Abstract
Introduction: The treatment of cutaneous leishmaniasis (CL) is based primarily on the use of pentavalent antimonials, which may lead to many side effects limiting their use. Photodynamic therapy (PDT) is an alternative for the treatment of CL, and some xanthene dyes have the potential for use in PDT.
Methods: The xanthenes rose bengal B (RB) and its derivatives rose bengal methyl ester (RBMET), and butyl ester (RBBUT) were analyzed for leishmanicidal activity against promastigotes and intracellular amastigotes of Leishmania amazonensis. Cytotoxicity was assessed in J774.A1 macrophages.
Results: RB derivates RBMET (IC50 9.83 μM), and RBBUT (IC50 45.08 μM) showed leishmanicidal activity, however, were toxic to J774.A1 macrophages, resulting in low selectivity index.
Conclusion: The RBMET and RBBUT showed to be effective against the L. amazonensis and the low selectivity index presented may not be a limitation for their use in PDT to CL treatment
Keywords: Cutaneous leishmaniasis, Photodynamic therapy, Leishmania amazonensis, Xanthenes, Rose Bengal B
Introduction
Cutaneous leishmaniasis (CL) is a zoonotic disease characterized by skin and mucosal involvement.1 Over the past 5 years, there were more than 1 million new cases of the disease, distributed on several continents with an incidence of 90% of cases in the Americas, Central Asia, and the Middle East.2 Brazil is one of the 6 countries that contribute more than two-thirds of the CL cases worldwide.3
Treatment of CL is mainly based on pentavalent antimonials such as meglumine antimoniate. However, these drugs give rise to various adverse effects that limit their use. The main adverse effect is the action on the cardiovascular system and can also lead to kidney and pancreatic failures, in which case the treatment should be discontinued.1 Given the difficulties encountered in the treatment of CL, it is important to search for new alternatives for treatment. The photodynamic therapy (PDT) is the therapeutic modality that has stood out and shown good results.4-8
PDT is based on the use of a photosensitizer that when irradiated with light at a suitable wavelength, releases reactive oxygen species (ROS) that induce cell death.9,10 Several substances can be used as a photosensitizer, such as colorants, including natural pigments and dyes. The leishmanicidal potential of substances such as methylene blue11 and toluidine blue,6 phthalocyanines5 and uroporphyrin12 have been demonstrated. The xanthene rose bengal B (RB), and its derivatives methyl ester (RBMET), and butyl ester (RBBUT) have favorable characteristics for use in PDT. To our knowledge, there are no reports of leishmanicidal activity of these xanthenes. Therefore, the aim of this study was to evaluate the leishmanicidal activity of RB and its esters derivatives, irradiated with LED as light source.
Methods
Illumination System
Illumination system was fixed on a surface so that the entire area of the plate was illuminated uniformly. The LED emission spectrum was obtained in a Varian-Cary Eclipse spectrofluorometer (Agilent Technologies, USA) and the light potencies were measured by a Handheld Laser Power Meter (Edmund Optics Inc., USA). This illumination system was positioned at 2 cm under the 96-well plate (Figure 1).
Figure 1.

Illumination System. It was composed of surface mounted diode (SMD) LED (575 nm/1.66 mW/cm-2).
Assay on Promastigotes and Intracellular Amastigotes
Promastigotes of Leishmania amazonensis (MHOM/BR/1977/LTB0016) were cultured in RPMI 1640 (Gibco, New York, NY, USA) pH 6.8 supplemented with 10% fetal calf serum, containing 100 IU/mL penicillin G and 0.1 mg/mL of streptomycin, until the exponential growth phase. For the evaluation of leishmanicidal activity, different concentrations of RB (370 mM) (Neon, São Paulo, Brazil) and its derivatives RBMET (122.5 mM) and RBBUT (56 mM) were diluted in twofold in 96-well plates and incubated with 4×107 L. amazonensis promastigotes/mL. The plates were incubated at 25°C for 30 minutes and after that, the plates were illuminated for 30 minutes with the illumination system under conditions described above. As controls, untreated promastigotes were used, treated and not lighted. After incubation at 25°C for 24, 48 and 72 hours, cell viability was measured by the XTT reduction method.13 The inhibitory concentration of 50% of the parasites (IC50) was calculated by linear regression equation.13 Peritoneal macrophages BALB/c mice were infected with L. amazonensis promastigotes (6 parasites/macrophage) as in Honda et al.14 Photosensitizers RBMET (8. 7 mM) and RBBUT (17.2 mM) were diluted in twofold, the protocol of incubation and illumination was the same as described above.
Cytotoxicity on Macrophages
Before the treatment, macrophages from cell line J774.A1 were cultured in RPMI 1640 (Gibco, New York, NY, USA) pH 7.6 supplemented with 10% fetal calf serum, containing 100 IU/mL penicillin G and 0.1 mg/mL of streptomycin. For the cytotoxicity assay J774.A1 macrophages were cultured in 96-well plates (5×104 macrophages/well) for 48 hours at 37°C with 5% CO2. After, RBMET (226 mM) and RBBUT (228 mM) were diluted in twofold and added to the wells. The lighting protocol was the same as used in antileishmanial assays. After the treatment, the plates were incubated for 24 hours at 37°C with 5% CO2 and cell viability was measured by the XTT reduction method. The 50% cytotoxic concentration (CC50) was calculated by the linear regression equation. The selectivity index (SI) was calculated as the ratio CC50/IC50.
Statistical Analysis
Statistical analysis was performed using Statistica 12.0 (StatSoft, Tulsam OK, USA) software, considering the significance level of 5%. The normal distribution of the data was verified by the Shapiro-Wilk’s W. The results of absorbance were analyzed using the Mann-Whitney U test.
Results
In PDT, the RBMET and RBBUT presented leishmanicidal activity to promastigotes (Figure 2), while the RB did not. The activity was only observed when photosensitizer was illuminated (P < 0.05). The illumination in the absence of photosensitizer did not result in leishmanicidal activity (P < 0.05).
Figure 2.
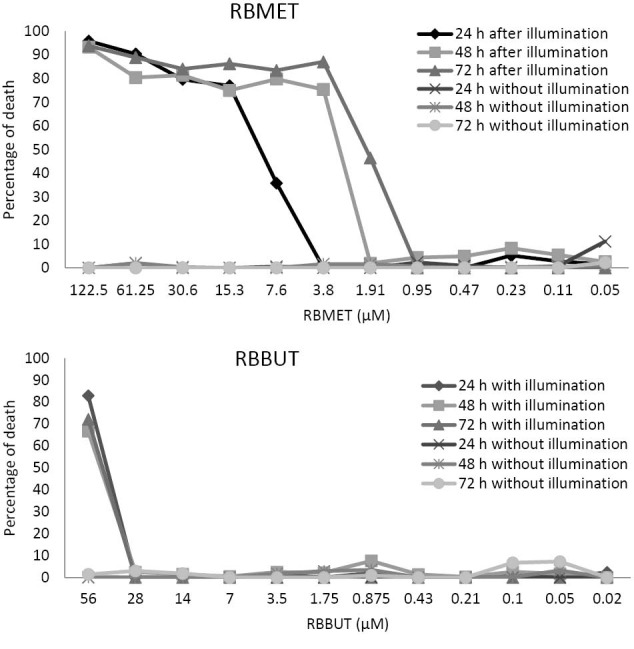
Antileishmanial Activity of Ester Derivatives of Rose Bengal B. Percentage of death after 24, 48 and 72 hours of Leishmania amazonensis promastigotes after incubation with RBMET or RBBUT for 30 minutes in the dark and illuminated for 30 minutes with LED SMD white. RBMET, rose bengal methyl ester; RBBUT, rosa bengal butyl ester. *P < 0.05 in relation to the treated and not illuminated group. #P < 0.05 compared to control that only was illuminated.
The RBMET was effective at concentrations of 7.6 μM to 122.5 μM (24 hours), the 3.8 μM to 122.5 μM (48 hours) and the 1.9 μM to 122.5 μM (72 hours). The RBBUT presented leishmanicidal activity at the concentration of 56 µM in the 3 intervals analyzed.
The IC50 of the RBMET was 9.83 μM, 4.72 μM and 3.31μM for 24, 48 and 72 hours, respectively. For the RBBUT, the IC50 was 45.08 μM, 49.00 μM, and 47.80 μM for 24, 48 and 72 hours, respectively (Table 1).
Table 1.
IC50, CC50 and Selectivity Index Values of the Rose Bengal Methyl Ester and Rose Bengal Butyl Ester 24 Hours of Treatment
The RBMET and RBBUT, in the evaluated concentrations, have led to lysis of macrophages and consequently it was not possible to evaluate the activity for intracellular amastigotes (Figure 3).
Figure 3.
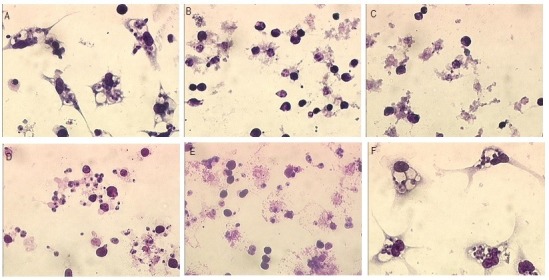
Peritoneal Macrophages of BALB/c Mice Infected With Leishmania amazonensis Promastigotes (6 Parasites/Macrophages). (A) Infected and illuminated control; (B and C) Treatment with RBMET 4.33 μM and 2.16 μM respectively; (D and E) Treatment with RBBUT 4.28 μM and 2.14 μM respectively; (F) Infected and not illuminated control. The slides were observed at ×1000 magnification.
PDT with RBMET and RBBUT was cytotoxic to J774.A1 macrophages. Toxicity was greater when the photosensitizer was illuminated (P < 0.05), however, also exhibited high cell death in the absence of light. There was no difference between illuminated and not illuminated controls (P > 0.05). The CC50 for PDT of RBMET was 3.44 μM and for the RBBUT was 3.50 μM. The selectivity index to RBMET and RBBUT were 0.34 and 0.07, respectively.
Discussion
The photosensitizers RBMET and RBBUT under LED light have activity against L. amazonensis. The IC50 for RBMET (9.83 μM) approximate the IC50 against L. amazonensis for amphotericin B (14.9 μM and 6.1 μM) found by Miranda et al,15 and Almeida et al,16 respectively. In contrast, to the IC50 for RBBUT (45.08 μM) which was higher than the amphotericin B.
However, these 2 photosensitizers were toxic to J774.A1 and mice peritoneal macrophages, but the CC50 value found for RBMET and RBBUT were similar to CC50 found by Cabanillas et al,17 for amphotericin B. They reported that a 4.3 μM concentration of this drug was able to kill 50% of peritoneal macrophages from BALB/c mice. On the other hand, pentamidine showed to be less toxic to macrophages compared to these photosensitizers.18,19
Consequently, RBMET and RBBUT presented low selectivity index. Reference drugs have selectivity index higher than photosensitizers in question. Garcia et al20 reported that the SI for pentamidine is 32, according to Cabanillas et al17 the SI is 29 for amphotericin B and for Dutra et al21 it is 7.16. Despite it, the low selectivity index found for the RBMET, and RBBUT may not be a limitation for their use in PDT. Since the PDT is applied on the lesion, it may even contribute to the destruction of infected cells, increasing parasite exposure to PDT action. Studies show that bengal B when illuminated by a light source with a specific wavelength helps in healing without causing toxicity.22,23 There are no studies like this with the esters derived from the RBMET and RBBUT, but we believe that they can behave similarly.
In contrast, the RB did not present antileishmanial activity. This result can be rationally explained by the fact that RB is dianionic while RBMET and RBBUT are monoanionic at aqueous physiological pH. The charges of RB turn this compound highly hydrophilic thus showing low interaction to biomembranes while the RBMET and RBBBUT are hydrophobic due to their low charge which increases the affinity to cells. Furthermore, the presence of more hydrophobic radicals (alkyl groups) in the RBMET and RBBUT can have facilitated their interaction with the cell membrane. The use of the pharmaceutical formulations can be an alternative to improve the photodynamic activity of these photosensitizers mainly RB.
Conclusion
PDT stands out as an alternative for the treatment of CL. It presents the advantage of topical administration, reducing the discomfort and adverse side effects to the patient. Our results show that RBMET and RBBUT under LED light have activity against L. amazonensis, thus, the RBMET and RBBUT are promising candidates for use as photosensitizers in PDT, to treat LC. Therefore, studies of absorption and toxicity in topical use of RBMET and RBBUT should be conducted to evaluate their potential use in the treatment of CL.
Ethical Considerations
All procedures were approved by the Committee on Ethical Conduct on the Use of Animals In Experiments of the State University of Maringá, Paraná, Brazil (Report 6441/2013).
Conflict of Interests
The authors declare no conflict of interest, financial or other exists.
Acknowledgments
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação Araucária.
Please cite this article as follows: Navasconi TR, dos Reis VN, Freitas CF, et al. Photodynamic therapy with bengal rose and derivatives against Leishmania amazonensis. J Lasers Med Sci. 2017;8(1):46-50. doi:10.15171/jlms.2017.09.
References
- 1. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Manual de Vigilância da Leishmaniose Tegumentar Americana. 2nd ed. Brasília: Editora do Ministério da Saúde; 2013. http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar_americana_2edicao.pdf.
- 2. Leishmaniasis. Word Health Organization website. http://goo.gl/iSw9KC. Accessed September 20, 2014. Published 2014.
- 3. Leishmaniose. Word Health Organization website. http://goo.gl/5mOxJT. Accessed September 20, 2014.
- 4.Evangelou G, Krasagakis K, Giannikaki E, Kruger-Krasagakis S, Tosca A. Successful treatment of cutaneous leishmaniasis with intralesional aminolevulinic acid photodynamic therapy. Photodermatol Photoimmunol Photomed. 2011;27:254–256. doi: 10.1111/j.1600-0781.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- 5.Dutta S, Waki K, Chang KP. Combinational sensitization of Leishmania with uroporphyrin and aluminum phthalocyanine synergistically enhances their photodynamic inactivation in vitro and in vivo. Photochem Photobiol. 2012;80:620–625. doi: 10.1111/j.1751-1097.2012.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbosa AF, Sangiorgi BB, Galdino SL, Barral-Netto M, Pitta IR, Pinheiro AL. Photodynamic antimicrobial chemotherapy (PACT) using phenothiazine derivatives as photosensitizers against Leishmania braziliensis. Lasers Surg Med. 2012;44:850–855. doi: 10.1002/lsm.22099. [DOI] [PubMed] [Google Scholar]
- 7.Pizinger K, Cetkovska P, Kacerovska D, Kumpova M. Successful treatment of cutaneous leishmaniasis by photodynamic therapy and cryotherapy. Eur J Dermatol. 2009;19(2):172–173. doi: 10.1684/ejd.2008.0587. [DOI] [PubMed] [Google Scholar]
- 8.Sbeghen MR, Voltarelli EM, Cmpois TG. et al. Topical and intradermal efficacy of photodynamic therapy with methylene blue and light-emitting diode in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis. J Lasers Med Sci. 2015;6(3):106–101. doi: 10.15171/jlms.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zollinger H. Color chemistry: syntheses, properties, and applications of organic dyes and pigments. Wiley-VCH; 1991.
- 10. Gilbert A, Baggott JE. Essentials of molecular photochemistry. CRC Press; 1991.
- 11.Peloi LS, Biondo CE, Kimura E. et al. Photodynamic therapy for American cutaneous leishmaniasis: the efficacy of methylene blue in hamsters experimentally infected with Leishmania (Leishmania) amazonensis. Exp Parasitol. 2011;128:353–356. doi: 10.1016/j.exppara.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Escobar P, Hernandez IP, Rueda CM, Martinez F, Paez E. Photodynamic activity of aluminium (III) and zinc (II) phthalocyanines in Leishmania promastigotes. Biomedica. 2006;26(1):49–56. [PubMed] [Google Scholar]
- 13.Demarchi IG, Tomazella MV, Terron MS. et al. Antileishmanial activity of essential oil and 6,7-dehydroroyleanone isolated from Tetradenia riparia. Exp Parasitol. 2015;157:128–137. doi: 10.1016/j.exppara.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Honda PA, Ferreira ICP, Cortez DAG. et al. Efficacy of components from leaves of Calophyllum brasiliense against Leishmania (Leishmania) amazonensis. Phytomedicine. 2010;17:333–338. doi: 10.1016/j.phymed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Abreu Miranda M, Tiossi RF, da Silva MR. et al. In vitro leishmanicidal and cytotoxic activities of the glycoalkaloids from Solanum lycocarpum (Solanaceae) fruits. Chem Biodivers. 2013;10(4):642–648. doi: 10.1002/cbdv.201200063. [DOI] [PubMed] [Google Scholar]
- 16.Almeida L, Alves KF, Maciel-Rezende CM. et al. Benzophenone derivatives as cysteine protease inhibitors and biological activity against Leishmania(L) amazonensis amastigotes. Biomed Pharmacother. 2015;75:93–99. doi: 10.1016/j.biopha.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Cabanillas BJ, Le Lamer AC, Olagnier D. et al. Leishmanicidal compounds and potent PPARgamma activators from Renealmia thyrsoidea (Ruiz & Pav) Poepp & Endl. J Ethnopharmacol. 2014;157:149–155. doi: 10.1016/j.jep.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Araujo MV, de Souza PS, de Queiroz AC. et al. Synthesis, leishmanicidal activity and theoretical evaluations of a series of substituted bis-2-hydroxy-1,4-naphthoquinones. Molecules. 2014;19(9):15180–15195. doi: 10.3390/molecules190915180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gontijo VS, Espuri PF, Alves RB. et al. Leishmanicidal, antiproteolytic, and mutagenic evaluation of alkyltriazoles and alkylphosphocholines. Eur J Med Chem. 2015;101:24–33. doi: 10.1016/j.ejmech.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Garcia M, Mozonte L, Schull R, Herrera P. Activity of Cuban Plants Extracts against Leishmania amazonensis. ISRN Pharmacol. 2012;2012:104540. doi: 10.5402/2012/104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutra LA, de Almeida L, Passalacqua TG. et al. Leishmanicidal activities of novel synthetic furoxan and benzofuroxan derivatives. Antimicrob Agents Chemother. 2014;58(8):4837–4847. doi: 10.1128/AAC.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao M, Yaroslavsky A, Henry FP, Redmond RW, Kochevar IE. Phototoxicity is not associated with photochemical tissue bonding of skin. Lasers Surg Med. 2010;42(2):123–131. doi: 10.1002/lsm.20869. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Yao M, De Martelaere SL, Redmond RW, Kochevar IE. Light-activated sutureless closure of wounds in thin skin. Lasers Surg Med. 2012;44(2):163–167. doi: 10.1002/lsm.21137. [DOI] [PubMed] [Google Scholar]



