Silva-Sousa YT, Souza-Gabriel AE, Sousa-Neto MD, Lorencetti KT, Silva SR. Debris and smear removal in flattened root canals after use of different irrigant agitation protocols. Microsc Res Tech. 2012;75(6):781–790. doi: 10.1002/jemt.21125. [DOI] [PubMed] [Google Scholar]
- 33.Guidotti R, Merigo E, Fornaini C, Rocca JP, Medioni E, Vescovi P. Er: YAG 2,940-nm laser fiber in endodontic treatment: a help in removing smear layer. Lasers Med Sci. 2014;29(1):69–75. doi: 10.1007/s10103-012-1217-x. [DOI] [PubMed] [Google Scholar]
- 34.Minamisako M, Kinoshita J, Matsimoto K, Stolf D, Morques J. A study on root canal cleaning by Nd:YAG laser with black dye solution. J Oral Laser Applications. 2009;9(2):101–109. [Google Scholar]
- 35.Asnaashari M, Safavi N. Disinfection of contaminated canals by different laser wavelengths, while performing root canal therapy. J Lasers Med Sci. 2013;4(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- 36.Santos C, Sousa-Neto MD, Alfredo E, Guerisoli DM, Pecora JD, Comelli Lia RF. Morphologic evaluation of the radicular dentine irradiated with Nd:YAG laser under different parameters and angles of incidence. Photomed Laser Surg. 2005;23:590–595. doi: 10.1089/pho.2005.23.590. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto H, Yoshimine Y, Akamine A. Visualization of irrigant flow and cavitation induced by Er: YAG laser within a root canal model. J Endod. 2011;37(6):839–843. doi: 10.1016/j.joen.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Zhang C, Yin X. Evaluation of bactericidal effect of Er;Cr:YSGG and Nd:YAG lasers in experimentally infected root canals. J Endod. 2007;33(7):830–832. doi: 10.1016/j.joen.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Hasheminia SM, Birang R, Feizianfard M, Nasouri M. A comparative study of the removal of smear layer by two endodontic irrigants and Nd: YAG Laser: a scanning electron microscopic study. ISRN Dent. 2012;2012:620951. doi: 10.5402/2012/620951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garberoglio R, Becce C. Smear layer removal by root canal irrigants: a comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1994;78(3):359–367. doi: 10.1016/0030-4220(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 41.Silveira LF, Silveira CF, Martos J, de Castro LA. Evaluation of the different irrigation regimens with sodium hypochlorite and EDTA in removing the smear layer during root canal preparation. J Microsc Ultrastructure. 2013;1(1):51–56. [Google Scholar]


