Abstract
Osteoporosis constitutes a major public health problem, through its association with age-related fractures, particularly of the hip, vertebrae, distal forearm and humerus. Substantial geographic variation has been noted in the incidence of osteoporotic fractures worldwide, with Western populations (North America, Europe and Oceania), reporting increases in hip fracture throughout the second half of the 20th century, with a stabilisation or decline in the last two decades. In developing populations however, particularly in Asia, the rates of osteoporotic fracture appears to be increasing. The massive global burden consequent to osteoporosis means that fracture risk assessment should be a high priority amongst health measures considered by policy makers.
The WHO operational definition of osteoporosis, based on a measurement of bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA), has been used globally since the mid-1990s. However, although this definition identifies those at greatest individual risk of fracture, in the population overall a greater total number of fractures occur in individuals with BMD values above threshold for osteoporosis diagnosis. A number of web-based tools to enable the inclusion of clinical risk factors, with or without BMD, in fracture prediction algorithms have been developed to improve the identification of individuals at high fracture risk, the most commonly used globally being FRAX®. Access to DXA, osteoporosis risk assessment, case finding and treatment varies worldwide, but despite such advances studies indicate that a minority of men and women at high fracture risk receive treatment. Importantly, research is ongoing to demonstrate the clinical efficacy and cost-effectiveness of osteoporosis case finding and risk assessment strategies worldwide. The huge burden caused by osteoporosis related fractures to individuals, healthcare systems and societies should provide a clear impetus for the progression of such approaches.
Keywords: Osteoporosis, epidemiology, assessment, geography, high fracture risk
Introduction
Over the last 3 decades, osteoporosis has been transformed from being viewed as an inevitable consequence of ageing to the disease which is readily assessed, and for which we now have a wide range of effective pharmacological therapies. The definitional approach to osteoporosis has changed markedly over this time. Thus the original histological definition was based on low bone mass and microarchitectural deterioration of bone tissue, resulting in bone fragility [1]. Clearly this is somewhat cumbersome in clinical practice, requiring, in the absence of high resolution peripheral quantitative computed tomography (HR-pQCT), a bone biopsy to make the diagnosis, and in the mid-1990s the World Health Organisation (WHO) convened a working group to generate an operational definition of osteoporosis, which could be used to provide a standardised case definition in epidemiological studies. Here osteoporosis is defined as a BMD (with the reference site now being the femoral neck) that is 2.5 standard deviations or more below the young adult female mean [2]. This operational definition has evolved into the clinical diagnostic definition of osteoporosis, and serves well where a diagnostic label is required. However it is readily apparent that although low BMD identifies individuals at increased risk of fracture, the majority of fragility fractures occur in individuals who have less marked reductions in bone mass or normal BMD, since although individually at lower risk, there are numerically far more in this population [3]. This consideration has led to more detailed assessments of bone itself, for example delineation of microarchitectural parameters using HR-pQCT, and more pragmatic approaches combining BMD with clinical risk factors partly independent of BMD in absolute risk calculators such as the web-based FRAX® algorithm [3]. In this review we will describe the variation in the burden of fragility fracture and high fracture risk globally, setting the worldwide context for the absolute necessity of both primary and secondary approaches to fracture risk assessment and subsequent treatment, before describing these approaches and highlighting some of the pressing issues currently facing clinicians and policymakers aiming to reduce the global impact of fragility fracture.
Epidemiology and geography of high fracture probability
The Global Burden of Disease study demonstrated a massive impact of musculoskeletal conditions on populations worldwide: the number of disability adjusted life years (DALYs) attributable to musculoskeletal disorders has increased by 17.7% between 2005 and 2013[4]. “Low back pain” ranked top, “neck pain” fourth, “other musculoskeletal” tenth, and “osteoarthritis” thirteenth in the WHO rankings of causes for years lived with disability worldwide in 2013[5], with osteoporotic fractures playing a major part in the “back pain” and “other musculoskeletal” categories. The 2004 US Surgeon General’s report estimated that 10 million Americans over the age of 50 have osteoporosis, leading to 1.5 million fragility fractures each year [6], with another 34 million Americans at risk of the disease. Economically, the cost to the US is around $17.9 billion per annum. In the EU, a report estimated that in 2010, 6.6% of men and 22.1% of women aged over 50 years had osteoporosis, and that there were 3.5 million fragility fractures [7]. The annual direct costs attributable to fracture treatment in the EU equate to approximately €24 billion, though when indirect costs such as long term care and facture prevention therapies are taken into account, this figure rises to €37 billion per year [7] (Table 1). A British study indicated similar population risks [8], with 1 in 2 women and 1 in 5 men aged 50 years expected to have an osteoporosis-related fracture in their remaining lifetime.
Table 1.
Impact of osteoporosis-related fractures across Europe. Data derived from (Hernlund et al, Archives of Osteoporosis, 2013)
| Hip | Spine | Wrist | |
|---|---|---|---|
| Lifetime risk in Women (%) | 23 | 29 | 21 |
| Lifetime risk in Men (%) | 11 | 14 | 5 |
| Cases / year | 620,000 | 810,000 | 574,000 |
| Hospitalization (%) | 100 | 2-10 | 5 |
| Relative survival | 0.83 | 0.82 | 1.00 |
| Costs: All sites combined ~ €37 billion | |||
Global variation in fracture rates
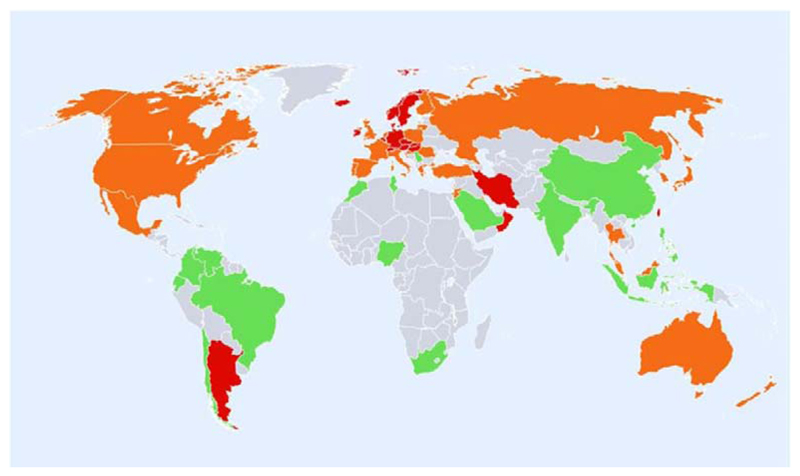
Global variation in fracture incidence is best documented for hip fracture, and studies have shown marked heterogeneity in annual age-standardised hip fracture rates. The largest systematic review, published in 2012, used a literature survey covering a 50 year period and UN data on population demography [9]. The highest annual age-standardised hip fracture incidences (per 100,000 person-years) were observed in Scandinavia (Denmark (574), Norway (563) and Sweden (539), plus Austria (501). The lowest were found in Nigeria (2), South Africa (20), Tunisia (58) and Ecuador (73). In general, there was a swathe of high risk countries in North Western Europe, Central Europe, the Russian Federation and Middle-Eastern countries such as Iran, Kuwait and Oman. Other high risk countries were Hong Kong, Singapore and Taiwan. Generally low risk regions included Latin America, (with the exception of Argentina), Africa, and Saudi Arabia, as shown in Figure 1. Discounting the rates for Nigeria and South Africa, which were from either old or unreliable sources, there was around a 10-fold range in hip fracture incidence worldwide, with the overall age-standardised incidence in men being half that of women. In general, the highest incidence of hip fracture is generally observed in countries furthest from the equator and in countries in which extensive coverage of the skin due to religious or cultural practices is the norm, suggesting that vitamin D status may be an important factor underlying this distribution.
Figure 1.
Hip fracture rates for men and women combined in different countries of the world categorised by risk, countries are coded red (annual incidence >250/100,000), orange (150-250/100,000) or green (<150/100,000) where estimates are available. (Reproduced with permission from Kanis et al, Osteoporosis International 2012 [9])
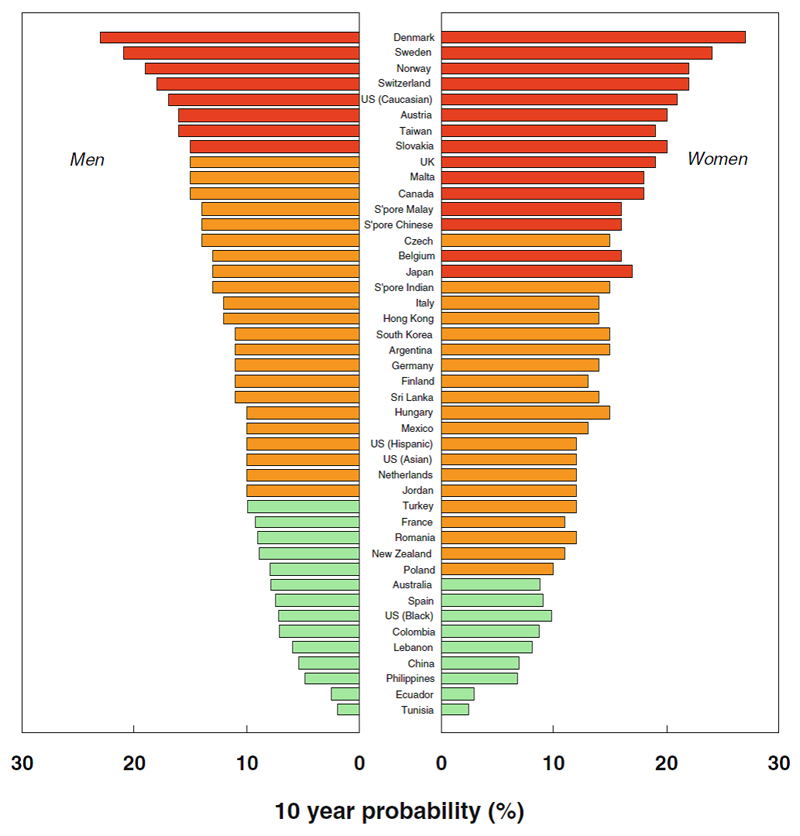
The 10 year probability of major osteoporotic fracture (hip, clinical vertebral, forearm or humeral fracture) was calculated for those countries where a FRAX model was available. These fracture probabilities are shown in Figure 2; in both men and women the lowest probabilities were found in Tunisia, Ecuador, Philippines and China, with the highest rates in Denmark, Sweden, Norway and Switzerland, with the USA (Caucasian population data only), fifth highest. Fracture probabilities, were, on average 23% higher in women than men, which contrasts with overall hip fracture incidence which was twofold higher in women than in men. This closer approximation between the sexes for the probability estimate (which included a BMD measurement) arises because the risk of hip and other osteoporotic fracture are roughly identical in men and women of the same age and femoral neck BMD [10–12].The slightly higher probability estimate seen in women reflects the lower death risk in women compared with men.
Figure 2.
Ten year probability of major fracture (in percent) in men and women aged 65 years with a prior fragiltity fracture and no other clinical risk factors, with a BMI of 24kg/m2 at the threshold of osteoporosis as judged by BMD at the femoral neck (i.e. T-score -2.5). (Reproduced with permission from Kanis et al, Osteoporosis International 2012[9])
The reasons for such large worldwide variation in age- and sex-adjusted hip fracture incidence worldwide are not clear. The authors of the systematic review on hip fracture incidence mention the possibilities of inaccurate coding and recording of fractures, the presumption that regional estimates (used in some countries) are representative of overall fracture risk, the fact that over 20% of the included studies were conducted more than a decade previously. Additionally, in some areas of the world, not all hip fracture cases come to medical attention (e.g. in Georgia, 75% of patients with hip fracture are not hospitalised, and in Kazakhstan and Kyrgyzstan 50% are not hospitalised [13] due to poor access to surgical services and affordable medical care). However, such problems would not undermine the principal finding of 10 fold differences in hip fracture risk, and in 10 year fracture probability worldwide. Genetic differences may go some way towards explaining the differences in fracture risk (for example, Black people in the USA have lower fracture probabilities than Caucasians), but the fact that immigrant populations show acclimatisation to local fracture rates (for example, the incidence of hip fracture in Black people in the USA is much higher than in Africans) [14], suggests that environmental factors are more important. Previously identified risk factors for osteoporosis (which will be discussed in more detail later in this review), such as low body mass index, low bone mineral density, poor calcium intake, reduced sunlight exposure, early menopause, smoking, alcohol use, low physical activity levels may explain effects within countries and communities but do not explain differences in risk between countries [15]. Socioeconomic prosperity is thought to be an important factor leading to lower levels of physical activity and the increased probability of falling onto hard surfaces (indeed a US$10,000 higher GDP per capita was associated with a 1.3% increase in hip fracture probability) [16]. However, within countries, higher socioeconomic status appears to have a protective effect against hip fractures in both the USA and UK [17, 18]. Calcium intake is another example of a risk factor whereby its role as a risk factor within populations (low calcium intake being an independent risk factor for osteoporosis) [19], appears to have opposite associations when countries are compared (high nutritional calcium intake countries having greater hip fracture risks) [20, 21]. It is not yet clear which factors overall are causally related to the heterogeneity in fracture risk worldwide.
Ethnic differences in bone health and fracture risk worldwide
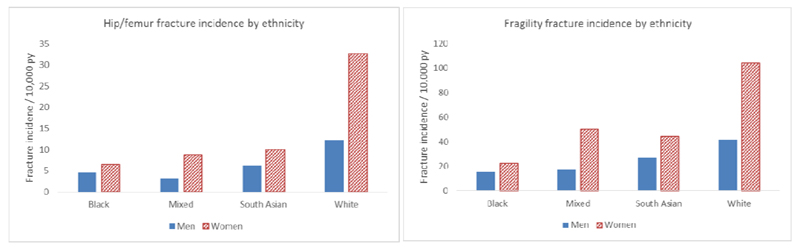
As described previously, differences in fracture rates worldwide are partly attributable to ethnic differences in bone resistance to fracture. Studies in the USA have demonstrated that the highest frequencies of hip fracture are observed in white women and the lowest in Black-American women [22]. Hip fracture rates in women of Hispanic and Asian ethnicity living in the USA are lower than those observed in white women, but higher than Black women [22]. In a recent study conducted in the UK, the lowest rates of fracture were observed in black individuals; rates of fragility fracture in white women were 4.7 times greater than in black women and 2.7 times greater in white men than black men. Those of mixed or South Asian ethnicity had hip fracture rates of less than half that of individuals of white ethnicity (Figure 3) [18]. This was in keeping with studies comparing Dundee, Scotland and Johannesburg, South Africa [23], and within California, USA [24]. In Singapore, lower BMD was noted in Chinese than Malay or Indian men [25] , and lower BMD in Chinese and Malay women compared with Indian women [26]. Differences by ethnicity in skeletal size and microarchitecture, peak bone mineral density and skeletal loss, in addition to differences in proximal femoral geometry are thought to underlie these differences in hip fracture rates [27] [28]. African-American women have higher areal BMD [29], greater bone area [27], increased trabecular thickness, cortical area and cortical thickness and reduced cortical porosity compared to Caucasian women, all of which will confer greater bone strength and resistance to fracture [27]. The differences in cortical and trabecular microarchitecture persisted after adjustment for BMD assessed by DXA.
Figure 3.
Incidence of hip / femur and fragility fractures by ethnicity in men and women aged over 50 years in the UK (Data from UK Clinical Practice Research Datalink, 1988-2012) (adapted with permission from Curtis et al Bone 2016[18])
Global trends in fracture incidence over time and future projections
Current estimates suggest that 12% of the world population are over the age of 60 years – a total of around 901 million people. Europe has the greatest percentage of its population aged 60+ years (24%), however, rapid ageing in other parts of the world means that by 2050 all continents except Africa will have nearly a quarter or more of their populations aged 60+ years. The number of older people in the world is projected to be 1.4 billion by 2030 and 2.1 billion by 2050, and could rise to 3.2 billion by 2100 [30].
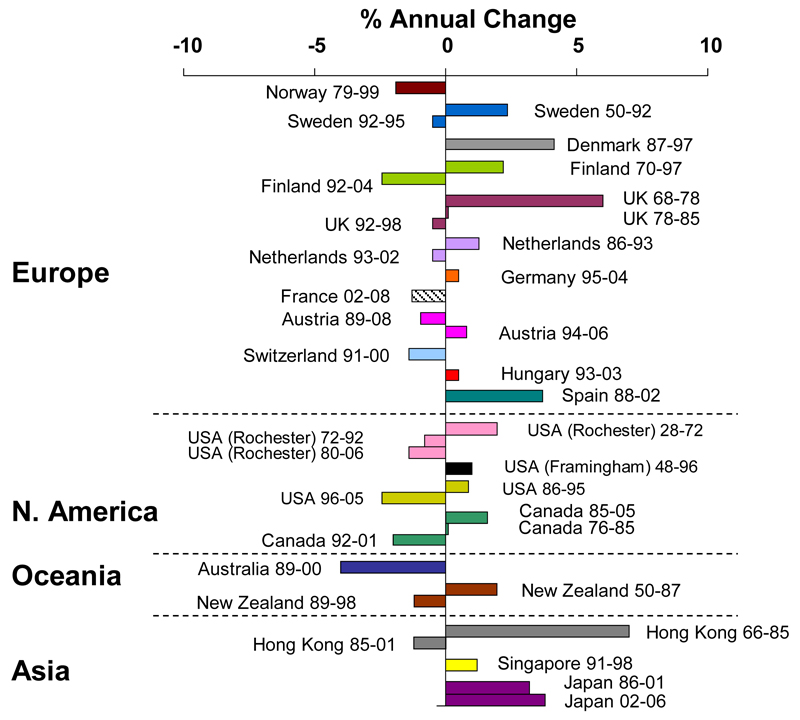
This growth in the world population and the increasing proportions of older people will substantially impact the number of hip fractures globally in coming decades, with a conservative estimate of the annual number of hip fractures increasing from 1.66 million in 1990 to 6.26 million in 2050, with the latter figure potentially over 20 million when known secular trends are considered [31, 32]. Alterations in age- and sex-adjusted incidence rates have been documented most robustly for hip fracture (Figure 4)[33]. Hip fracture rates appeared to have plateaued or decreased in the last 1-2 decades in many developed countries, following a rise in preceding years; however in the developing world, age- and sex-specific rates are still rising in many areas [33]. A recent study of UK fracture incidence showed little change in fracture incidence overall from 1990 to 2012, though a small increase in male hip fracture rates was seen (10.8 to 13.4 per 10,000 py) [34]. In Asia, secular trends in hip fracture rates are heterogeneous - rates in Hong Kong China, appeared to have stabilised between 1985-1995, following a steep increase in incidence up to this point [35]. Conversely, rates in Beijing have increased by around 33% between 1988 and 1992 from being among the lowest in the world, though this may be due to improvements in the completeness and accuracy of reporting in hospitals [36]. In Singapore, one of the most urbanised parts of Asia, hip fracture incidence appeared to be increasing by around 1% per year between 1991 and 1998 in comparison with rates derived from 1965 [37]. In Japan, ongoing age- and sex-specific increases in hip fracture rates of around 3.8% per year were recorded in 2006, and a 32% increase in age- and sex-standardised fracture rates was observed between the periods 1992-1994 and 2010-2012 [38]. Such increases, as seen in Singapore, Japan and Hong Kong, appear to be in line with rapid increases in urbanisation, with attendant changes in physical activity and nutrition.
Figure 4.
Trends in hip fracture worldwide over time: annual change in age and sex-adjusted hip fracture incidence (Reproduced with permission from Cooper et al, Osteoporosis International 2011 [33])
Secular trends in other types of fragility fractures are less well documented. A UK study over the period 1990 to 2012 demonstrated considerable variation in fracture rates over time by fracture site, with radius and ulna fractures becoming less frequent in women but stabilising in men [34], in keeping with European and North American trends [33]. In terms of vertebral fractures, a study of residents of Rochester, Minnesota showed an 80% increase in rates 1950 to 1965, followed by a plateau and stabilisation until 2005 [39, 40]. A Swedish study on radiologically diagnosed thoracic and vertebral compression fractures showed an increase in age- and sex-specific incidence rates between the periods 1950 to 1952 and 1982 to 1983 [41], whilst a more recent Korean study using an insurance database also documented an increase over a 5 year period 2008 to 2012 [42]. Recent work in the UK showed increases in clinical vertebral fracture in women, but a stabilisation in men [34], and a general stabilisation in vertebral fracture rates was also seen in an Icelandic study covering the period 1989 to 2008 [43].
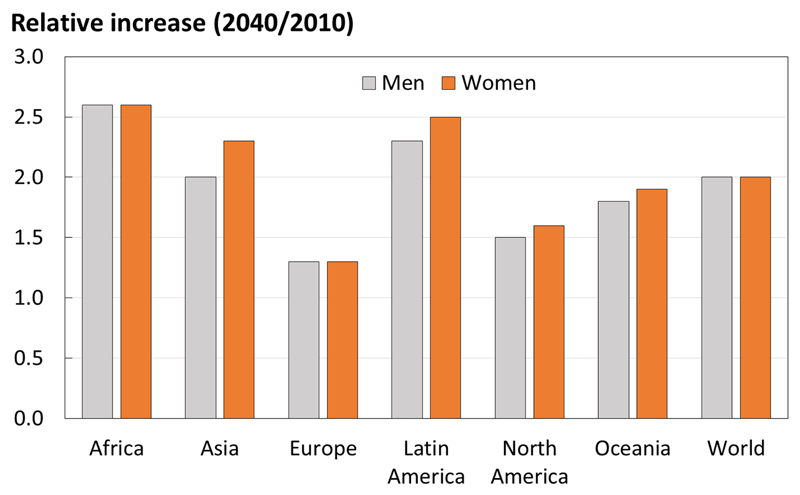
Whilst the burden of osteoporosis can be assessed in terms of consequent fracture, there is value in identifying the number of individuals at high fracture risk to help to inform future health resource allocation. Using this approach, it has been estimated that in 2010 there were 21 million men and 137 million women aged 50 years or greater at high fracture risk, and that this number is expected to double by 2040, with the increase predominantly borne by Asia [44] , demonstrated in Figure 5. Such increases in the burden of osteoporosis across the world highlights the need for effective primary and secondary prevention strategies, driven by fracture risk assessment.
Figure 5.
Number of men and women at high fracture risk in 2040 relative to 2010, by world region. (With permission from Oden et al, Osteoporosis International 2015 [44]).
Identification of patients at high risk of fracture
It is apparent from the evidence described above, that osteoporotic fracture is places a huge burden on societies across the world. It is well known that osteoporosis is a silent disease until a fracture occurs. Patient perception of fracture risk is often underestimated [45, 46], so initiation of primary prevention is usually reliant on health care practitioners. It is unsurprising therefore that secondary prevention (identifying individuals for treatment on the basis of a low trauma fragility fracture occurring), is the approach most often taken as the starting point for fracture prevention. However, whatever approach is taken to the reduction of fracture risk, it is critically important to place this within the context of local factors, such as the background population fracture risk, prevalent patterns and risk factors, funding constraints and willingness of healthcare providers to pay for treatment.
Secondary fracture prevention
Following attendance to a healthcare practitioner with a new fracture, it is important to assess fracture risk in a straightforward way, and to treat if appropriate. Several methods have been explored – some staff based, some IT-based and others a combination of the two. The most successful systems usually focus on a multi-disciplinary Fracture Liaison Service [47, 48], incorporating orthogeriatricians, rheumatologists and fracture liaison clinical nurse specialists. They work in a multidisciplinary team to ensure that medical management of patients admitted with fracture is optimised, both whilst in hospital, and for future fracture prevention, ideally with a lead clinician responsible for coordinating the team [49]. The International Osteoporosis Foundation has recently instituted “a global campaign to facilitate the implementation of coordinated, multi-disciplinary models of care for secondary fracture prevention.” The “Capture the Fracture” (http://www.capturethefracture.org/) initiative has provided guidance on secondary fracture prevention, and also a global map, with a quality grading scheme, on which, subject to application, secondary fracture prevention services can be documented [50]. There is currently huge variation, not only between, but also within countries, and in the availability, scope and quality of secondary prevention facilities. For example, a prospective observational study of over 60,000 older women recruited from primary care practices in 10 countries showed that more than 80% of women with a fragility fracture did not receive osteoporosis treatment [51]. The Capture the Fracture initiative, aimed at raising the quality and coverage of fracture liaison services providing secondary prevention for osteoporosis, should provide a clinically valuable and cost effective contribution to service improvement [52].
Further important initiatives around case finding of fragility fractures centre around vertebral fractures – around 12% of postmenopausal women with osteoporosis have at least one vertebral deformity, with less than a third of these individuals coming to clinical attention [53]. Primary care based screening strategies [54], and history-taking strategies distinguishing back pain likely to relate to vertebral fracture from other types of back pain may facilitate detection of these fractures [55]. In addition, consistent reporting of radiographs, CT scans and the incorporation of vertebral fracture assessment in DXA scans will help with secondary fracture prevention in individuals with prevalent osteoporotic vertebral fracture.
Primary fracture prevention
In osteoporosis, as in any non-communicable chronic disease, there is clearly a balance between the benefits of a systematic screening approach leading to widespread treatment, with associated increased cost and risk of side-effects, a case-finding strategy focused on those at greatest individual risk, with associated problems of under-treatment. Although, DXA screening is standard in the US (at the age of 65 years in women, and age 70 in men, and in individuals over the age of 50 who have suffered an adult fracture) [56], in the majority of countries, population screening is not judged to be cost-effective and primary prevention is focused more on opportunistic case-finding, triggered by the presence of clinical risk factors https://www.nice.org.uk/guidance/cg146 [57–59]. A seven-centre randomised controlled trial of the effectiveness and cost-effectiveness of screening older women in primary care for the prevention of fractures (the UK SCOOP study), in which approximately 12,500 older women were randomised to either normal care or screening and subsequent treatment (based upon the FRAX risk assessment tool), has demonstrated encouraging findings with regard to a reduction in hip fracture [60, 61].
Tools for osteoporosis risk assessment
Measurement of BMD alone
The WHO operational definition of osteoporosis is based on a DXA measurement of BMD; there is evidence that fracture risk approximately doubles for every standard deviation decrease in BMD [62]. In recent years however, it has been increasingly recognised that low BMD should be viewed as a risk factor for fragility fracture rather than as a disease in itself. Furthermore, other features independent of BMD, such as the geometric and microarchitectural properties of the bone itself, and an individual’s clinical risk factors, clearly contribute to fracture risk. A small proportion of the population is identified by a T-score of -2.5 or below, and in terms of total numbers, more fractures in later life may occur in individuals who have a BMD in the normal or osteopenic than osteoporotic range. For example, in a study of 8065 post-menopausal women in the USA, 243 women experienced a hip fracture over the 5 year study period, and only 46% of these women had a T-score ≤-2.5 at baseline screening [63]. In this case, if BMD alone is used to determine treatment thresholds, then many women at risk of fracture will not be offered intervention. Newer techniques including peripheral quantitative computed tomography (pQCT) and HR-pQCT can provide a more detailed assessment of bone structure. However, their use in clinical practice is limited by the expense and availability of instruments, a lack of population-based reference data and, indeed, any convincing evidence of their superiority, in terms of risk stratification, over traditional densitometry.
Fracture risk assessment tools encompassing BMD and clinical risk factors
The use of clinical risk factors (CRFs) in addition to BMD measurement has been demonstrated to increase the accuracy of hip and major osteoporotic fracture risk assessment [64]. As such, a number of tools have been developed to calculate an individual’s risk of fracture, either based on clinical risk factors alone (QFracture), or in combination with BMD measurement (FRAX, Garvan). The most widely used tool is the WHO Fracture Risk Assessment Tool, FRAX® (www.shef.ac.uk/FRAX) [65], which has been developed across a large number of population-based cohorts worldwide. Two further calculators developed from single cohorts are also available: The Australian Garvan Fracture Risk Calculator https://www.garvan.org.au/bone-fracture-risk and QFracture (www.qfracture.org) [66]. The attributes of the 3 algorithms are summarised in Table 2.
Table 2.
Characteristics of FRAX, QFracture and Garvan fracture risk calculators. Adapted with permission [67].
| FRAX | Garvan fracture risk calculator | QFracture | |
|---|---|---|---|
| Output | 10 year probability of major osteoporotic and hip fracture | 5 and 10 year probability of major osteoporotic fracture and hip fracture | 1-10 year probability of major osteoporotic fracture and hip fracture |
| BMD assessment | Can be used both with and without | Can be used both with and without | × |
| Trabecular bone score assessment | Can be used both with and without | × | × |
| Clinical risk factors included: | |||
| • Age | ✔ | ✔ | ✔ |
| • Sex | ✔ | ✔ | ✔ |
| • Ethnicity | 68 population specific calculators available, four for the USA and three for Singapore | × | ✔ |
| • BMI or weight | ✔ | ✔ | ✔ |
| • Prior fragility fracture | ✔ | ✔ | ✔ |
| • Glucocorticoid use | ✔ | × | ✔ |
| • Rheumatoid arthritis | ✔ | × | ✔ |
| • Parental hip fracture | ✔ | × | ✔ |
| • Current Smoking | ✔ | × | ✔ |
| • Alcohol intake | ✔ | × | ✔ |
| • Falls history | × | ✔ | ✔ |
| • Other | Diabetes, living in a care home, dementia, cancer, asthma, COPD, Cardiovascular disease, chronic liver disease, chronic kidney disease, Parkinson’s disease, SLE, malabsorption, endocrinopathy, epilepsy, antidepressant use, HRT use | ||
| Mortality risk accounted for | ✔ | × | × |
FRAX®
FRAX®, developed by the WHO Collaborating Centre for Metabolic Bone Diseases at the University of Sheffield, UK is the most comprehensively developed risk assessment tool [65]. It estimates 10-year major osteoporotic (vertebral, hip, forearm and proximal humerus) and hip fracture probability, either with or without inclusion of BMD measurement, for individuals between the ages of 40-90 years. Clinical risk factors were selected on the basis of intuitive linkage to fracture risk, with at least partial independence from BMD, representing a risk that was amenable to pharmacological treatment and being readily available from standard clinical sources. The algorithm was developed through a series of meta-analyses of prospective cohort studies from Europe, North America, Asia and Australia including nearly 45,000 individuals, and have subsequently been validated in a similar number of individuals in other independent cohorts. FRAX is only algorithm which incorporates the competing hazard of death with fracture risk to yield a 10-year probability of fracture. Sixty-eight country (population)-specific FRAX calculators have since been developed to account for geographical variations in fracture incidence and mortality, incorporating inter-ethnic differences in risk within the USA and Singapore for example, taking into account migration effects [68]. The freely available internet based calculator is available in 32 languages; the fact that the model does not require BMD [69, 70] is of benefit to low resource settings where availability of DXA is limited. The website currently handles about 2.8 million calculations per year but is not the sole portal for the calculation of fracture probabilities; for example, FRAX is available in BMD equipment, on smartphones and, in some countries, through handheld calculators (e.g. Poland and Russia) [68, 71]. In healthcare settings where trabecular bone score is available, this can also be incorporated into the fracture risk calculation.
Clearly not all CRFs for osteoporotic fracture are included in the FRAX algorithm (this being limited by which data were available globally in population-based cohorts) and many of the included CRFs have a dose-response element that is not incorporated into the model. Details of glucocorticoid exposure (e.g. dose, duration) were not available in the original FRAX cohorts so that the relationship again assumes an average exposure; this will lead to an underestimation of fracture risk for recipients of higher daily doses of steroids, and overestimation for low daily doses [72]. Based on the assumption that the average exposure in the FRAX cohorts probably lay within the range of 2-5-7.5mg daily, an adjustment to the calculated fracture risk has been proposed based on the relative fracture risks according to steroid dose, [73, 74]. Furthermore, although like all risk assessment tools FRAX has not been validated in patients who have received anti-osteoporotic treatment, there is some evidence that it may still provide a useful guide in terms of continuation or cessation of therapy [75]. In addition, only current smoking is considered, whereas a past history of smoking also increases fracture risk above that of a lifetime non-smoker [76], and the calculator assumes an average daily consumption for all current smokers. Further adjustment for differences between the femoral neck and lumbar spine BMD [77] and for past falls may also be made; indeed, whilst the lack of falls as an input variable has been a criticism of FRAX, the output probability has been shown to predict risk of incident falls [78].
Garvan Fracture Risk Calculator and QFracture
The Garvan Fracture Risk Calculator (Australia) and QFracture (UK) provide country specific alternatives to FRAX. The Garvan calculator and QFracture generate cumulative fracture risk, as opposed to FRAX, which yields probability of fracture adjusted for the competing hazard of death. That is, the outputs are based on fundamentally different concepts. The Garvan calculator was derived using the Australian Dubbo cohort of around 2000 individuals and includes men and women [79]. It yields absolute fracture risk as a percentage over 5 or 10 years for osteoporotic fracture or hip fracture, based on age, sex, prior fracture, falls and bone mineral density. The 5-year fracture risk is sometimes felt to be useful at older ages, particularly as, like QFracture, this algorithm does not incorporate the competing hazard of death. QFracture takes a different approach, with a statistically driven identification of multiple clinical risk factors, many more than FRAX (30 in total, and including falls), which are derived from a primary care database [66, 80]. Although the first version of QFracture was validated in an independent UK cohort [80], the second version (which now includes prior fracture) has been tested and validated in random subsets of the same overall cohort [66, 81], with further evidence of calibration in UK CPRD [82]. It is critically important to realise that there are differences in the calibration of these instruments, particularly for major osteoporotic fracture, and thus the outputs cannot be used interchangeably. Indeed, in the case of QFracture, there are several concerns with regard to calibration, its being based on a primary care data set, in which the prevalence of past fracture and family history of fracture are markedly lower than those expected from meta-analysis of comparable populations [83]. An example of a further specific concern is that at the age of 85 years, the risk of hip fracture and major osteoporotic fracture (spine, humerus, distal forearm and hip) are identical, the implication being that individuals of this age do not experience fractures of the spine, humerus or distal forearm, a proposition that is somewhat at variance with clinical experience [83].
Thresholds for intervention
Clearly, fracture risk assessment tools provide information on fracture probability which is not the same as a diagnosis of future fracture: a proportion of individuals predicted to be at low probability will still experience an incident fracture, and a proportion at high probability will not; this is not due to failure of the risk assessment model. Neither FRAX itself, nor the Garvan calculator or QFracture, inform treatment decisions by themselves, so the threshold risk at which treatment may be given to reduce the risk of future fracture will depend on many considerations. These include both those at the level of the individual, and also in terms of cost-benefit of fracture prevention and how much an individual country is prepared to pay for each year of quality-adjusted life-year saved.
There are a number of country-specific treatment threshold guidelines available, many of which advocate the use of fracture prediction models (usually FRAX) for case-finding approaches [84, 85]. Even between the USA and UK guidance, there is marked heterogeneity. The National Osteoporosis Foundation in the USA suggests BMD assessment in women and men aged ≥65 years or 70 years respectively, or at younger ages if they have had a prior fracture, and treatment for those with either a history of vertebral or hip fracture, osteoporosis on BMD assessment, or osteopenia and a 10-year FRAX-calculated probability of a hip fracture ≥3% or major osteoporotic fracture ≥20% [86]. Conversely, the UK National Osteoporosis Guideline Group (NOGG) recommends the use of FRAX with or without BMD as the first step in risk assessment, with prior fragility fracture at older ages usually a sufficient basis for treatment regardless of other risk factors. Where a 10-year probability has been generated by FRAX, threshold graphs are subsequently used to guide appropriate intervention. The possible outcomes include patient reassurance with further risk calculation at a later date (low risk), BMD assessment (intermediate risk), or immediate treatment without the need for BMD assessment (high risk) [87]. Once BMD has been performed, the 10-year probability of fracture is plotted by age, either above or below a single treatment threshold, which is set at the 10-year fracture probability conferred by having had a previous fragility fracture, corresponding to older UK national guidance. The treatment threshold thus increases with age, but even so, the proportion of women potentially eligible for treatment rises from 20% to 40% across the age range assessed. Differences in access to health care, cost of medications, health policy in terms of willingness to pay for quality adjusted life years saved, fracture epidemiology, other disease burdens, and the implications of fracture at the individual and societal level within different countries will reflect the threshold and overall strategy employed. Taking into account these considerations, and particularly the huge difference in background fracture risk and thus in the probability of future fracture conferred by a past fracture, it is apparent that simply using a threshold because it has been used elsewhere may lead to counterintuitive health policies. For example intervening in China at a threshold of 20% for FRAX major osteoporotic fracture, a threshold used in the USA, would lead to only a very tiny proportion of the population treated [85]. Clearly there is no one correct approach to threshold setting, which depends as much on philosophy as science, but the important message is that it should not be assumed that one size will fit all. Accordingly, the International Osteoporosis Foundation has published guidance relating to osteoporosis and corticosteroid-induced osteoporosis, which can be readily modified to reflect national priorities and subsequent treatment thresholds [57–59].
Healthcare policies and osteoporosis assessment
Osteoporosis, in comparison with comparable non-communicable diseases, has often not attracted proportionate levels of attention from healthcare providers and governments, and an individual nation’s policy on access to DXA and its reimbursement will greatly influence the assessment and treatment of this disease. The IOF has published various regional audits covering the European Union, Eastern Europe and Central Asia, Latin America, North America, the Middle East and Africa, Asia Pacific in terms of epidemiology, burden and costs of osteoporosis. Taking Asia Pacific as an example, whilst Australia, Hong Kong, Japan, New Zealand, Republic of Korea and Singapore had 12-24 DXA machines per million of population, China, India, Indonesia, Pakistan, Phillipines, Sri Lanka and Vietnam were greatly under-resourced with less than 1 DXA machine per million of population. In addition, BMD testing and osteoporosis treatment were not fully reimbursed by insurance or healthcare policies in many countries, which served as a barrier to accessing treatment.
In Europe, it was assumed that 11 DXA machines per million of population were needed to provide adequate osteoporosis care. 16 European countries fell into this category, and 9 countries were considered to have very inadequate provision with less than 8.4 DXA units per million (Bulgaria, Czech Republic, Hungary, Latvia, Lithuania, Luxembourg, Poland, Romania and the UK). Table 3 shows the number of DXA units per million of population in the EU27 countries [88]. Reimbursement for DXA scans was extremely variable between EU member states in terms of the criteria required and level of imbursement awarded – interestingly in some countries reimbursement for DXA was only offered if the BMD turns out to show osteoporosis (Bulgaria and Switzerland), only if after fracture (Germany), or only if seen by a specialist (Poland).
Table 3.
Number of central DXA units available in the EU27 countries per million of the general population. Adapted with permission [88]
| COUNTRY | DXA UNITS/MILLION | COUNTRY | DXA UNITS/MILLION | COUNTRY | DXA UNITS/MILLION |
|---|---|---|---|---|---|
| AUSTRIA | 28.7 | GERMANY | 21.1 | NETHERLANDS | 10.7 |
| BELGIUM | 53.0 | GREECE | 37.5 | POLAND | 4.3 |
| BULGARIA | 1.2 | HUNGARY | 6.0 | PORTUGAL | 26.9 |
| CYPRUS | 23.9 | IRELAND | 10.0 | ROMANIA | 2.4 |
| CZECH REPUBLIC | 5.2 | ITALY | 18.6 | SLOVAKIA | 10.7 |
| DENMARK | 14.6 | LATVIA | 4.9 | SLOVENIA | 27.1 |
| ESTONIA | 8.9 | LITHUANIA | 3.4 | SPAIN | 8.4 |
| FINLAND | 16.8 | LUXEMBURG | 2.0 | SWEDEN | 10.0 |
| FRANCE | 29.1 | MALTA | 9.7 | UK | 8.2 |
Though no official IOF audit is available for North America, reimbursement for treatment also varies greatly depending on each individual patient’s health insurance plan. However, healthcare reform is evolving in the USA from fee for service to supporting improved quality, prevention and care coordination with financial incentives to encourage healthcare professionals or systems to report on or improve patient outcomes. However, performance measures on osteoporosis assessment remain low compared to other major chronic diseases, and a major drop in reimbursement for DXA scans in the office setting has led to a fall in the number of DXA providers and more than 1 million fewer DXA scans performed per annum [89] .
The Osteoporosis Treatment Gap
Despite many advances in the diagnosis of osteoporosis, the assessment of fracture risk, the development of therapies to reduce the risk of fractures, and the production of best practice guidelines, many studies indicate that a minority of men and women at high fracture risk actually receive treatment. Even in patients who sustain a fragility fracture, fewer than 20% actually receive therapies to reduce the risk of fracture in the year following the fracture [90, 91], with particularly poor rates of treatment for older women and those who live in long term care. Disparities in use of fracture risk assessment tools such as FRAX vary one thousand-fold worldwide, with a far greater variability than the 30-fold range of crude, or 10-fold range of age-standardised hip fracture worldwide, indicating a large gap in service provision. Limitations in access to the internet, lack of national assessment guidelines for osteoporosis in many countries, and the availability of alternative assessment algorithms may partially explain these differences [68]. Not only is lack of assessment and lack of treatment of those at very high risk of further fracture such as hip fracture a concern, most worrying is the downward trend in people being treated after hip fracture, demonstrated both in the USA and UK populations [92, 93]. The precise causes for this trend are likely to be several, including the recent reimbursement changes in the US, and the massive inflation of concerns regarding potential red side effects of long-term bisphosphonate treatment such as osteonecrosis of the jaw and atypical femoral shaft fractures. Despite these events not even being definitely causally related to bisphosphonate treatment, and in absolute terms being very rare (with incidences in the range of 1/100,000 to 1/10,000 per year) [94], media stories focusing on these outcomes have been common in recent years. Reassuringly, a recent study in the Danish population has demonstrated that users of alendronate still have a reduced risk of fracture compared with matched controls even after 10 years use, and that the number of hip fractures prevented is still greater than the number of subtrochanteric fractures occurring even by the end of a decade of bisphosphonate treatment [95].
Summary
Osteoporosis and its associated fragility fracture are globally common conditions, contributing significantly to morbidity, mortality and healthcare spending. Although there is some evidence for a plateauing of fracture incidence in the developed world, an aging population and adoption of westernised lifestyles in transitioning populations is leading to an increasing burden of osteoporosis globally. Whilst the clinical definition of osteoporosis has been based solely on BMD, the prediction of fracture at the individual level has been improved by incorporation of clinical risk factors, derived from a greater understanding of the epidemiology of osteoporosis. Although fracture prediction tools are now available, the most widely used being FRAX, which can be used to stratify risk and guide treatment, studies are required to demonstrate cost-effectiveness and clinical efficacy of these approaches. Greater efforts are also required from healthcare funders and providers to shift attention towards the identification and treatment of those at highest fracture risk with the aim of closing the osteoporosis treatment gap.
Acknowledgements
We would like to thank Medical Research Council (UK), National Institute for Health Research, Wellcome Trust, Arthritis Research UK, National Osteoporosis Society (UK), International Osteoporosis Foundation, supporting this work.
Footnotes
Disclosures:
EMC and RM have no disclosures. NH has no disclosures directly related to this work, and has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare and Internis Pharma; CC has no disclosures directly related to this work, and has received consultancy, lecture fees and honoraria from AMGEN, GSK, Alliance for Better Bone Health, MSD, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Study G. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. 1994 [PubMed] [Google Scholar]
- 3.Kanis JA, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville MD: 2004. [PubMed] [Google Scholar]
- 7.Hernlund E, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden : A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1-2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Staa TP, et al. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–56. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan B, et al. Relationship of femoral neck areal bone mineral density to volumetric bone mineral density, bone size, and femoral strength in men and women. Osteoporos Int. 2012;23(1):155–62. doi: 10.1007/s00198-011-1822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanis JA, et al. Towards a diagnostic and therapeutic consensus in male osteoporosis. Osteoporos Int. 2011;22(11):2789–98. doi: 10.1007/s00198-011-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnell O, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 13.The Eastern European & Central Asian Regional Audit Epidemiology, costs & burden of osteoporosis in 2010. International Osteoporosis Foundation; Nyon: 2011. [Google Scholar]
- 14.Cauley JA, et al. Official Positions for FRAX(R) clinical regarding international differences from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R) J Clin Densitom. 2011;14(3):240–62. doi: 10.1016/j.jocd.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Elffors I, et al. The variable incidence of hip fracture in southern Europe: the MEDOS Study. Osteoporos Int. 1994;4(5):253–63. doi: 10.1007/BF01623349. [DOI] [PubMed] [Google Scholar]
- 16.Johnell O, et al. Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int. 2007;18(3):333–7. doi: 10.1007/s00198-006-0245-4. [DOI] [PubMed] [Google Scholar]
- 17.Bacon WE, Hadden WC. Occurrence of hip fractures and socioeconomic position. J Aging Health. 2000;12(2):193–203. doi: 10.1177/089826430001200203. [DOI] [PubMed] [Google Scholar]
- 18.Curtis EM, et al. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnell O, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res. 1995;10(11):1802–1815. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Passmore R. Calcium supplementation of the diet--I. Bmj. 1989;298(6667):137–40. doi: 10.1136/bmj.298.6667.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanis JA, Passmore R. Calcium supplementation of the diet--II. Bmj. 1989;298(6668):205–8. doi: 10.1136/bmj.298.6668.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright NC, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27(11):2325–32. doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]
- 23.Solomon L. Osteoporosis and fracture of the femoral neck in the South African Bantu. J Bone Joint Surg Br. 1968;50(1):2–13. [PubMed] [Google Scholar]
- 24.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1988;78(11):1482–3. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang PL, et al. Associations between ethnicity, body composition, and bone mineral density in a Southeast Asian population. J Clin Endocrinol Metab. 2013;98(11):4516–23. doi: 10.1210/jc.2013-2454. [DOI] [PubMed] [Google Scholar]
- 26.Goh JC, Low SL, Das De S. Bone mineral density and hip axis length in Singapore's multiracial population. J Clin Densitom. 2004;7(4):406–12. doi: 10.1385/jcd:7:4:406. [DOI] [PubMed] [Google Scholar]
- 27.Putman MS, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013;28(10):2177–85. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araujo AB, et al. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18(7):943–53. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 29.Looker AC, et al. Prevalence and trends in low femur bone density among older US adults: NHANES 2005-2006 compared with NHANES III. J Bone Miner Res. 2010;25(1):64–71. doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nations U. World Population Prospects: The 2015 Revision. United Nations; New York: 2015. [Google Scholar]
- 31.Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 32.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–13. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 33.Cooper C, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22(5):1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Velde RY, et al. Secular trends in fracture incidence in the UK between 1990 and 2012. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau EM, et al. Hip fracture in Hong Kong over the last decade--a comparison with the UK. J Public Health Med. 1999;21(3):249–250. doi: 10.1093/pubmed/21.3.249. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, et al. Very low rates of hip fracture in Beijing, People's Republic of China the Beijing Osteoporosis Project. Am J Epidemiol. 1996;144(9):901–907. doi: 10.1093/oxfordjournals.aje.a009024. [DOI] [PubMed] [Google Scholar]
- 37.Koh LK, et al. Hip fracture incidence rates in Singapore 1991-1998. Osteoporos Int. 2001;12(4):311–8. doi: 10.1007/s001980170121. [DOI] [PubMed] [Google Scholar]
- 38.Tsukutani Y, et al. Epidemiology of fragility fractures in Sakaiminato, Japan: incidence, secular trends, and prognosis. Osteoporos Int. 2015;26(9):2249–55. doi: 10.1007/s00198-015-3124-z. [DOI] [PubMed] [Google Scholar]
- 39.Cooper C, et al. Secular trends in the incidence of postmenopausal vertebral fractures. Calcif Tissue Int. 1992;51(2):100–104. doi: 10.1007/BF00298496. [DOI] [PubMed] [Google Scholar]
- 40.Islam S, et al. Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: analysis of a large insurance claims database, 2000-2005. Menopause. 2009;16(1):77–83. doi: 10.1097/gme.0b013e31817b816e. [DOI] [PubMed] [Google Scholar]
- 41.Bengner U, Johnell O, Redlund-Johnell I. Changes in incidence and prevalence of vertebral fractures during 30 years. Calcif Tissue Int. 1988;42(5):293–6. doi: 10.1007/BF02556362. [DOI] [PubMed] [Google Scholar]
- 42.Kim TY, et al. Trends of Incidence, Mortality, and Future Projection of Spinal Fractures in Korea Using Nationwide Claims Data. J Korean Med Sci. 2016;31(5):801–5. doi: 10.3346/jkms.2016.31.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siggeirsdottir K, et al. Epidemiology of fractures in Iceland and secular trends in major osteoporotic fractures 1989-2008. Osteoporos Int. 2014;25(1):211–9. doi: 10.1007/s00198-013-2422-6. [DOI] [PubMed] [Google Scholar]
- 44.Oden A, et al. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26(9):2243–8. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 45.Grover ML, et al. Fracture risk perception study: patient self-perceptions of bone health often disagree with calculated fracture risk. Womens Health Issues. 2014;24(1):e69–75. doi: 10.1016/j.whi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Gregson CL, et al. Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int. 2014;25(1):85–95. doi: 10.1007/s00198-013-2438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisman JA, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep. 2013;11(1):52–60. doi: 10.1007/s11914-012-0130-3. [DOI] [PubMed] [Google Scholar]
- 49.Drew S, et al. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteoporos Int. 2016;27(5):1719–27. doi: 10.1007/s00198-015-3452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akesson K, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24(8):2135–52. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenspan SL, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455–61. doi: 10.1111/j.1532-5415.2011.03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell P, et al. Implementation of Models of Care for secondary osteoporotic fracture prevention and orthogeriatric Models of Care for osteoporotic hip fracture. Best Pract Res Clin Rheumatol. 2016;30(3):536–558. doi: 10.1016/j.berh.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Cooper C, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7(2):221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 54.Clark EM, et al. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) J Bone Miner Res. 2012;27(3):664–71. doi: 10.1002/jbmr.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark EM, Gooberman-Hill R, Peters TJ. Using self-reports of pain and other variables to distinguish between older women with back pain due to vertebral fractures and those with back pain due to degenerative changes. Osteoporos Int. 2016;27(4):1459–67. doi: 10.1007/s00198-015-3397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosman F, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanis JA, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lekamwasam S, et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23(9):2257–76. doi: 10.1007/s00198-012-1958-1. [DOI] [PubMed] [Google Scholar]
- 59.Lekamwasam S, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7(1-2):25–30. doi: 10.1007/s11657-012-0070-7. [DOI] [PubMed] [Google Scholar]
- 60.Shepstone L, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23(10):2507–15. doi: 10.1007/s00198-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 61.McCloskey ELE, Clarke S, Fordham R, Gittoes N, Harvey I, Holland R, Howe A, Marshall T, Peters T, Kanis J, O’Neill T, et al. Screening based on FRAX fracture risk assessment reduces the incidence of hip fractures in older community-dwelling women – results from the SCOOP study: Abstracts of Osteoporosis Conference 2016. Osteoporosis International. 2016;27(2):609–685. [Google Scholar]
- 62.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainwright SA, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90(5):2787–93. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- 64.Kanis JA, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 65.Kanis JA, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. Bmj. 2012;344:e3427. doi: 10.1136/bmj.e3427. [DOI] [PubMed] [Google Scholar]
- 67.Moon RJ, Harvey NC. Identification of patient profile for treatment. Best Pract Res Clin Endocrinol Metab. 2014;28(6):767–82. doi: 10.1016/j.beem.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Kanis JA, et al. Worldwide uptake of FRAX. Arch Osteoporos. 2014;9(1):166. doi: 10.1007/s11657-013-0166-8. [DOI] [PubMed] [Google Scholar]
- 69.Leslie WD, et al. Fracture risk assessment without bone density measurement in routine clinical practice. Osteoporos Int. 2012;23(1):75–85. doi: 10.1007/s00198-011-1747-2. [DOI] [PubMed] [Google Scholar]
- 70.Olmez Sarikaya N, et al. Agreement between FRAX scores calculated with and without bone mineral density in women with osteopenia in Turkey. Clin Rheumatol. 2014 doi: 10.1007/s10067-014-2491-8. [DOI] [PubMed] [Google Scholar]
- 71.McCloskey EV, et al. FRAX updates 2016. Curr Opin Rheumatol. 2016;28(4):433–41. doi: 10.1097/BOR.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 72.van Staa TP, et al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39(12):1383–9. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 73.van Staa TP, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res JID - 8610640. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 74.Kanis JA, et al. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22(3):809–16. doi: 10.1007/s00198-010-1524-7. [DOI] [PubMed] [Google Scholar]
- 75.Leslie WD, et al. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27(6):1243–51. doi: 10.1002/jbmr.1582. [DOI] [PubMed] [Google Scholar]
- 76.Kanis JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 77.Kanis JA, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395–411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 78.Harvey NC, et al. FRAX predicts incident falls in elderly men: findings from MrOs Sweden. Osteoporos Int. 2016;27(1):267–74. doi: 10.1007/s00198-015-3295-7. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen ND, et al. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10):1431–44. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 80.Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper C, Harvey NC. Osteoporosis risk assessment. BMJ. 2012;344:e4191. doi: 10.1136/bmj.e4191. [DOI] [PubMed] [Google Scholar]
- 82.Hippisley-Cox J, Coupland C, Brindle P. The performance of seven QPrediction risk scores in an independent external sample of patients from general practice: a validation study. BMJ Open. 2014;4(8):e005809. doi: 10.1136/bmjopen-2014-005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanis JA, et al. SIGN Guidelines for Scotland: BMD Versus FRAX Versus QFracture. Calcif Tissue Int. 2016;98(5):417–25. doi: 10.1007/s00223-015-0092-4. [DOI] [PubMed] [Google Scholar]
- 84.Leslie WD, Schousboe JT. A review of osteoporosis diagnosis and treatment options in new and recently updated guidelines on case finding around the world. Curr Osteoporos Rep. 2011;9(3):129–40. doi: 10.1007/s11914-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 85.Kanis JA, et al. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.National Osteoporosis Foundation. Clinician's Guide to the Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington DC: 2013. [Google Scholar]
- 87.Compston J, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas; 2013. [DOI] [PubMed] [Google Scholar]
- 88.Kanis JA, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos. 2013;8:144. doi: 10.1007/s11657-013-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Overman RA, et al. DXA Utilization Between 2006 and 2012 in Commercially Insured Younger Postmenopausal Women. J Clin Densitom. 2015;18(2):145–9. doi: 10.1016/j.jocd.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giangregorio L, et al. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Kanis JA, et al. The osteoporosis treatment gap. J Bone Miner Res. 2014;29(9):1926–8. doi: 10.1002/jbmr.2301. [DOI] [PubMed] [Google Scholar]
- 92.Solomon DH, et al. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–37. doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Velde RY, et al. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: Variation by age, sex, geographic location and ethnicity. Bone. 2016;94:50–55. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adler RA, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abrahamsen B, et al. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. Bmj. 2016;353:i3365. doi: 10.1136/bmj.i3365. [DOI] [PMC free article] [PubMed] [Google Scholar]