Abstract
Background and Objectives:
Vulvovaginal candidiasis is a common fungal infection among women during reproductive ages. Although, Candida albicans is accounted as the main etiologic agent of vaginitis, non-albicans species have arisen during last years. Resistant to antifungal drugs especially, fluconazole has been more reported by researchers from around the World. The aims of this study were to determine the prevalence of vulvovaginal candidiasis among suspected patients with vaginitis, the frequency of Candida species, and the susceptibility profiles of isolates to caspofungin, fluconazole and clotrimazole.
Materials and Methods:
One hundred and twenty suspected women with vaginitis were examined by specialist physician and sampled using moisture swabs. Swabs were inoculated on CHROMagar Candida plates, incubated at 35°C and detected all isolated Candida species using morphological, microcopy and molecular methods. The antifungal susceptibility tests with caspofungin, fluconazole and clotrimazole were applied using microdilution and Resazurin dye methods against all isolated yeasts.
Results:
The cultures were positive for 34(28.3%) samples and three Candida species including; C. albicans (88.2%), C. glabrata (8.8%) and C. kefyr (2.9%). Our study shows that only one isolate of C. albicans was resistant to caspofungin at the concentration of 2 μg/ml after 24h incubation that increased to 2 isolates after 48h incubation. All isolates were sensitive to fluconazole at the MIC ranges of 1-0.25 μg/ml, while 88.2% of them were inhibited at 0.25 μg/mL of clotrimazole. Candida albicans remains the most common agent of fungal vaginitis.
Conclusion:
Although all of Candida isolates were susceptible to fluconazole in vitro, it should be used with caution for empirical therapy due to more resistant rates in clinic. In addition, due to valuable sensitivity of all tested strains to caspofungin, it potentially can be presented as the first line therapy for Candida vaginitis.
Keywords: Caspofungin, Fluconazole, Clotrimazole, Candida vaginitis, Candida albicans
INTRODUCTION
Vaginitis or vulvovaginal candidiasis is a common fungal infection among women during reproductive ages with worldwide distribution (1). In addition, immunocompromised patients (especially HIV positive) are usually associated with chronic or persistent forms of vaginitis (2). External factors such as, IUD using, antibacterial and corticosteroid therapy, type II diabetes, and psychosocial stress are important risk factors for Candida vaginitis (3–5). The frequency of disease varies in reports from 62.1% (6), 49% (2) and, 28.2% (7). Although, several antifungal drugs were applied for vulvovaginal candidiasis, the rate of disease did not decrease during several last decades. Many reports have shown that 75% of healthy women experiences at least one symptomatic vulvovaginal candidiasis during their lifetime (8). On the other hand, some reports indicated that vulvovaginal candidiasis is an important problem among pregnant women (9). In these cases, neonates (particularly, low birth weight and prematurity) are usually exposed to systemic candidiasis after delivery (9).
Candida albicans was accounted as the first vulvovaginal candidiasis causative agent followed by C. glabrata and C. tropicalis (10–12). Others non-albicans species included; C. africana (13), C. dubliniensis (13), C. parapsilosis (14), C. guilliermondii (3), C. nivariensis (15), C. bracarensis (15), C. kefyr (4), Saccharomyces cerevisiae (16), C. pintolopesii (7) and C. krusei (17). An increasing from 10% to 20% was reported from 1970s to 1980s for C. glabrata and C. tropicalis as Candida vaginitis agents (3). Clotrimazole and fluconazole are two azole antifungal drugs that are usually prescribed for Candida vaginitis treatment. Although, fluconazole was routinely used for the treatment of vaginal candidiasis, the rate of resistance varies in different studies, 11.8% to 94% (6, 18, 19). The sensitivity of C. tropicalis and C. glabrata to miconazole is 10 times less than C. albicans (3).
Some studies have shown that vaginitis due to C. krusei is resistant to fluconazole (5). Furthermore, clotrimazole resistant strains (1.8%) were also reported (18). Caspofungin is a new echinocandin antifungal that has been used during last two decades for invasive fungal infection in immunocompromised patients (20) and, transplant recipients (21). It inhibits cell wall 1,3-β-D-glucan synthesis (22) and there are few available reports associated with resistance to this antifungal. Caspofungin has an excellent safety profile with good efficacy for antifungal therapy among patients with invasive aspergillosis and candidiasis (23, 24). On the other hand, few reports evaluated the efficacy of caspofungin against vaginal Candida isolates in vitro. The aims of this study were to determine the prevalence of vulvovaginal candidiasis among patients suspected to vaginitis, the frequency of Candida species, and the susceptibility profiles of isolates to caspofungin, fluconazole and clotrimazole.
MATERIALS AND METHODS
Patients and sampling
This project was approved in the ethical committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS. REC.1394.285). All patients were signed the consent form before sampling. Demographic details and pre-disposing factors for each patient were recorded. In the present study 120 suspected women with vaginitis were examined by the specialist physician and sampled using moisture swabs. The presence of signs and symptoms including, erythema, edema, excoriation, discharge, pruritus, soreness, and burning were defined as common signs and symptoms of Candida vaginitis. Vaginal swabs were inoculated on CHROMagar Candida (CHROMagar Candida, France) plates, incubated at 35°C for 4 days, aerobically. All plates were evaluated for the yeast growth and colony colour every day. Direct microscopy slides were prepared from each colony for yeast confirmation. All yeast isolates were duplicately subcultured on Sabouraud dextrose agar (SDA, Merck, Germany) plates and kept at room temperature for future mycological analyses.
Laboratory procedures
After isolation from CHROMagar Candida medium, all yeast strains were subsequently identified using classical mycological tests for the genus Candida, such as germ tube formation on fresh human serum at 37°C, microscopic morphology on cornmeal agar (High Media, India) with 1% Tween 80 (Merck, Germany) and growth at 42–45°C for 24–48h (25).
PCR-RFLP method
For molecular identification, a loopful of fresh colony of each strain was suspended in a 1.5 ml microtube contained 100 μl of deionized distilled water and heated at 100°C for 10 minutes. Then, microtubes were centrifuged at 4000 g for 10 minutes. The supernatants were transferred to a new microtube and kept at −20°C as DNA. The nuclear ribosomal ITS1-5.8S-ITS2 regions of the strains were amplified through PCR from extracted DNAs using the ITS1/ITS4 primer pair (26). The obtained products were then subjected to restriction analysis with MspI enzyme (Thermo Fisher Scientific, Waltham, MA, USA). For final identification of the isolates, the restriction products were electrophoresed through 2% agarose gel, and size of digested fragments were compared with those determined in the previous report (27).
Antifungal stock solutions
A stock solution of antifungal drugs including; caspofungin (Sigma-Aldrich, Germany) 1.25 mg/ml, fluconazole (Serva, USA) 32 mg/ml and clotrimazole (Sigma-Aldrich, Germany) 32 mg/ml was prepared in dimethyl sulf-oxide (DMSO, Fluka, Germany). Antifungal stocks were kept at −20°C until use.
RPMI 1640 containing Resazurin
The 0.01g of Resazurin (Sigma-Aldrich, Germany) was completely dissolved in 100 ml of distilled water and then sterilized using a syringe filter (0.45μm). Stock solution was kept in a brown bottle at 4°C until use.
Standard suspension preparation
A suspension of overnight culture of each isolate on SDA incubated at 35°C was prepared in sterile PBS. Suspensions were adjusted to 0.5 McFarland standard (1.5 × 108 CFU/ml) using a spectrophotometer. The suspensions were diluted as 1:50 with RPMI 1640 containing Resazurin in sterile condition.
In vitro antifungal susceptibility test
The antifungal susceptibility tests with caspofungin, fluconazole and clotrimazole were applied using microdilution and Resazurin dye methods against all vaginal isolates (28, 29). Briefly, 100 μl of serial dilution of each antifungal and 100 μl of standard suspension were added to microplate wells. Serial dilutions were as 4 to 0.03125μg/ml for caspofungin, 32 to 0.25μg/ml for fluconazole and 16 to 0.125μg/ml for clotrimazole. Positive and negative controls were also included in test as RPMI 1640 with and without fungal suspension, respectively. The minimum inhibitory concentration (MIC) ranges and geometric means (GM) were determined after 24 and 48h of incubation. Furthermore, the MIC50 and MIC90 values were calculated for those species with 10 or more isolates (25).
RESULTS
Demographic results
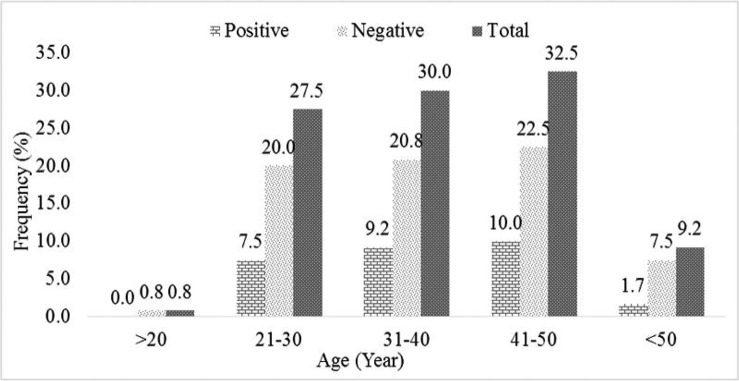
Totally 120 women suspected to vulvovaginal candidiasis were physically examined and sampled for fungal infection. Patients ages ranged from >20 to <50 years, however most of the patients were in the 41–50 years old groups. Table 1 shows the details of predisposing factor among 120 sampled patients. As shown several predisposing factors were found in 65% of sampled patients. The most frequent predisposing factor was history of vaginitis, in 54 cases out of 120 suspected patients (45%).
Table 1.
Predisposing factors among 120 sampled patients
| Predisposing factors | Age groups (Year) | Total | ||||
|---|---|---|---|---|---|---|
| >20 | 21–30 | 31–40 | 41–50 | <50 | ||
| Contraceptive use | 0(0.0%) | 2(2.6%) | 4(5.1%) | 0(0.0%) | 0 (0.0%) | 6(7.7%) |
| Antibiotic therapy | 0(0.0%) | 4(5.1%) | 3(3.8%) | 4(5.1%) | 1(1.3%) | 12(15.4%) |
| Previous vaginitis | 0(0.0%) | 18(23.1%) | 15(19.2%) | 14(17.9%) | 7(9.0%) | 54(69.2%) |
| Diabetes | 1(1.3%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 4(5.1%) | 5(6.4%) |
| Cancer | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 1(1.3%) | 1(1.3%) |
| Total | 1(1.3%) | 24(30.8%) | 22(28.2%) | 18(23.1%) | 13(16.7%) | 78(100%) |
Fig. 1.
The frequency of positive and negative samples for Candida strains among different age groups
Culture results
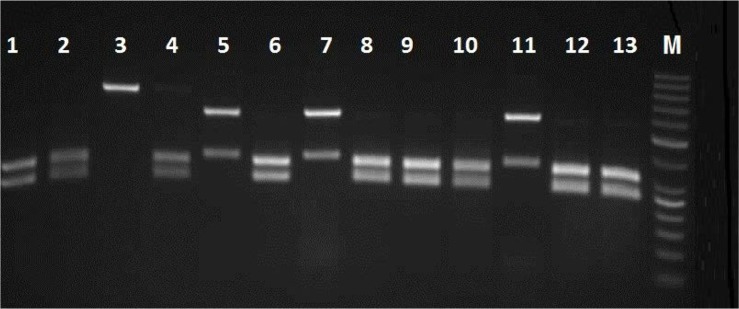
The culture results show that 28.3% (34 of 120) of sampled patients were yielded different species of Candida. According to classical and molecular identification techniques, 30(88.2%) isolates of C. albicans, 3(8.8%) isolates of C. glabrata and one (2.9%) isolate of C. kefyr were detected. Fig. 2 shows the PCR-RFLP results for identification of some study isolates representative of three species of Candida.
Fig. 2.
Agarose gel electrophoresis of ITS-rDNA RFLP profiles by MspI for representative isolates of C. albicans, C. glabrata and C. kefyr
Lanes 1, 2, 4, 6, 8, 9, 10, 12 and 13; clinical isolates of C. albicans, Lanes 5,7 and 11; C. glabrata and lane 3; C. kefyr, M; 50 bp DNA marker
Susceptibility test results
Different concentrations of caspofungin, clotrimazole and fluconazole were applied against 34 isolates of Candida recovered from Candida vaginitis. As shown in the Table 2, only one isolate of C. albicans were resistant to caspofungin at the concentration of 2 μg/ml after 24h incubation that increased to 2 isolates after 48h incubation. MIC50 and MIC90 for all isolates, albicans and non-albicans Candida species, were found to be at 1 μg/ml. All C. albicans isolates were sensitive to fluconazole at the MIC ranges 1-0.25 μg/ml, while non-albicans species of C. glabrata and C. kefyr, were associated to 0.25 μg/ml (Table 2). The 88.2% of isolates were inhibited at 0.25 μg/ml of clotrimazole (sensitive to clotrimazole), whereas three isolates found to be dose dependent at the concentration of 0.5 μg/ml. Only one isolate (2.9%) was inhibited at 1 μg/ml of clotrimazole which considered as resistant to drug (Table 2).
Table 2.
Geometric mean MIC, MIC range, MIC50 and MIC90 (μg/ml) of caspofungin, fluconazole and clotrimazole for 34 tested Candida strains
| Caspofungin (μg/ml) | C. albicans (30) | C. glabrata (3) | C. kefyr (1) | Total (34) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 24h | 48h | 24h | 48h | 24h | 48h | ||
| R | 2 | 1 | 2 | - | - | - | - | 1 | 2 |
| 1 | 24 | 26 | - | - | - | 1 | 24 | 27 | |
| S | 0.5 | 3 | 2 | - | - | - | - | 3 | 2 |
| 0.25 | 2 | - | - | - | - | - | 2 | - | |
| 0.125 | - | - | 3 | 3 | 1 | - | 4 | 3 | |
| MIC50 | 1 | 1 | - | - | - | - | 1 | 1 | |
| MIC90 | 1 | 1 | - | - | - | - | 1 | 1 | |
| MICGM | 0.87 | 1 | - | - | - | - | 0.69 | 0.83 | |
| Fluconazole (μg/ml) | |||||||||
| 1 | 2 | 5 | - | - | - | - | 2 | 5 | |
| S | 0.5 | 7 | 6 | - | - | - | - | 7 | 6 |
| 0.25 | 21 | 19 | 3 | 3 | 1 | 1 | 25 | 23 | |
| 0.125 | - | - | - | - | - | - | - | - | |
| MIC50 | 0.25 | 0.25 | - | - | - | - | 0.25 | 0.25 | |
| MIC90 | 0.5 | 1 | - | - | - | - | 0.5 | 1 | |
| MICGM | 0.32 | 0.36 | - | - | - | - | 0.31 | 0.35 | |
| Clotrimazole (μg/ml) | |||||||||
| R | 1 | 1 | 3 | - | - | - | - | 1 | 3 |
| DD | 0.5 | 3 | 2 | - | - | - | - | 3 | 2 |
| S | 0.25 | 26 | 25 | 3 | 3 | 1 | 1 | 30 | 29 |
| 0.125 | - | - | - | - | - | - | - | - | |
| MIC50 | 0.25 | 0.25 | - | - | - | - | 0.25 | 0.25 | |
| MIC90 | 0.5 | 0.5 | - | - | - | - | 0.5 | 0.5 | |
| MICGM | 0.29 | 0.32 | - | - | - | - | 0.28 | 0.29 | |
MIC: Minimum Inhibitory Concentration; GM: Geometric Mean; R, Resistant; DD: Dose dependent; S, sensitive
DISCUSSION
Vulvovaginal candidiasis is one of the most common fungal infections among adult women during bearing child period. Several authors have believed that 75% of women are affected at least once during lifetime (3, 30). Furthermore, chronic vaginitis and recurrent vulvovaginal candidiasis were more reported among several groups of women (31–34). Both forms of diseases are problematic conditions for patients. The healthy women vagina is containing several normal microflora including C. albicans, and patients associated factors, organism pathogenic factors and external factors are interference in involving disease. In our study, the overall prevalence of Candida vaginitis was found to be 28.3% which is similar to Fornari et al. (35), Rasti et al. (9), and Hedayati et al. (7), while it is in contrast to Mohamadi et al. (6). Furthermore, 61.8% of cases had at least one episode of Candida vaginitis that similar to previous study (11). Our results show that in the studied population, antibiotic treatment, diabetes and contraceptive use were linked with vulvovaginal candidiasis as described before (11, 31, 36).
Although, C. albicans is considered as a vaginal mycoflora and the main causative agent of vaginal candidiasis (2, 37), non-albicans species have increased during last decades (3, 37, 38). Besides, a report from India indicated that C. glabrata was isolated from 50.4% patients with vulvovaginal candidiasis (39). Most reports have shown that C. glabrata, C. tropicalis and C. krusei were accounted as the second, third and fourth common agents of disease, respectively (14, 37, 39). Though, in the study by Mohammadi et al. C. kefyr was reported as the third agent of fungal vulvovaginitis in Iran (27). Furthermore, some researchers reported C. dubliniensis as the third causative agent (7, 14). Consistent with these facts, the frequency of C. albicans in our study was 88.2% followed by C. glabrata (8.8%). C. kefyr is an uncommon vulvovaginal candidiasis agent that previously was reported by Hedayati et al., (8.2%) (7), Mohammadi et al. (5.8%) (27), Fornari et al. (2.5%) (35), and Alfouzan et al. (1.9%) (16). The rate of C. kefyr isolation in our study was nearly similar to above reports (2.9%).
Although, topical clotrimazole, nystatin and miconazole are usually prescribed for vulvovaginal candidiasis, however a single dose of oral fluconazole is more acceptable in some patients both for prophylaxis and cure. On the other hand, resistance to fluconazole among less sensitive Candida species (especially non-albicans such as, C. glabrata, C. tropicalis and C. krusei) has been increased during last decades (30, 40). Mohanty et al. have shown that 70% and 30% of vaginal isolates of Candida were sensitive (MIC ≤ 8 μg/ml) and dose dependent (MIC, 16–32 μg/ml) to fluconazole, respectively and 77.8% of dose dependent isolates were C. glabrata (39). On the other hand, resistant rate for fluconazole was 57.1% for C. krusei strain in the study by Guzel et al. (30). Previously, we reported that 34.8% of vaginal isolates of Candida were resistant to fluconazole in vitro (14).
The resistance rate to C. albicans was 4.7% for caspofungin in vaginal isolates in China (8). In a clinical trial, no significant difference was observed in response of patients with Candida vaginitis after treatment with fluconazole and clotrimazole. On the other hand, disease recurrence was 6.9% and 9.7% for fluconazole and clotrimazole groups, respectively (41). In our study, all isolates were highly sensitive to fluconazole as MIC for 73.5% of them including all non-albicans was 0.25 μg/ml. Although, resistant to antifungal is not common, it increased during several last decades due to widespread prophylaxis use as well as to be over counter some antifungals. Furthermore, some strains of C. krusei and C. glabrata are genetically resistant to some antifungals. Diaz et al. and Moges et al. studies shown that 6 and 4 isolates of Candida species were respectively resistant to clotrimazole (42, 43). Balashov et al. have believe that resistance to caspofungin is linked to mutations in FKS1 gene (44). In our study, only one isolate of C. albicans had MIC 2 μg/ml (Resistant) for caspofungin after 24h incubation that increased into two strains after 48h. Whereas, resistant to clotrimazole (MIC = 1μg/ml) was found in one and three isolates of C. albicans after 24h and 48h incubation.
CONCLUSION
In conclusion, 28.3% of examined patients had positive cultures for three different species of Candida. The most common agent was C. albicans followed by, C. glabrata and C. kefyr. Although all of Candida isolates were susceptible to fluconazole in vitro, it should be used with caution for empirical therapy due to more resistant rates in clinic. In addition, due to valuable sensitivity of all tested strains to caspofungin, it can be presented as first line therapy for Candida vaginitis. However, there is not a suitable caspofungin formulation for the treatment of vaginal candidiasis due to lack of data about effect of drug on causative agents. According to our study, we can claim that caspofungin has an effective efficacy against vaginal agents. However, several clinical trials are needed for confirm our hypothesis.
ACKNOWLEDGEMENT
We would like to thank the Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences for their support. This study was an MSc thesis (Shokoofe Shafiei) supported by a grant (No: OG-94139) from the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
REFERENCES
- 1.Shokri H, Khosravi AR, Yalfani R. Antifungal efficacy of propolis against fluconazole-resistant Candida glabrata isolates obtained from women with recurrent vulvovaginal candidiasis. Int J Gynaecol Obstet 2011;114:158–159. [DOI] [PubMed] [Google Scholar]
- 2.Zarei Mahmoudabadi A, Najafyan M, Alidadi M. Clinical study of Candida vaginitis in Ahvaz, Iran and susceptibility of agents to topical antifungal. Pak J Med Sci 2010;26:607–610. [Google Scholar]
- 3.Diba K, Namaki A, Ayatolahi H, Hanifian H. Rapid identification of drug resistant Candida species causing recurrent vulvovaginal candidiasis. Med Mycol J 2012;53:193–198. [DOI] [PubMed] [Google Scholar]
- 4.Ray A, Ray S, George AT, Swaminathan N. Interventions for prevention and treatment of vulvovaginal candidiasis in women with HIV infection. Cochrane Database Syst Rev 2011(8):CD008739. [DOI] [PubMed] [Google Scholar]
- 5.Mendling W, Brasch J, German Society for G, Obstetrics, Working Group for I, Infectimmunology in G et al. Guideline vulvovaginal candidosis (2010) of the German society for gynecology and obstetrics, the working group for infections and infectimmunology in gynecology and obstetrics, the German society of dermatology, the board of German dermatologists and the German speaking mycological society. Mycoses 2012;55 Suppl 3:1–13. [DOI] [PubMed] [Google Scholar]
- 6.Mohamadi J, Havasian MR, Panahi J, Pakzad I. Anti-fungal drug resistance pattern of Candida. spp isolated from vaginitis in Ilam-Iran during 2013–2014. Bioinformation 2015;11:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedayati MT, Taheri Z, Galinimoghadam T, Aghili SR, Yazdani Cherati J, Mosayebi E. Isolation of different species of Candida in patients with vulvovaginal candidiasis from Sari, Iran. Jundishapur J Microbiol 2015;8(4):e15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi XY, Yang YP, Zhang Y, Li W, Wang JD, Huang WM, et al. Molecular identification and antifungal susceptibility of 186 Candida isolates from vulvovaginal candidiasis in southern China. J Med Microbiol 2015;64(Pt 4):390–393. [DOI] [PubMed] [Google Scholar]
- 9.Rasti S, Asadi MA, Taghriri A, Behrashi M, Mousavie G. Vaginal candidiasis complications on pregnant women. Jundishapur J Microbiol. 2014;7(2):e10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G, et al. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015;58 Suppl 1:1–15. [DOI] [PubMed] [Google Scholar]
- 11.Gamarra S, Morano S, Dudiuk C, Mancilla E, Nardin ME, de Los Angeles Mendez E, et al. Epidemiology and antifungal susceptibilities of yeasts causing vulvovaginitis in a teaching hospital. Mycopathologia 2014;178(3–4):251–258. [DOI] [PubMed] [Google Scholar]
- 12.Zarei Mahmoudabadi A, Najafyan M, Moghimipour E, Alwanian M, Seifi Z. Lamisil versus clotrimazole in the treatment of vulvovaginal candidiasis. Iran J Microbiol 2013;5:86–90. [PMC free article] [PubMed] [Google Scholar]
- 13.Shan Y, Fan S, Liu X, Li J. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Med Mycol 2014;52:636–640. [DOI] [PubMed] [Google Scholar]
- 14.Seifi Z, Zarei Mahmoudabadi A, Zarrin M. Extra-cellular enzymes and susceptibility to fluconazole in Candida strains isolated from patients with vaginitis and healthy individuals. Jundishapur J Microbiol 2015;8(3):e20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Shan Y, Fan S, Liu X. Prevalence of Candida nivariensis and Candida bracarensis in vulvovaginal Candidiasis. Mycopathologia 2014;178(3–4):279–283. [DOI] [PubMed] [Google Scholar]
- 16.Alfouzan W, Dhar R, Ashkanani H, Gupta M, Rachel C, Khan ZU. Species spectrum and antifungal susceptibility profile of vaginal isolates of Candida in Kuwait. J Mycol Med 2015;25:23–28. [DOI] [PubMed] [Google Scholar]
- 17.Wang FJ, Zhang D, Liu ZH, Wu WX, Bai HH, Dong HY. Species distribution and In vitro antifungal susceptibility of vulvovaginal Candida isolates in China. Chin Med J 2016;129:1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XP, Fan SR, Peng YT, Zhang HP. Species distribution and susceptibility of Candida isolates from patient with vulvovaginal candidiasis in Southern China from 2003 to 2012. J Mycol Med 2014;24:106–111. [DOI] [PubMed] [Google Scholar]
- 19.Nasrollahi Z, Yadegari MH, Roudbar Mohammadi S, Roudbary M, Hosseini Poor M, Nikoomanesh F, et al. Fluconazole resistance Candida albicans in females with recurrent vaginitis and Pir1 overexpression. Jundishapur J Microbiol 2015;8(9):e21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva A, Gotuzzo E, Arathoon EG, Noriega LM, Kartsonis NA, Lupinacci RJ, et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med 2002;113:294–299. [DOI] [PubMed] [Google Scholar]
- 21.Veroux M, Macarone M, Fiamingo P, Cappello D, Gagliano M, Di Mare M, et al. Caspofungin in the treatment of azole-refractory esophageal candidiasis in kidney transplant recipients. Transplant Proc 2006;38:1037–1039. [DOI] [PubMed] [Google Scholar]
- 22.Mihu MR, Pattabhi R, Nosanchuk JD. The impact of antifungals on toll-like receptors. Front Microbiol 2014;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leon-Gil C, Ubeda-Iglesias A, Loza-Vazquez A, de la Torre MV, Raurich-Puigdevall JM, Alvarez-Sanchez B, et al. Efficacy and safety of caspofungin in critically ill patients. ProCAS Study. Revista Espanola de Quimioterapia 2012;25:274–282. [PubMed] [Google Scholar]
- 24.Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis 2004;39:1563–1571. [DOI] [PubMed] [Google Scholar]
- 25.Zarei Mahmoudabadi A, Rezaei-Matehkolaei A, Ghanavati F. The susceptibility patterns of Candida species isolated from urine samples to posaconazole and caspofungin. Jundishapur J Microbiol 2015;8(3):e24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: nPCR - Protocols and Applications - A Laboratory Manual. Academic Press, Cambridge, Massachusetts, USA: pp.315–3322. [Google Scholar]
- 27.Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, Ghahri M, Shidfar MR, Jalalizand N, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol 2013;51:657–663. [DOI] [PubMed] [Google Scholar]
- 28.Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 2016;38:1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex JH, Alexander BD, Andes D, Arthington-Skaggs B, Brown SD, Chaturvedi V, et al. Refrence method for broth dilution abtifungal suceptibility testing of yeasts; approved standard-third edition. M27-A3 2008;28(14). [Google Scholar]
- 30.Guzel AB, Aydin M, Meral M, Kalkanci A, Ilkit M. Clinical characteristics of Turkish women with Candida krusei vaginitis and antifungal susceptibility of the C. krusei isolates. Infect Dis Obstet Gynecol 2013;2013:698736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donders GG, Bellen G, Mendling W. Management of recurrent vulvovaginal candidosis as a chronic illness. Gynecol Obstet Invest 2010;70:306–321. [DOI] [PubMed] [Google Scholar]
- 32.Zhu YX, Li T, Fan SR, Liu XP, Liang YH, Liu P. Health-related quality of life as measured with the Short-Form 36 (SF-36) questionnaire in patients with recurrent vulvovaginal candidiasis. Health Qual Life Outcomes 2016;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016;214:15–21. [DOI] [PubMed] [Google Scholar]
- 34.Akimoto-Gunther L, Bonfim-Mendonca Pde S, Takahachi G, Irie MM, Miyamoto S, Consolaro ME, et al. Highlights regarding host predisposing factors to recurrent vulvovaginal candidiasis: chronic stress and reduced antioxidant capacity. PloS One 2016;11(7):e0158870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornari G, Vicente VA, Gomes RR, Muro MD, Pinheiro RL, Ferrari C, et al. Susceptibility and molecular characterization of Candida species from patients with vulvovaginitis. Braz J Microbiol 2016;47(2):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malazy OT, Shariat M, Heshmat R, Majlesi F, Alimohammadian M, Tabari NK, et al. Vulvovaginal candidiasis and its related factors in diabetic women. Taiwan J Obstet Gynecol 2007;46(4):399–404. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Reprod Biol 2011;155(2):199–203. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. Vaginitis due to Candida krusei: epidemiology, clinical aspects, and therapy. Clin Infect Dis 2002;35:1066–1070. [DOI] [PubMed] [Google Scholar]
- 39.Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE. Prevalence and susceptibility to fluconazole of Candida species causing vulvovaginitis. Indian J Med Res 2007;126:216–219. [PubMed] [Google Scholar]
- 40.Bennett JE, Izumikawa K, Marr KA. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother 2004;48:1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoursandi M, Modares Gilani M, Khosravi A. Recovery and recurrence of vaginal candidiasis after oral and intravaginal treatment. J Qazvin Univ Med Sci 2000(14):25–29. [Google Scholar]
- 42.Diaz MC, Camponovo R, Araya I, Cerda A, Santander MP, Carrillo-Munoz AJ. [Identification and in vitro antifungal susceptibility of vaginal Candida spp. isolates to fluconazole, clotrimazole and nystatin]. Revista Espanola de Quimioterapia 2016;29:151–154. [PubMed] [Google Scholar]
- 43.Moges B, Bitew A, Shewaamare A. Spectrum and the In Vitro Antifungal Susceptibility Pattern of Yeast Isolates in Ethiopian HIV Patients with Oropharyngeal Candidiasis. Int J Microbiol 2016;2016:3037817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother 2006;50:2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]