Abstract
Current evidence is mixed on the role of progesterone and its metabolites in perinatal mood and anxiety disorders. We measured second and third trimester (T2 and T3) progesterone (PROG) and allopregnanolone (ALLO) levels by ELISA and postpartum depression (PPD) by clinician interview (DSM-IV criteria) in 60 pregnant women with a prior diagnosis of a mood disorder. Methods included multivariate and logistic regression with general linear mixed effect models. We found that, after adjustment, every additional ng/mL of T2 ALLO resulted in a 63% (95% CI 13% to 84%, p = 0.022) reduction in the risk of developing PPD. Our findings extend previous work connecting ALLO and depression within pregnancy, and indicate that the relationship between pregnancy ALLO and PPD is worth further exploration in a larger sample.
Keywords: Hormones, reproductive, pregnancy, postpartum, depression, anxiety
1. Introduction
1.1 Hormones and Postpartum Depression
Postpartum depression (PPD) is a debilitating illness that affects up to 15% of women in the general population (Yonkers et al., 2011), carries a high mortality (with suicide accounting for up to 20% of all postpartum deaths) (Shadigan and Bauer 2005), and has long-term and devastating effects on children and on the parent-child relationship (Yonkers et al. 2011). There are both biological and psychosocial risk factors for PPD. While now increasingly focused on the immune system as well as genetic and epigenetic factors, the bulk of the existing literature on biological factors has focused on the hormonal fluctuations (especially of estrogen and progesterone) that occur in the immediate postpartum period (Chrousos et al., 1998; Glynn et al., 2013; Moses-Kolko et al., 2009). Our group has previously argued for a “reproductive subtype of depression” (Payne et al., 2009), which affects vulnerable women during reproductive transitions including premenstrual, postpartum, and perimenopausal periods. There is evidence that estrogen fluctuations can trigger mood disturbance throughout the reproductive life cycle in susceptible women (Douma et al., 2005). Similarly, progesterone has long been thought to be anxiolytic and protective against depression (Gilbert Evans et al., 2005; Yim et al., 2015), with some studies suggesting that progesterone withdrawal may be associated with PPD (Harris et al., 1994). Bloch et al.’s pioneering study (2000) demonstrated that vulnerable women have a sensitivity to hormonal fluctuation; in this study, 62% of women with a history of PPD developed mood symptoms in response to a blinded withdrawal of supraphysiological levels of estrogen and progesterone, compared to none of controls.
While a few early studies have pointed to a relationship between hormone levels and postpartum blues or depression (Abou-Saleh et al., 1999; Feksi et al., 1984; Harris et al., 1994), the bulk of the evidence indicates no relationship between either hormone levels themselves or the amplitude of hormonal fluctuation and the development of mood symptoms (Chatzicharampolous et al., 2011; Harris et al., 1996; Heidrich et al., 1994; Klier et al., 2007; Kuevi et al., 1983; for an excellent review, see Yim et al., 2015). Schiller et al. (2015) have recently summarized the literature supporting a hormone-sensitive variant of PPD, pointing not only to hormonal fluctuation but also to consequent variation in the regulation of GABA-A receptors as a factor in PPD.
There are a number of gaps in this literature. First, the published studies have primarily included hormonal measurements only in late gestation (Chatzicharalampous et al., 2011), when levels for all women are elevated 1000-fold over pre-pregnancy levels, as well as the postpartum (Epperson et al., 2006), when levels for all women are at their nadir. Might levels earlier in pregnancy, when changes from baseline are more moderate (Gilbert Evans, 2005), predict PPD? Second, many studies have focused on the hormones themselves rather than on any downstream mediators, such as their metabolites.
1.2 Progesterone, Allopregnanolone, and PPD
The neuroprotective, anxiolytic, and sedative properties of progesterone may be due in part to the actions of allopregnanolone, one of its 3α-reduced metabolites and a strong allosteric modulator of the GABA-A receptor (Bali and Jaggo 2014; Bristot et al., 2014; Schumacher et al., 2014). There is considerable animal research on the anxiolytic effects of allopregnanolone (see Schule et al., 2014, for an overview). There is also significant research linking low levels of allopreganolone with depressed mood (Backstrom et al., 2014; Eser et al., 2006; Le Melledo and Baker, 2004; Nappi et al., 2001; Padberg et al., 2002; Pinna et al., 2006; Romeo et al., 1998; Schule et al., 2014; Strohle et al., 1999, 2000; Uzunova et al., 1998, 2006), and several studies have demonstrated that antidepressant treatment can increase allopregnanolone levels (Romeo et al., 1998; Strohle et al., 1999, 2000; Uzunova et al., 1998). Other studies have also demonstrated alterations in the ratio of allopregnanolone to its precursors among depressed women, those with a history of depression, or those with Premenstrual Dysphoric Disorder (PMDD) (Girdler et al., 2001, 2012; Klatzkin et al., 2006a; Lindgren et al., 2012; Pearson Murphy et al., 2001; Sundstrom Poromaa et al., 2002; Schiller et al., 2014), indicating that the metabolism of progesterone may be altered in women with depressive symptoms.
Allopregnanolone and other progesterone metabolites change significantly across pregnancy and after childbirth (Biggio et al., 2009; Mostallino et al., 2009). Gilbert-Evans et al. (2005) found that absolute levels of allopregnanolone (and other metabolites) rise steadily through pregnancy before a sudden drop in the postpartum, and that the ratio of allopregnanolone to its precursor progesterone begins to drop even before delivery. They and others (Maguire and Mody 2008) hypothesize that the rising levels of allopregnanolone in pregnancy may down-regulate GABA-A receptors, and that the lag in restoration of receptor function following the abrupt decrease in hormones at birth may play an etiological role in PPD. Failure to regulate receptors appropriately in pregnancy may be a risk factor for the development of PPD.
A number of studies have directly examined the relationship between progesterone and its metabolites and PPD. Hellgren et al. (2014) found that women in late pregnancy with elevated depressive symptoms (Montgomery Asberg Depression Scale ≥ 13) had significantly lower levels of ALLO than those with normal-range scores, while Deligiannidis et al. (2013) found no relationship between hormone levels in late pregnancy or the early postpartum and the development of PPD in a small sample. Crowley et al. (2016) found that lower levels of a progesterone and of a combined measure of ALLO + pregnanolone in the second trimester were associated with greater negative emotional responses to stress, whereas Deligiannidis et al. (2016) found no differences in ALLO but higher levels of PROG and pregnanolone in women at risk for PPD compared to healthy controls. The present study builds upon this prior work. We hypothesized that women who went on to develop PPD would have lower levels of ALLO when measured during the second and third trimesters (T2 and T3). We also sought to investigate PROG at the same time points, as prior studies (Crowley et al. 2016, Deligiannidis et al. 2016) have had contradictory results concerning PROG.
2. Material and Methods
2.1 General Study Procedures
This was a prospective study conducted at The Johns Hopkins University School of Medicine in Baltimore, Maryland, and in collaboration with the University of North Carolina (UNC) at Chapel Hill. The study was approved by the Institutional Review Boards at The Johns Hopkins University and UNC Chapel Hill. Detailed methods were published in Kimmel et al. 2015. Briefly, we followed 93 pregnant women with a history of a mood disorder across pregnancy and the postpartum. Participants could enroll at any trimester during pregnancy and were managed clinically by their treating psychiatrist. Sixty women had data from at least one pregnancy and one postpartum visit and were deemed to be eligible for inclusion in this analysis. Of these sixty women, 40 had both hormone and mood data at the second trimester visit, and 58 had data from the third trimester. The mean gestational age at the second trimester visit was 19.73 weeks (SD 3.94), and mean gestational age at the third trimester visit was 33.39 weeks (SD 3.00). Data collection included mood scales, measures of stress, clinical history, personality measures, sleep quality measures, medication use, blood, and clinician assessment for the presence of a mood episode based on DSM-IV criteria.
2.2. Blood Analysis
Participant blood was collected at each visit in four 10ml EDTA tubes. Blood samples were non-fasting, and collection times were arranged at the convenience of the participant. All occurred during the working day (9:00 am to 5:00 pm). Samples were immediately centrifuged at 4 degrees C for 30 minutes. The plasma was then aliquotted in 2 ml microcentrifuge tubes, snap frozen on dry ice, and immediately stored in a −80° freezer. Blood was analyzed with the allopregnanolone EIA kit from Arbor Assays LLC Cat 3 KC44-H1 and progesterone EIA kit from Alpco catalogue # PROHU-E01. All samples were run in duplicate and the coefficient of variation (CV) among samples was <10%.
2.3 Statistical Methods
All significance tests were conducted as 2-sided tests with a 0.05 statistical significance level. Demographic and clinical history characteristics were compared between women included in this analysis (n=60) and those in the parent study (n=93) to assess for systematic differences. Demographic and clinical history characteristics were also compared between subjects who had second trimester samples (n=40) and those with third trimester samples (n=58). Relationships between PROG and ALLO levels and study outcomes by visit were assessed using scatter plots and locally weighted regression smoothers to assess whether linear fit was reasonable. Depression at each visit and postpartum was defined as clinician assessment of a major depressive episode based on DSM-IV criteria. Generalized linear mixed effects models with random intercept were used to estimate the relationship between PROG and ALLO levels and both concurrent depression and PPD while accounting for within-person correlation of outcomes over time and adjusting for potential confounders (gestational age at time of blood draw, age of participant, and sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI)). We used similar models to examine relationships between PPD and 1) the ratio of ALLO to PROG during pregnancy and 2) the rate of change of ALLO and PROG across pregnancy; in these models we used random slope for time period during pregnancy to allow for heterogeneity in outcome trajectories over time across women. The models were compared using Akaike Information Criterion (AIC). We used multivariate regression and logistic regression models to test the relationship between the PPD and the change in ALLO and PROG from 3rd trimester to postpartum. We also explored and tabulated patterns of missing data. Assuming missingness at random, we performed multiple imputation using the Markov chain Monte Carlo (MCMC) data augmentation method for multivariate normal model for women who provided blood samples. We generated 40 datasets; the estimates across the imputed datasets were combined using Rubin’s rules (Rubin 1987). All analyses were performed using STATA 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.).
3. Results
3.1 Population
This sample was drawn from a larger study of 93 women. All participants were pregnant women between the ages of 18 and 45. The larger sample was a very ill population previously described in Kimmel et al. (2015). The majority of women in the parent study (57%) had a remitting course of illness; 32.6% had a prior hospitalization; 73% met criteria for more than one psychiatric disorder; 76% used psychiatric medications at some point during the study; and 75% met criteria for a major depressive episode at some point during the study. When demographic and clinical history characteristics were compared between women included in this analysis (n=60) and those not included (n=33), the only significant difference was in education levels (p = 0.023). Excluded subjects were more likely to have less than a high school education (33.3% vs. 8.3% of those included) and less likely to have a graduate or professional degree (21.2% vs. 41.7%).
For the group reported on in this paper, the majority (69.9%) were Caucasian, and 21.5% were African American. Approximately 50% were employed. All women had a previously diagnosed mood disorder. The majority (67.7%) carried a DSM-IV diagnosis of MDD; other diagnoses included BPI, BPII, and BP-NOS. Table 1 shows demographic and clinical history for the women included in this analysis. We compared demographic and clinical characteristics for those with data available from the second trimester (T2, N=40) and compared them to those with third trimester (T3, N=58) data. Thirty-eight women had data at both time points. At the T2 blood draw, 65% of subjects were using antidepressants and 12.5% were using mood stabilizers. At the T3 blood draw, 53.4% were using antidepressants and 12.1% were using mood stabilizers. Rates of obstetric complications were low overall, with the exception of fetal stress, which was noted in 35% of participants with T2 data and 27.6% of those with T3 data. Similar proportions met criteria for an episode of depression at either T2 (37.5%) or T3 (43.1%), and similar proportions of those with T2 data (47.5%) and those with T3 data (43.1%) went on to develop PPD.
Table 1.
Demographic and Clinical Characteristics of Population
| Subjects with T2 Data (N=40) |
Subjects withT3 Data (N=58) |
|||
|---|---|---|---|---|
| Age | ||||
| <20 | 0 | 0.00% | 1 | 1.70% |
| 20–25 | 4 | 10.00% | 8 | 13.80% |
| 26–30 | 9 | 22.50% | 15 | 25.90% |
| 31–35 | 12 | 30.00% | 14 | 24.10% |
| 36–40 | 14 | 35.00% | 18 | 31.00% |
| 41+ | 1 | 2.50% | 2 | 3.40% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Race | ||||
| Asian | 0 | 0.00% | 3 | 5.20% |
| Black | 7 | 17.50% | 12 | 20.70% |
| other | 4 | 10.00% | 3 | 5.20% |
| White | 29 | 72.50% | 40 | 69.00% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Education | ||||
| <high school | 3 | 7.50% | 5 | 8.60% |
| high school | 6 | 15.00% | 9 | 15.50% |
| AA | 3 | 7.50% | 6 | 10.30% |
| BA | 11 | 27.50% | 12 | 20.70% |
| Prof/Grad | 16 | 40.00% | 25 | 43.10% |
| 1 | 2.50% | 1 | 1.70% | |
| Total | 40 | 100.00% | 58 | 100.00% |
| Diagnosis | ||||
| Bipolar 1 | 6 | 15.00% | 8 | 13.80% |
| Bipolar 2 | 3 | 7.50% | 5 | 8.60% |
| Bipolar NOS | 3 | 7.50% | 3 | 5.20% |
| MDD | 28 | 70.00% | 42 | 72.40% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Marital Status | ||||
| Married | 32 | 80.00% | 42 | 72.40% |
| In a relationship | 6 | 15.00% | 10 | 17.20% |
| Single | 1 | 2.50% | 5 | 8.60% |
| Divorced | 1 | 2.50% | 1 | 1.70% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Antidepressants | ||||
| NO | 14 | 35.00% | 26 | 44.80% |
| YES | 26 | 65.00% | 31 | 53.40% |
| missing | 0 | 0.00% | 1 | 1.70% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Mood Stabilizers | ||||
| NO | 35 | 87.50% | 50 | 86.20% |
| YES | 5 | 12.50% | 7 | 12.10% |
| missing | 0 | 0.00% | 1 | 1.70% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Concurrent MDE | ||||
|---|---|---|---|---|
| NO | 22 | 55.00% | 33 | 56.90% |
| YES | 15 | 37.50% | 25 | 43.10% |
| missing | 3 | 7.50% | 0 | 0.00% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Subsequent PPD | ||||
| NO | 19 | 47.50% | 27 | 46.60% |
| YES | 19 | 47.50% | 25 | 43.10% |
| missing | 2 | 5.00% | 6 | 10.30% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Preeclampsia | ||||
| NO | 34 | 85.00% | 48 | 82.80% |
| YES | 1 | 2.50% | 1 | 1.70% |
| missing | 5 | 12.50% | 9 | 15.50% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Gestational Diabetes | ||||
| NO | 35 | 87.50% | 48 | 82.80% |
| YES | 0 | 0.00% | 1 | 1.70% |
| missing | 5 | 12.50% | 9 | 15.50% |
| Total | 40 | 100.00% | 58 | 100.00% |
| High-Risk Pregnancy | ||||
| NO | 35 | 87.50% | 49 | 84.50% |
| YES | 5 | 12.50% | 9 | 15.50% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Fetal Stress | ||||
| NO | 22 | 55.00% | 34 | 58.60% |
| YES | 14 | 35.00% | 16 | 27.60% |
| missing | 4 | 10.00% | 8 | 13.80% |
| Total | 40 | 100.00% | 58 | 100.00% |
| Postpartum Hemorrhage | ||||
| NO | 35 | 87.50% | 49 | 84.50% |
| YES | 5 | 12.50% | 9 | 15.50% |
| Total | 40 | 100.00% | 58 | 100.00% |
| PROM | ||||
| NO | 34 | 85.00% | 48 | 82.80% |
| YES | 6 | 15.00% | 10 | 17.20% |
| Total | 40 | 100.00% | 58 | 100.00% |
3.2 Hormone Levels and PPD
There was no relationship between either of the hormones measured at either time point and concurrent clinician-assessed major depressive episodes. There was no relationship between PROG at either time point and PPD. There was no relationship between ALLO measured at T3 and PPD. Lower levels of ALLO in the 2nd trimester, however, were associated with clinician-assessed PPD measured in the postpartum period. In an unadjusted model, every additional ng/mL of T2 ALLO was associated with a 52% reduction in the likelihood of developing PPD (95% CI: 5% to 76%, p = 0.034). We considered whether numerous confounders, including asthma, smoking, medication use, diagnosis, infection, obstetrical risk factors, and other hormones, might affect these results. We performed tests for statistical association among these confounders, mood outcomes, and the exposure variables (ALLO and PROG) and found no significant relationships; we therefore excluded them from the model.
Age and sleep quality were also found to be significantly related to PPD, so we adjusted our analysis for age and sleep quality. The reduction in risk of developing PPD was increased to 63% for every additional ng/mL of T2 ALLO (95% CI 13% to 84%, p = 0.022) in the adjusted analysis (Figure 1). When we restricted our analyses to the small group who had no depression in pregnancy (n=15), our findings lost statistical significance.
Figure 1.
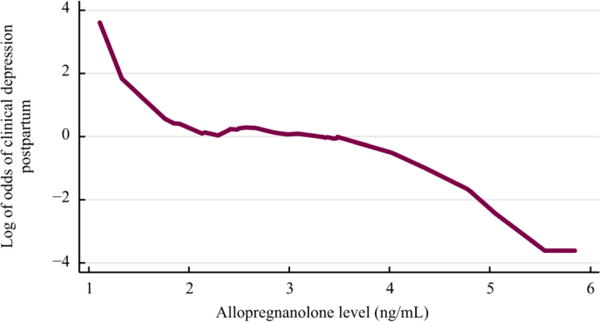
ALLO and PPD. Logit transformed smooth line graph showing the log of the odds of developing clinical postpartum depression on the y axis and ALLO level in ng/mL on the x axis (p=.022).
We also examined the relationship between longitudinal hormone change and clinician-assessed depression. We examined relationships between clinical depression and three longitudinal factors: 1) the ratio of ALLO to PROG at each time point across pregnancy; 2) the rate of change of ALLO and PROG across pregnancy; and 3) the change in ALLO and PROG from third trimester to the postpartum. We found no significant relationships. We repeated all analyses using imputation for missing data and the results did not change.
Discussion and Conclusions
Our principal finding was a significant association between low levels of ALLO in the second trimester and the development of PPD (a clinician-assessed major depressive episode using DSM-IV criteria in the postpartum) in women with a history of mood disorders. We did not find any such relationships between ALLO and concurrent depression in pregnancy, nor with PROG and concurrent or future depression, nor with the ratio of ALLO to PROG or the rate of change of ALLO across pregnancy.
Our findings differ from some previous research (Deligiannidis et al., 2013), in that we found a relationship between postpartum mood state and absolute levels of a metabolite of a reproductive hormone during pregnancy. One explanation for this could be the fact that we measured hormone levels earlier in pregnancy, rather than at their third trimester high point. Our lack of significant predictive findings in the third trimester is more consistent with previous research. In addition, all of our participants had a prior diagnosis of a mood disorder, with high rates of clinician-assessed depression at most points during the study – which may mean that our population is not comparable to a more general population. In fact, when we restricted our analyses to those who were not depressed during pregnancy, our findings lost statistical significance. This may, however, be related to the small sample size (n=15) of non-depressed women, for ALLO was not related to concurrent depression at either time point in pregnancy but rather only predictive of PPD, indicating that it cannot simply be considered a marker of chronic depression.
This is an exploratory study, an as such there are significant limitations. The sample size is small, and most participants were Caucasian with high income and education levels. The power of this sample size to answer these particular questions was not calculated, and we had missing data (although results did not differ when we imputed for missing data). We were unable to control for some clinical confounders that could have affected our results, including body mass index, levels of other hormones, and other medical conditions. Blood was not collected at the same time point during the day for each subject, and it is possible that our results were affected by diurnal variations. (Prior published studies indicate diurnal variation in estradiol (McGregor et al. 1999), but evidence is lacking for diurnal variation in progesterone and progesterone metabolites.) We did not collect information about fetal sex so were unable to examine any differences in either mood or hormone level by sex of the fetus.
Our findings can nevertheless point us to future directions of study. Are other progesterone metabolites similarly altered in women with low ALLO? If rising ALLO in normal pregnancy leads to down-regulation of GABA-A receptors, as some prior research has suggested (see Gilbert Evans et al. 2005), what is the receptor profile of women whose ALLO is lower than normal during pregnancy? Might PPD vulnerability be conferred by failure to regulate receptors appropriately? If ALLO levels measured in the 2nd trimester predict PPD, might ALLO measured in the first trimester – or before pregnancy – also be predictive? Or might combining ALLO with other biological variables improve our ability to predict PPD? Our group has reported separately on epigenetic biomarkers (HP1BP3 and TTC9B) that predict PPD with an AUC of 80%, and found that the change in ALLO from 2nd to 3rd trimester is correlated with those epigenetic biomarkers (Osborne et al. 2015). The direction of methylation for HP1BP3 differed between antenatally depressed and euthymic women, and was associated with the number of inflammatory cell-type elevations in the antenatally depressed women. This may mean that ALLO could be a more useful predictor of PPD if combined with markers of inflammation. The present work, when coupled with such work in the future, may therefore represent one small step in the path toward greater understanding of the biological mechanisms that underlie the peripartum depressive symptoms that affect the functioning and happiness of so many mothers and children.
Acknowledgments
Dr. Payne receives research support from Sage Therapeutics and holds a patent for epigenetic biomarkers of postpartum depression.
Funding sources: This work was supported by the National Institutes of Health (NIMH K23 MH074799, Jennifer Payne, PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: On behalf of all other authors, the corresponding author states that there is no additional conflict of interest.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Bhai I. Hormonal aspect of postpartum depression. Psychoneuroendocrinology. 1998;28(5):465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Stromberg J, Timby E, van Broekhoven F, van Wingen G. Allopregnanolone and mood disorders. Progress in Neurobiology. 2014;113:88–94. doi: 10.1016/j.pneurobio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggo AS. Multifunctional aspects of allopregnanolone in stress and related disorders. Progress in Neuro-Psychophparmacology and Biological Psychiatry. 2014;48:64–78. doi: 10.1016/j.pnpbp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Biggio G, Cristina Mostallino M, Follesa P, Concas A, Sanna E. GABA(A) receptor function and gene expression during pregnancy and postpartum. Int Rev Neurobiol. 2009;85:73–94. doi: 10.1016/S0074-7742(09)85006-X. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bristot G, Ascoli B, Gubert C, Panizzutti B, Kapczinski F, Rosea AR. Progesterone and its metabolites and therapeutic targets in psychiatric disorders. Expert Opin Ther Targets. 2014;18(6):679–690. doi: 10.1517/14728222.2014.897329. [DOI] [PubMed] [Google Scholar]
- Chatzicharalampous C, Rizos D, Pliatsika P, Leonardou A, Hasiakos D, Zervas I, Alexandrou A, Creatsa M, Konidaris S, Lambrinoudaki I. Reproductive hormones and postpartum mood disturbances in Greek women. Gynecological Endocrinology. 2011;27(8):543–550. doi: 10.3109/09513590.2010.501886. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–40. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Crowley SK, O’Buckley TK, Schiller CE, Stuebe A, Morrow AL, Girdler SS. Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: a pilot study. Psychopharmacology. 2016;233:1299–1310. doi: 10.1007/s00213-016-4217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, Kosma CA, Rothschild AJ, Moore CM. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: A preliminary study. Journal of Psychiatric Research. 2013;47:816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, Hall JE, Barton BA, Rothschild AJ, Shaffer SA. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 2016;70:98–107. doi: 10.1016/j.psyneuen.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. Adv Nurs Sci. 2005;28(4):364–75. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186(3):425–33. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Feksi A, Harris B, Walker RF, Riad-Fahmy D, Newcombe RG. ‘Maternity Blues’ and hormone levels in saliva. Journal of Affective Disorders. 1984;6:351–355. doi: 10.1016/s0165-0327(84)80013-0. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reducted neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecological Endocrinology. 2005 Nov;21(5):268–279. doi: 10.1080/09513590500361747. 2005. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow D, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47(6):363–70. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Harris B, Lovett L, Newcombe RG, Read GF, Walker R, Riad-Fahmy D. Maternity blues and major endocrine changes: Cardiff puerperal mood and hormone study II. BMJ. 1994;1994:308. doi: 10.1136/bmj.308.6934.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B, Lovett L, Smith J, Read G, Walker R, Newcombe R. Cardiff puerperal mood and hormone study. III. Postnatal depression at 5 to 6 weeks postpartum, and its hormonal correlates across the peripartum period. British Journal of Psychiatry. 1996;168:739–744. doi: 10.1192/bjp.168.6.739. [DOI] [PubMed] [Google Scholar]
- Heidrich A, Schleyer M, Spingler H, Albert P, Knoche M, Fritze J, Lanczik M. Postpartum blues: relationship between not-protein bound steroid hormones in plasma and postpartum mood changes. Journal of Affective Disorders. 1994;30:93–98. doi: 10.1016/0165-0327(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Hellgren C, Akerud H, Skalkidou A, Backstrom T, Sundstrom-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69:147–153. doi: 10.1159/000358838. [DOI] [PubMed] [Google Scholar]
- Kimmel M, Hess E, Roy PS, Palmer JT, Meltzer-Brody S, Meuchel JM, Bost-Baxter E, Payne JL. Family history, not lack of medication use, is associated with the development of postpartum depression in a high-risk sample. Arch Womens Ment Health. 2015;18(1):113–121. doi: 10.1007/s00737-014-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006;71(1):2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Klier CM, Muzik M, Dervic K, Mossaheb N, Benesch T, Ulm B, Zeller M. The role of estrogen and progesterone in depression after birth. Journal of psychiatric research. 2007;41:273–279. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kuevi V, Causon R, Dixson AF, Everard DM, Hall JM, Hole D, Whitehead SA, Wilson CA, Wise JC. Plasma amine and hormone changes in ‘post-partum blues’. Clinical Endocrinology (Oxford) 1983;19:39–46. doi: 10.1111/j.1365-2265.1983.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL, Girdler SS. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor JA, Hastings C, Roberts T, Barrett J. Diurnal variation in saliva estriol level during pregnancy: a pilot study. Am J Obstet Gynecol. 1999;180(1 Pt 3):S223–5. doi: 10.1016/s0002-9378(99)70705-2. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516–29. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABA A receptors during pregnancy and after delivery. Psychoneuroendocrinology. 2009;34S:S74–S83. doi: 10.1016/j.psyneuen.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97(1):77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Kimmel M, Clive M, Guintivano J, Brown T, Cox O, Judy J, Brier A, Beckmann MW, Kornhuber J, Fasching PA, Goes F, Payne JL, Gispen F, Binder E, Kaminsky Z. Replication of Epigenetic postpartum depression biomarkers and variation with hormone levels. Neuropsychopharmacology. 2016;41(6):1648–1658. doi: 10.1038/npp.2015.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg F, di Michele F, Zwanzger P, Romeo E, Bernardi G, Schule C, Baghai TC, Ella R, Pasini A, Rupprecht R. Plasma concentrations of neuroactive steroids before and after repetitive transcranial magnetic stimulation (rTMS) in major depression. Neuropsychopharmacology. 2002;27:874–878. doi: 10.1016/S0893-133X(02)00355-X. [DOI] [PubMed] [Google Scholar]
- Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: Conceptualizing models and moving toward etiology. Harv Rev Psychiatry. 2009;17(2):72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson Murphy BE, Steinberg SI, Fen-Yun H, Allison CM. Neuroactive ring α-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5α-dihydroprogesterone in depressed patients during the latter half of pregnancy. The Journal of Clinical Endocrinology & Metabolism. 2001;86(12):5981–598. doi: 10.1210/jcem.86.12.8122. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl) 2014;231(17):3557–67. doi: 10.1007/s00213-014-3599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20(1):48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Shadigan E, Bauer ST. Pregnancy-associated death: a qualitative systematic review of homicide and suicide. Obstet Gynecol Surv. 2005;60(3):183–90. doi: 10.1097/01.ogx.0000155967.72418.6b. [DOI] [PubMed] [Google Scholar]
- Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2105;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3a,5a-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. GABA(A) receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry. 2002;159:145–147. doi: 10.1176/appi.ajp.159.1.145. [DOI] [PubMed] [Google Scholar]
- Sundstrom Poromoaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Men Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186(3):351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. 2011;117(4):961–77. doi: 10.1097/AOG.0b013e31821187a7. [DOI] [PubMed] [Google Scholar]


