Abstract
BACKGROUND
Dr. Athina Markou sought treatments for a common neural substrate shared by depression and drug dependence. Antagonists of corticotropin-releasing factor (CRF) receptors, a target of interest to her, have not reached the clinic despite strong preclinical rationale and sustained translational efforts.
METHODS
We explore potential causes for the failure of CRF1 antagonists and review recent findings concerning CRF-CRF1 systems in psychopathology.
RESULTS
Potential causes for negative outcomes include: 1) poor safety and efficacy of initial drug candidates due to bad pharmacokinetic and physicochemical properties 2) specificity problems with preclinical screens, 3) the acute nature of screens vs late-presenting patients, 4.) positive preclinical results were limited to certain models and conditions with dynamic CRF-CRF1 activation not homologous to tested patients, 5) repeated CRF1 activation-induced plasticity that reduces the importance of ongoing CRF1 agonist stimulation, 6) therapeutic silencing may need to address CRF2 receptor or CRF-BP molecules, constitutive CRF1 activity, or molecules that influence agonist-independent activity or to target structural regions other than the allosteric site bound by all drug candidates We describe potential markers of activation towards individualized treatment, human genetic and functional data that still implicate CRF1 systems in emotional disturbance, sex differences, and suggestive clinical findings for CRF1 antagonists in food craving and CRF-driven HPA-axis overactivation.
CONCLUSION
The therapeutic scope of selective CRF1 antagonists now appears narrower than had been hoped. Yet, much remains to be learned about CRF’s role in the neurobiology of dysphoria and addiction and the potential for novel anti-CRF therapies therein.
Keywords: CRF or CRH or corticotropin-releasing hormone or corticotropin-releasing factor or CRF1 receptor antagonist or CRH1 receptor antagonist, drug addiction or alcoholism or alcohol dependence or alcohol use disorder or binge drinking or binge eating or food addiction, major depression, generalized anxiety disorder or panic disorder or post-traumatic stress disorder, antidepressant or anxiolytic, irritable bowel syndrome, translation or clinical trial or treatment
In an early, influential contribution, Dr. Athina Markou, with Drs. Kosten and Koob, cited comorbidity data, preclinical findings on the neurobiological consequences of substances of abuse, and similar neurotransmitter alterations to propose that drug dependence and major depression share a common neurobiological substrate. In this conceptual model, drug use is motivated by negative reinforcement mechanisms to relieve depression-like symptoms – the so-called “self-medication” hypothesis (Markou et al 1998; Paterson et al 2007). From that time on, a thrust of research in their and other laboratories has been to identify novel compounds with antidepressant and anxiolytic activity (Markou and Cryan 2012) in order to reduce the suffering of emotional disorders and of the abstinent state in drug-dependent individuals. Relatedly, novel compounds (e.g., mGluR5 antagonists; Markou 2007; Stoker et al 2012) and recognized antidepressants, such as fluoxetine, bupropion and desipramine (Lin et al 1999; Harrison and Markou 2001; Harrison et al 2001; Cryan et al 2003a; Bruijnzeel and Markou 2003; Takamatsu et al 2006, 2011; Paterson et al 2008a, b; Paterson et al 2007) have been used as pharmacological tools to understand better the neurobiology of drug dependence.
At the same time that the “shared neurobiology and self-medication hypothesis” was published, there was mounting interest in the therapeutic potential of corticotropin-releasing factor (CRF) receptor antagonists to treat stress-related psychiatric disorders. Preclinical studies during the previous 15 years had strongly supported the hypothesis that CRF was a key physiological mediator of not only neuroendocrine, but also behavioral, responses to psychosocial stress, and stress was a known etiologic factor in depression, anxiety disorders and addiction. The cloning of a second CRF receptor subtype in 1995 (CRF2) raised uncertainty as to the roles of each subtype in mediating the actions of CRF (Lovenberg et al 1995), including vis-à-vis the “depressed” neural substrate hypothesized to be common to major depression and drug dependence (Macey et al 2000).
In this context, there was much interest to determine the role of each CRF receptor subtype (CRF1, CRF2) in mediating dysphoria and, by inference, the anti-dysphoria therapeutic potential of subtype-selective CRF receptor antagonists (Zorrilla et al 2002; Henry et al 2006; Cryan et al 2003b). In one project, Dr. Markou organized a collaboration between colleagues at The Scripps Research Institute and a pharmaceutical partner at Novartis-Basel to determine whether antalarmin, a recently identified, first-generation, small molecule CRF1 antagonist (see Figure 1), had anxiolytic-like activity in the rat. The findings were among the first to show that selective CRF1 antagonists reduced naturally-occurring anxiety-like behavior (Zorrilla et al 2002), joining reports that a structurally-related CRF1 antagonist, CP-154,526, had antidepressant-and anxiolytic-like activity in rodent models (Mansbach et al 1997; Kehne et al 2000).
Figure 1.
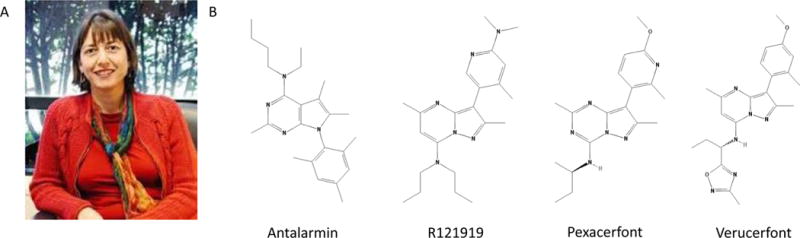
A. Dr. Athina Markou, one of the pioneers in the preclinical study of selective CRF1 antagonists for potential therapeutic use in emotional disorders B. The chemical structure of antalarmin (CAS: 157284-96-3), an early CRF1 antagonist shown to have anxiolytic-like activity by Dr. Markou and colleagues, and similar structures of CRF1 antagonists that have been evaluated in clinical trials, including compounds R121919 (CAS: 195055-03-9), pexacerfont (CAS: 459856-18-9), and verucerfont (CAS: 885220-61-1)
In the intervening 15 years, an enormity of medicinal chemistry, preclinical testing, and clinical trials concerning CRF1 antagonists has been performed. Unfortunately, since initial promising results of an open-label Phase IIa trial of the CRF1 antagonist R121919 for the treatment of major depression reported in 2000 (Zobel et al 2000; see Figure 1), a series of disappointing clinical failures have followed. Table 1 summarizes the many drug-like, small molecule CRF1 antagonists that have failed to successfully complete double-blind, placebo-controlled trials for a wide range of stress-related psychiatric disorders. We and others have reviewed details of these trials previously (Zorrilla et al 2013; Shaham and De Wit 2016; Sanders and Nemeroff 2016). In light of these setbacks, a recent commentary noted that CRF1 receptor antagonists, which were considered by many to have some of the strongest preclinical evidence of recent therapeutic candidates for psychiatric disorders, appeared to have been lost in translation from the laboratory to the bedside (Shaham and De Wit 2016). Here, we analyze, and in some cases revisit (Zorrilla and Koob 2010; Koob and Zorrilla 2012; Zorrilla et al 2013; Shaham and De Wit 2016), possible explanations for the negative outcomes, in order to assess constructively the most current state of the field. In the spirit of avoiding translational obstacles, we also review recent (2014 to present) findings in humans and non-human primates, many of which continue to implicate a role for CRF1 receptors in psychiatric conditions.
Table 1.
Clinical trial outcomes of small-molecule CRF1 antagonists in selected samples with stress-like psychiatric symptoms
| Trial for | Drug | Notes | Refs |
|---|---|---|---|
| Major depression | R121919 | Reduced anxiety and depression; normalized sleep EEG But, withdrawn due to liver enzyme elevations | Held et al 2004; Kunzel et al 2005; Kunzel et al 2003; Zobel et al 2000; Neurocrine Press Release April 5, 2000 |
| PF-00572778 | Withdrawn due to liver enzyme elevations | NCT00580190 | |
| ONO-2333Ms | Lacked efficacy | NCT00514865; Ono Pharmaceutical Co Ltd, 2008 | |
| CP-316,311 | Lacked efficacy | Binneman et al 2008 | |
| SSR125543 | Lacked efficacy | NCT01034995; Sanofi Report DFI5687, September 2011 | |
| Verucerfont (NBI-77860/GSK561679A) |
Lacked efficacy | Tellew et al 2010; GlaxoSmithKline Results Summary for CRS106139, 2010 | |
| Pexacerfont (BMS-562,086) |
Study completed October, 2007; no results reported | NCT00135421 | |
| Generalized | Pexacerfont | Lacked efficacy | Coric et al 2010 |
| Anxiety Disorder | (BMS-562,086) | ||
| Post-traumatic stress disorder | Verucerfont (NBI-77860/GSK561679A) |
Lacked efficacy | NCT01018992 |
| Suicidal ideation in anxious patients | Pexacerfont (BMS-562,086) |
Lacked efficacy | Coric et al 2010 |
| Social anxiety disorder | Emicerfont (GW876008) |
Study completed January, 2008; no results reported | NCT00555139 |
| Verucerfont (NBI-77860/GSK561679A) |
Study completed January, 2008; no results reported | NCT00555139 | |
| Alcohol dependence | Pexacerfont (BMS-562,086) |
Lacked efficacy to reduce alcohol craving, emotional responses to alcohol- or stress-related imagery, or anxiety | Kwako et al 2015 |
| Verucerfont (NBI-77860/GSK561679A) |
Lacked efficacy to reduce alcohol craving, emotional responses to alcohol- or stress-related imagery, or anxiety in anxious alcoholic women. | Schwandt et al 2016 | |
| Irritable bowel syndrome | Emicerfont (GW876008) |
Lacked efficacy | GlaxoSmithKline Results Summary for CRI105626, 2008 |
| Pexacerfont (BMS-562,086) |
Lacked efficacy | Sweetser et al 2009 |
Performance in animal models
Given the perception that CRF1 antagonists performed well in preclinical models but have performed poorly in the clinic, one might prematurely conclude that existing preclinical models are invalid predictors of clinical efficacy in psychiatric disorders (see also Hyman 2012) This alarmist interpretation is not well-supported. In the alcohol research literature, for example, Shaham and De Wit (2016) noted that other drugs that were effective in animal models of stress-induced reinstatement (e.g., alpha-2 adrenoceptor agonists such as clonidine and lofexidine) translated to showing efficacy against stress-induced drug craving in human laboratory studies (Mantsch et al 2016; Sinha et al 2011). Similarly, acamprosate and the opioid receptor antagonists naltrexone and nalmefene reduce operant oral ethanol self-administration in rats under a variety of conditions (Rassnick et al 1992; Heyser et al 1998; Heyser et al 2003; Sabino et al 2006; Ji et al 2008; Gilpin et al 2008; Walker and Koob 2008) and, analogously, show some efficacy to mitigate alcohol use disorders (see Stevenson et al 2015; Keating 2013; Rösner et al 2010; Plosker 2015; but see Palpacuer et al 2015). Gabapentin reduced both the anxiogenic-like behavior and the increased ethanol self-administration observed in withdrawn, ethanol dependent rats, but not non-dependent rats (Roberto et al 2008; Besheer et al 2016; Watson et al 1997) and was found to improve emotional function and reduce insomnia and alcohol use in abstinent alcoholics (Bonnet et al 2007; Malcolm et al 2007; Brower et al 2008; Myrick et al 2009; Mason et al 2014). Most recently, the glucocorticoid receptor antagonist mifepristone, which like CRF1 antagonists more efficaciously reduces ethanol intake in dependent rodents during abstinence than in non-dependent rodents (Yang et al 2008; Simms et al 2012; Vendruscolo et al 2012; Vendruscolo et al 2015), was found to reduce alcohol-cued craving in the laboratory as well as naturalistic measures of alcohol use in a double-blind, placebo-controlled study of 56 alcohol-dependent human subjects (NCT01548417; Vendruscolo et al 2015). Thus, the preclinical models do show predictive sensitivity to detect effective treatments.
On the other hand, Haller et al., have pointed out that, like CRF1 antagonists, 40% of compounds that showed activity in so-called “classical” or “popular” animal models of anxiety-like behavior (which are used in ~90% of anxiety studies), ultimately failed to show therapeutic activity in humans (Haller et al 2013). Accordingly, many neurokinin, cholecystokinin, and 5-hydroxytryptamine type 3 receptor antagonists that showed activity in these preclinical models and were developed contemporaneously with CRF1 receptor antagonists also were then found to be ineffective to treat anxiety disorders. Thus, even when preclinical models show predictive sensitivity to detect therapeutic compounds (i.e., ~60% of compounds that advanced to human trials based on promising results in anxiety models did ultimately show efficacy), they may have suboptimal specificity. Identical issues hamper preclinical models of antidepressant activity. Solutions to improve not only the sensitivity, but also specificity, of preclinical anxiety and depression models have been proposed and apply similarly to all psychiatric domains in which CRF1 antagonists have yet to show clinical efficacy (Haller et al 2013; Griebel et al 2013; Stewart et al 2015; Belzung 2014).
The concept of predictive, validity, in the literature currently is often used to refer, to whether an effective treatment is detected by a model In reality, however, predictive validity refers to whether a model distinguishes effective vs, ineffective, treatments, which jointly reflects the identification of true positives and true negatives in a summary measure of accuracy. Analogous to how positive and negative predictive value jointly determine the accuracy of diagnostic tests in receiver-operating-characteristics (ROC) analyses, both sensitivity and specificity must be considered to determine the predictive validity of animal models. From a screening perspective, the joint use of an animal model with high predictive sensitivity with another having high predictive specificity may yield better outcomes than current screening approaches that focus more on predictive sensitivity (see Abruzzo et al 2015 for analogous approaches with diagnostic tests). The suboptimal specificiiy of commonly used models of depression and anxiety disorders may reflect an incomplete implementation of the pathognomonic constructs and pathophysiological bases of these disorders, in contrast with more recently developed models for alcohol use disorder.
Another consideration is the reality that CRF1 antagonists did not show activity in some models or conditions under which some clinically efficacious treatments do. For example, CRF1 antagonists did not reduce substance- or cue-induced reinstatement of substance-seeking in animal models, so it is not surprising that they did not reduce alcohol cue-induced craving in human laboratory studies (Schwandt et al 2016; Kwako et al 2015). Similarly, CRF1 antagonists did not reduce and even exacerbated fear-potentiated acoustic startle responses in rat models (Walker et al 2009). Accordingly, the CRF1 antagonist GSK561679 ultimately increased fear-potentiated acoustic startle reactivity in 31 healthy women (Grillon et al 2015). Thus, for a few endpoints, the “negative” clinical results may actually translate from the preclinical findings.
Along the same lines, whereas several clinically effective treatments like naltrexone, nalmefene, and acamprosate reduce alcohol self-administration in rat models of non-dependent alcohol self-administration, including in rats genetically selected for high alcohol preference or in outbred rats receiving alcohol under intermittent schedules of alcohol access (Rassnick et al 1992; Heyser et al 1998; Heyser et al 2003; Sabino et al 2006; Ji et al 2008; Gilpin et al 2008; Walker and Koob 2008), CRF1 antagonists frequently did not (Sabino et al 2006; Gilpin et al 2008; Sabino et al 2013). Rather, they differentially or more strongly showed effects in rats that had been made dependent on alcohol due to chronic intermittent exposure (but, see also some positive findings in non-dependent rat (Simms et al 2014; Cippitelli et al 2012) and mouse models (Lowery et al 2010; Sparta et al 2009; Lowery et al 2008; Sparta et al 2008)). Similarly, unlike benzodiazepines, CRF1 antagonists did not typically show activity under baseline conditions in most models of anxiety-like behavior, including the elevated plus-maze (see Zorrilla and Koob 2004). Likewise, unlike tricyclic antidepressants and noradrenergic- or serotonergic-reuptake inhibitors, CRF1 antagonists did not consistently show activity under baseline conditions in rodent forced swim tests and several other models that have been used to screen for antidepressant-like compounds Rather, they required environmental, pharmacological, or genetic manipulation to induce a stress-like phenotype during testing (or, for some antidepressant-predictive models, did not show activity even under those conditions; see Zorrilla and Koob 2010). One skeptical interpretation of these results might have been that, even though the forced swim test is subject to false positive results (specificity issue, low PPV+), perhaps the predictive value of a negative result in the model is high (sensitivity issue, high NPV+) CRF1 antagonists might thereby have not been expected to show antidepressant-like activity. Instead, the collective findings were regarded as being conceptually appealing and heralded as evidence that pathological substance use and dysphoria are associated with recruitment of otherwise quiescent CRF-CRF1 synaptic transmission, a hypothesis also supported by molecular and electrophysiological studies in preclinical models.
Revisionist hypotheses and their implications
In light of the negative findings to date in clinical studies, revised hypotheses concerning the manner of “CRF-CRF1 recruitment” needed for therapeutic activity have been offered For example, CRF1 antagonists were proposed to be effective in “specific psychiatric disorders in which stress was a dynamic rather than chronic condition” (to include, for example, PTSD, panic and addiction disorders and exclude major depression and generalized anxiety disorder; Koob and Zorrilla 2012) or, in which central CRF overactivation was explicitly present. Neither of these hypotheses has been fully evaluated yet, but they raise several testable predictions.
One interpretation of the “dynamic” revision is not only that only certain types of stress-related disorders may be treatable by CRF1 antagonists, but also that a given patient may be more sensitive to CRF1 antagonist treatment earlier in the course of their disorder (before CRF activation has become chronic). The “dynamic” revision also suggests that sensitivity to CRF1 antagonists might decrease with greater chronicity in preclinical models and that repeated CRF1 activation (as in chronic stress) may lead to plasticity within or downstream of CRF1 receptor signaling that comes to perpetuate the maladaptive behavior comparatively less dependent on subsequent acute CRF1 agonist stimulation. Possible mechanisms for such plasticity have been described, including kindling, priming, heterologous sensitization, altered G-protein coupling, altered splicing, and long-term potentiation (Lee et al 2008; Ray et al 2011; Sajdyk et al 1999; Rainnie et al 2004; Narla et al 2016; Zmijewski and Slominski 2010; Dunn et al 2016; Magalhaes et al 2010; Bunson et al 1998; Rajbhandari et al 2015; Huang et al 2010; Krishnan et al 2010). It may be that patients present for treatment later in their disease course, after more such plasticity has occurred, as compared to preclinical models, which are designed for expeditious testing. Finally, the “dynamic” revision suggests that patients may be more responsive to CRF1 antagonists during particular circumstances or stages of their disorder during which stress responses play a greater role in driving symptoms. In support of the final proposition, oral CRF1 antagonist emicerfont administration (and not placebo) selectively reduced BOLD fMRI signal in the hypothalamus, amygdala, hippocampus, insula, anterior cingulate, and orbitomedial prefrontal cortices in patients with irritable bowel disease who were actively experiencing anxiety in anticipation of visceral pain (Hubbard et al 2011). Similarly, the CRF1 antagonist R317573/JNJ19567470/CRA5626 decreased regional glucose utilization in the amygdala (Schmidt et al., 2010) and anxiety responses to 7.5% acute CO2 inhalation challenge in a double-blind, placebo-controlled trial with healthy men (Bailey et al 2011).
Relatedly, the “CRF overactivation” revision suggests that CRF1 antagonists may be effective in patients who show high activity in CRF-CRF1 systems (on either a trait or state basis). This could be probed via biochemical (e.g., high CSF CRF), neuroimaging (e.g., altered CRF1 receptor availability), endophenotypic (e.g., increased REM sleep/pressure signs of high CRF drive; see also. Heilig et al 2016, and Heilig and Leggio 2016), or genetic means (e.g., functional single nucleotide polymorphisms [SNPs] in CRF system molecules; Holsboer and Ising 2010; Zorrilla et al 2013; Sanders and Nemeroff 2016, Treutlein et al 2006, Barr et al 2008, Nelson, et al 2010, Heilig et al 2011) Significant limitations of the often cited initial clinical study that obtained promising results with R121919 in. patients with major depression (Zobel et al 2000) include that it was small, not double-blind, and did not show a significant cross-sectional difference between subjects treated with the high-versus low-escalating dose schedule. With regard to potential markers of treatment response, however, a reanalysis of the study found that patients with increased rapid eye movement (REM) sleep density during the first half of the baseline night showed a greater reduction of Hamilton-Depression scores with high-dose R121919 treatment than those that did not. Low-dose R121919 treatment was ineffective in all groups (Held et al 2004). On the other hand, several SNPs for CRF1 and CRF-binding protein (CRF-BP) did not predict treatment response to pexacerfont in patients with generalized anxiety disorder (Coric et al 2010). Thus, validating therapeutically prognostic markers of CRF-CRF1 activation in double-blind, placebo-controlled studies may be key.
Targeting and validating drug action in humans
Clinical trial failures alternatively might reflect the inadequacy of prioritized drug candidates to quiet central CRF1 signaling in humans. Indeed, early CRF1 antagonists had unacceptably high lipophilicities and other physiochemical properties not characteristic of successful CNS drugs, leading to high toxicity potential and poor bioavailability (see Zorrilla and Koob, 2010). Some compounds were suggested to yield negative results because they had lower CRF1 affinity than R121919 (e.g., CP 316,311; Holsboer and Ising 2010). More recently, it was proposed that a long duration of receptor residency may be key for efficacy (Fleck et al 2012; Zorrilla et al 2013), because R121919 had slower receptor dissociation rates than compounds that had failed clinically. In support of the importance of this property for antagonist action in vivo, verucerfont (NBI-77860; see Figure 1), a high-affinity, drug-like (Zorrilla and Koob, 2010), CRF1 antagonist with long receptor residence, was found, like R121919, and unlike the faster-dissociating compounds pexacerfont and CP316,311, to reduce circulating adrencocorticotropic hormone (ACTH) in adrenalectomized rats. In anxious individuals with alcohol use disorder, verucerfont also reduced ACTH and cortisol responses to dexamethasone/CRF challenge and blunted right amygdala fMRI activation responses to fearful faces, activities that had not been seen for the faster-dissociating pexacerfont (Schwandt et al 2016; Kwako et al 2015). These findings validate the drug’s pharmacological action Also, as expected, verucerfont did not reduce alcohol cue-induced craving or anxiety, negative findings consistent with the lack of action of CRF1 antagonists in animal models of cue-induced craving Unexpectedly, however, verucerfont still did not reduce alcphol craving or anxiety induced by public speaking or by stress-related guided imagery; rather, it worsened anxiety associated with the New Trier social stress test of public speaking (Schwandt et al 2016). Thus, although the verucerfont trial was negative, the confirmation that receptor residence, a previously underappreciated property, was key for pharmacological action, supports the possibility that not all mechanisms critical for achieving desired CRF1 silencing in humans have been identified.
To the degree that the biology of human vs rodent CRF1 receptors differ (or that of several, molecular partners that influence CRF1 signaling; Dunn et al 2016; Bonfiglio et al 2013; Bangasser et al 2010 Walther et al 2015), cryptic species differences also may be impeding therapeutic silencing of CRF1 receptors in humans. Furthermore, selective CRF1 antagonists that have been tested to date have no activity at CRF2 receptors or the CRE-binding protein (CRF-BP). The CRF2 subtype in rodents has often been regarded as having a net null or perhaps even anxiolytic-like action, but as Dr. Markou and others showed, stimulation of CRE2 receptors in the lateral septum is anxiogenic in rodents (Bakshi et al 2007, Henry et al 2006, Anthony et al 2014). Furthermore, in contrast to rodents, which mainly express only CRF1 receptors in the central nucleus of the amygdala (CeA) (Van Pett et al 2000), primates also express substantial numbers of CRF2 receptors of unknown behavioral significance in the CeA (Sanchez et al 1999). Third, humans, but not rodents, possess a unique CRF2 gamma subtype (Kostich et al 1998).Finally,CRF2receptors interact with the CRF-B.P to produce actions independent from CRF1 (Wang et al 2007, Ungless et al 2003, Slater et al 2016a, Slater et al 2016b, Milan-Lobo et al 2009).
In this context, the CRF-BP initially had been regarded as serving an inhibitory role in the CRF system; but, it has increasingly been recognized to have other modulatory roles in the brain (Westphal and Seasholtz 2006). Indeed, the CRF-BP has recently received attention as a potential target for its role in alcohol use disorder (Haass-Koffler et al 2016, Ketchesin et al 2016); and, particularly, its role in the escalation of alcohol drinking may involve interaction with CRF2 receptors (Albrechet-Souza et al 2015, Quadros et al 2016). Receptor activity modifying proteins (RAMPs) are other molecules that interact directly with the CRF system, as RAMP2 binds CRF1 and increases its surface expression and signaling sensitivity (Wootten et al 2013). But CRF1 antagonists to date likewise have not explicitly considered CRF1-RAMP2 complexes.
Thus, perhaps small molecules that: 1) act as non-selective antagonists at both CRF subtypes, 2) exhibit inverse agonist activity, or 3) also modulate activities of other CRF system molecules, such as CRF-BP or receptor activity modifying protein-2 (RAMP2) (Wooten et al 2013; Weston et al 2016; Gingell et al 2016) would have greater therapeutic activity than the many selective CRF1 neutral antagonists tested to date. Similarly, the structural manner of binding the receptor may be important; perhaps small molecules that bind differentially to certain residues of the atypical allosteric binding site (e.g., compare MTIP vs. CP-376395; Xu et al 2015) may slow the antagonist’s escape kinetics (Bai et al 2014) or direct its anti-signaling pathway bias (Suen et al 2014; Zhang et al 2016). Alternatively, a small molecule that binds to the orthosteric (agonist)-binding site, rather than to the atypical, deep allosteric binding site that is bound by all clinically-evaluated CRF1 antagonists to date (see Zorrilla and Koob, 2010; Hollenstein et al 2013; Hausch 2013) may yield different pharmacological effects. Finally, there are different degrees of “inactivity” in terms of receptor confirmation, and a recent study showed that a cooperative, “double antagonist” approach (one at orthosteric, one at allosteric site) led to the most inactive state for the CC chemokine receptor 2, another G-protein coupled receptor (Zheng et al 2016; Miao and McCammon 2016).
The unavailability of a CRF1 radiotracer for PET/SPECT imaging to confirm adequate receptor occupancy in humans, despite continuing efforts to obtain one that exhibits specific binding in vivo (Stehouwer et al 2015a; Stehouwer et al 2015b; Lodge et al 2014) as well as the uncertain density of CRF1 receptors in human brain vs. other species (see discussion in Lodge et al 2014) also may be leading to suboptimal prioritization of drug candidates and dosing. A surrogate, non-PET biomarker approach (Schwandt et al 2016) used to predict central receptor occupancy found that even with high estimated (~90%) occupancy, verucefont still did not produce therapeutic action The development of CRF1 PET/SPECT radioligands could further validate and refine such surrogate approaches for estimating receptor occupancy.
Recent genetic and molecular findings in humans
In addition to results already cited, several genetic and molecular findings since 2014 in humans and non-human primates continue to implicate CRF-CRF1 systems in emotional dysfunction, addiction, or stress-related phenotypes (see also Zorrilla et al 2013, for review of earlier studies of CRF system SNPs in addiction).
As prelude, major limitations of the genetic variant studies to date are that many of them have not been replicated and, for most, their molecular effect, if any, on the CRF1 system (as opposed to a putative surrogate marker of CRF1 system activity) is unknown. Additionally, in general, individual genetic variants are associated with a low percentage of psychiatric disease prevalence. Furthermore, with only a few recent exceptions (see Clarke et al 2014, Crist et al 2016, Crist et al 2013 Heinzerling et al 2013) genetic variants have not yet reliably and reproducibly predicted treatment response in psychiatric diseases (Jones and Comer 2015 Berrettini 2016 Qedegaard et al 2016) A molecular, and not only phenotype-based, understanding, of a variant’s functional effect (if any) may ultimately be needed to understand its prognostic relation to CRF1 antagonist treatment response.
Major Depression
With respect to major depression, CRHR1 SNPs of rs7209436, rs110402, and rs242924 previously had been associated with peak cortisol responses to the Trier Social Stress Test in healthy adults (Mahon et al 2013). Recently it was reported that a TATGA haplotype combination that includes the above 3 polymorphic loci (rs17689966, rs173365, rs7209436, rs110402, and rs242924) increased the risk for major depression by 68% in a community-based study in South Spain (Ching-Lopez et al 2015). Furthermore, the T allele at CRHR1 rs242941, which forms part of a haplotype that previously had been linked to major depression and antidepressant response (Liu et al 2006; Licinio et al 2004; Liu et al 2007) was linked to family history of mental illness (Tan et al 2015). Smoller recently reviewed genome-wide association studies that implicate CRHR1 genotype X early childhood maltreatment environmental interactions for major depression risk (Smoller 2016). Finally, an A-Deletion-A CRHR1 haplotype (rs77032924, rs3832590, rs6159) was associated with a trend for poorer response to treatment with mirtazapine or escitalopram in patients with major depression not experiencing stressful life events, perhaps suggesting a role for this haplotype in driving CRF-mediated intrinsic dysphoria that is resistant to non-CRFergic intervention (Chang et al 2015).
Suicidality
CRF-CRF1 systems also were linked to suicidality, with ~2-fold increased CRF mRNA in the anterior cingulate of depressed patients who committed suicide as opposed to those who died from natural causes (Zhao et al 2015). Allele C at the Crhr1 rs878886 locus was overrepresented in suicide attempters from both Russian and Tatar ethnicity samples (Khalilova et al 2014). In addition, CRHR1 loci that mitigated (rs2664008) or tended to potentiate suicide risk (rs1724425, rs1526123, rs6503447, rs11655764) were identified in a case-control study of individuals with bipolar disorder, the former especially buffering the effects of early childhood abuse (Breen et al 2015)
Anxiety disorders
With respect to anxiety disorders, a recent case-control study found that the minor (A) allele of CRHR1 rs17689918 and, relatedly, a CGTGA haplotype (rs7209436, rs4458044, rs12936181, rs3785877, rs17689918) increased risk for panic disorder selectively in women. Unexpectedly, post-mortem studies showed that this risk allele was associated with decreased CRF1 mRNA in human forebrains and amygdala. Neuroimaging studies found that allele carriers showed a pattern of altered fMRI signal in the prefrontal cortex and amygdala that was interpreted to reflect overgeneralization of fear conditioning and underprocessing of safety signals; allele carriers also showed less “flight” and more “anxious apprehension” behaviors in response to fear provoking-stimuli. While the results implicate CRF1 in anxious processing in panic disorder, they do so in an unexpected direction and also indicate that pharmacogenetic selection of patients in which CRF1 receptors play a role may need to be considered in a sex-specific manner (Weber et al 2016). An additional concern here as well is if a patient has fewer CRF1 receptors, then they have less available drug target.
In apparent opposition to the findings in people with panic disorder, Kalin, Oler and colleagues observed that viral vector mediated overexpression of CRF in the CeA of young rhesus monkeys led to increased anxious temperament (freezing, cooing and cortisol reactivity in response to a human intruder). The increase in anxious temperament correlated directly with increased fluorodeoxyglucose metabolism (by PET) and fMRI functional connectivity within a circuit that included the dorsal amygdala, orbital proisocortex/anterior insula and hippocampus (Kalin et al 2016).
Addiction
With respect to addiction, the CRHBP rs1875999 locus was associated with risk for both cocaine and heroin addiction in African Americans in a study of heroin addicts (n = 314), cocaine addicts (n = 281), and healthy controls (n = 208) (Levran et al 2014). SNPs in the CRHBP (10kD) fragment, rs10055255, rs10062367, and rs7728378 were each shown to be associated with increased risk of alcohol drinking and/or anxiety in patients with alcohol use disorder (Haass-Koffler et al 2016).
Irritable bowel syndrome
Several recent human studies further implicated CRF-CRF1 activity in irritable bowel syndrome (IBS), a heterogeneous diagnosis that involves abdominal pain, altered bowels habits, gastrointestinal (GI) symptom-specific anxiety, and altered, stress-sensitive brain-gut interactions, often in association with comorbid anxiety or mood disorders. First, men with IBS showed increased sensitivity to intravenous CRF infusion, with greater right amygdala activation (by H215O-PET) and peripheral noradrenaline secretion than in healthy controls (Tanaka et al 2016). Second, the major alleles of CRHR1 rs110402, rs242924 and rs720943 (all C) were associated with increased risk for IBS and, within IBS patients, increased GI-symptom-related anxiety. Unexpectedly, IBS patients with the same risk alleles showed reduced acoustic startle responses vs. healthy controls, whereas those with the minor alleles did not, indicating a complex influence of the CRHR1 SNPs on different symptoms of anxiety per diagnostic group (Orand et al 2016). Finally, a separate study of young Japanese individuals observed that the CRHR1 rs10474485 locus was associated with increased psychometric scores for depression, perceived stress or state/trait anxiety in IBS patients with diarrhea or mixed symptoms, with the CRHR1 variant more predictive of differences in emotional scales in women than in men (Sasaki et al 2016). Two. CRHR2. variants, rs4722999 and rs3779250, have also been associated with genotype frequency of IBS and the distribution of the major allele was significantly different in IBS patients compared to controls (Komuro et al 2016).
Other emotion-related phenotypes
A recent neuroimaging study of school-age children was performed involving a genetic profile score that predicts HPA-axis reactivity, wherein 5 of the 10 SNPs used to calculate the genetic profile score involved CRHR1 loci (rs4792887, rs110402, rs242941, rs242939, rs1876828). Higher genetic profile scores were found to predict greater amygdala and hippocampal fMRI activational responses to facial stimuli in pubertal, but not non-pubertal, children. In pubertal children, differential activation to fearful faces was seen in girls and to neutral faces in boys (Pagliaccio et al 2015). The results again are consistent with the possibility of sex differences in the functional significance of CRF1 genetic variants in a manner that depends upon developmental milestones associated with puberty. Indeed, gonadal hormones influence the regulation of CRF system molecules in both male and female rats in a puberty-relevant manner (Bangasser and Valentino 2012; Gomez et al 2004). Furthermore, Valentino and colleagues have described sex differences in the signaling pathway bias of CRF1 receptors in rodents (Valentino et al 2013a; Valentino et al 2013b), a mechanistic difference that may be relevant to some of the reviewed sex differences in the functional impact of CRHR1 genetic variants.
A separate imaging study at the Duke Neurogenetics Center examined the widely studied CRHR1 locus rs110402, which, as reviewed above, has been associated with increased HPA-axis reactivity, in interaction with a locus relevant to a gene encoding fatty acid amide hydrolase, an enzyme that degrades the endocannabinoid anandamide. Individuals with a genetic background of increased CRF1 signaling (A homozygotes) in combination with increased anandamide inhibitory tone (FAAH 385A carriers) showed decreased habituation of the BOLD fMRI response of the basolateral amygdala during emotional facial processing. The blunted amygdala habituation, in turn, was associated with increased risk for an anxiety disorder (Demers et al 2016). Interestingly, the rs110402 locus also was associated recently with premature decline in working memory, but not other measures of neuropsychological function, across the lifespan. The finding has been interpreted to reflect a chronic deleterious influence of stress reactivity (Grimm et al 2015).
Finally, the GGA haplotype at polymorphic loci of the CRHR1 gene (rs4458044, rs242924, and rs1768996) was associated with aggressive behavior towards others as determined in a Han Chinese sample of violent criminals (Chen et al 2014).
Summary
Variants of the CRHR1 and CRHBP genes continue to be associated with the diagnoses, phenomenology and/or non-CRFergic treatment response of major depression, suicidality, panic disorder, and irritable bowel syndrome. Findings also implicate other stress-related (endo)phenotypes, including not only HPA-axis reactivity and startle reactivity, but also more novel findings, such as altered amygdalar habituation or activation during facial processing, premature impairment of working memory, and physical aggression. A new development includes the finding that there may be sex differences or developmental (pubertal) moderation of the predictive relation of some genetic variants, and that for some phenotypes (e.g., panic disorder, startle reactivity between IBS patients and controls). Particular CRF1 variants also appeared to have effects opposite to those anticipated from a simple model of greater CRF1 activation always having anxiogenic action. The latter result may reflect that CRF1 activation in some brain regions may have anxiolytic-like effects, including via circuit action to inhibit anxiogenic-like effects of CRF1 activation elsewhere (Sztainberg et al 2011; Walker et al 2009). These results may also relate to why CRF1 antagonists even exacerbated anxious or fearful symptomatology in two human studies (Grillon et al 2015; Schwandt et al 2016).
Variants that have been replicated across studies and which, at the least, associate with an endophenotype of CRF1 activation, such as rs7209436, rs110402, or rs242924, are hypothesized to be more likely to predict better treatment response to CRF1 antagonists. However, as alluded to previously, important criticisms of these genetic variant studies include that their associated odds ratios, even when significant, have been modest (Levran et al 2014), and many genetic variants have unknown molecular effects, if any, on the. CRF1 system. Additionally, many genetic variant studies failed to replicate (Buttenschøn et al 2016, Ventura-Juncá et al 2014) or have not yet been replicated. Finally, the finding that variants of CRHBP and CRHR2 have been associated with psychiatric and stress-related disease lends support to the hypothesis that other components of the CRF system may be involved and warrants further exploration of polymorphs of CRHBP, CRHR2, and/or RAMP2.
Recent promising CRF1 antagonist trials in humans
Two clinical trials with CRF1 antagonists reported potentially promising results during 2016. First, in a randomized, double-blind, placebo-controlled study that was stopped by the NIH. IRB for reasons unrelated to adverse drug effects or efficacy (reinterpretation of the Common Rule for human snbject protection under HHS, 45 CFR 46A), pexacerfont was found to produce effect sizes consistent with reduction of food craving and laboratory stress-induced eating in a small sample of healthy individuals with restrained eating. Although statistical significance was not seen, the study was stopped prematurely and thereby only had 30% power to detect the stated effect size of.interest; thus, it would be inappropriate to interpret it as a negative result and observed effect sizes may inform whether future appropriately-powered studies are warranted. The effect size for pexacerfont’s reduction of laboratory stress-induced eating was r = 0.30 (counternull r = 0.55; Rosenthal and Rubin 1994) and for its reduction of craving for sweet foods (brownies and Swedish fish) ranged from r = 0.28 to 0.49 (counternull rs = 0.52–0.79). Furthermore, in bogus taste tests designed to mask the true dependent measure of interest (intake), pexacerfont reduced eating of palatable foods independent of which imagery script was presented before food access (neutral, food cue, stress) with an effect size of r = 0.34 (counternull r = 0.61). Finally, nightly Yale Food Addiction Scores were consistently lower in subjects receiving pexacerfont (vs. placebo) beginning the evening after the first loading dose of pexacerfont with an effect size of r = 0.39 (counternull r = 0.68). Because the study was stopped prematurely (n = 11–13/group for laboratory studies and n = 13–17/group for YFAS ratings), only the YFAS result was significant at the p < 0.05 level. However, Bayes factor analysis, a ratio that relates to the relative probability of an effect actually being present vs. the null effect (Goodman 1999), and counternull analysis, which describes the effect size as likely to be true as the null, indicate a strong positive potential of CRF1 antagonists to reduce palatable food craving and eating in restrained eaters (Epstein et al 2016) and justify a well-powered clinical trial in this domain. A concern with these results is that the YFAS scores had changed as early as 24-hours post-treatment, and the degree of CNS exposure obtained at that time is uncertain, leading one to question the CRF1 antagonist mechanism of action Still, the preliminary results concord with preclinical studies showing that systemic administration of CRF1 antagonists reduce overeating of a palatable, high sucrose diet in rats receiving intermittent access to the diet (Cottone et al 2009) and also reduce stress-induced reinstatement of palatable food-seeking (Ghitza et al 2006). Why the clinical results differ from those obtained for alcohol craving with pexacerfont in anxious alcoholics (Kwako et al 2015) is unclear.
The second promising clinical result involved a phase Ib, single-blind, placebo-controlled, fixed-sequence, single-dose trial of verucerfont, (NBI-77860; see Figure 1) for 21-hydroxylase deficiency, the most common cause of congenital adrenal hyperplasia. In 21-hydroxylase deficiency, the cortisol synthetic pathway is impaired, leading to loss of glucocorticoid negative feedback over the HPA-axis (similar to adrenalectomy) and consequent hypothalamic CRF-driven hypersecretion of ACTH, with accumulation of upstream precursors of cortisol, including 17α-hydroxyprogesterone (17OHP). Because 17OHP cannot be processed to cortisol, it instead is converted along the androgen pathway, leading to clinical manifestations of congenital adrenal hyperplasia. Following CRF1 antagonist treatment, dose-dependent reductions of ACTH and/or 17OHP were observed in six of eight subjects, with overall mean reductions of 41–43% for ACTH and, at the higher antagonist dose, 27% for 17OHP. Thus, the results validate the reviewed clinical finding with verucerfont (Schwandt et al 2016) that CRF1 antagonists with long receptor residence can reduce CRF-driven chronic overactivation of the HPA-axis and indicate one possible therapeutic indication for this action (Turcu et al 2016). This positive finding also is consistent with the revisionist hypotheses that robust pathophysiological overactivation of CRF signaling is a key for therapeutic potential.
Conclusion
We reviewed a range of issues that may explain why CRF1 antagonists could be said to have been lost in translation from the bench to the bedside. These include not only potential specificity limitations of the preclinical models themselves, but also the reality that, for some predictive endpoints, CRF1 antagonists produced therapeutic-like results only under certain circumstances (e.g., high stress, withdrawal), unlike some clinically effective compounds that act more generally. In some models, CRF1 antagonists had null or even exacerbating actions, the latter consistent with analogous findings in gene variant and clinical studies. Recognition of this leads to the revised view that the efficacy of CRF1 antagonists may be correspondingly circumscribed to particular psychiatric disorders or symptoms, patient subgroups, or circumstances in which the activation of pro-stress-like CRF-CRF1 circuits is dynamically heightened. We described genetic and non-genetic markers that could be evaluated as markers of such activation towards individualized treatment and obstacles that remain for such approaches (e.g.., CRF1 in vivo radiotracer; molecularly validated, functional SNPs). We also discussed both solved and unresolved issues concerning whether small molecules that have been advanced to the clinic adequately engaged human CRF system molecules in the manner needed to attain therapeutic silencing. We also noted mechanisms via which CRF1 stimulation-induced plasticity within and downstream of CRF1 receptors may reduce the need for high ongoing CRF1 agonist stimulation to perpetuate the maladaptive behavior. Finally we review promising, recent human trials which suggest that CRF1 antagonists may have potential to reduce craving for and stress-induced eating of palatable food as well as CRF-driven overactivation of the HPA-axis. One must be cognizant of the significant opportunity cost in continuing to pursue selective CRF1 antagonists for therapeutic use, but given the increasing understanding in the field, several therapeutie avenues with these or, especially, novel anti-CRF compounds remain underexplored Thus, while it appears that the therapeutic scope of selective CRF1 antagonists is narrower than had been hoped when Dr. Markou organized those early studies of antalarmin, much remains to be learned about the shared molecular roles of CRF receptors in the neurobiology of stress, dysphoria and addictive behavior in humans and the potential individualized role of novel anti-CRF approaches therein.
Acknowledgments
The authors thank Michael Arends for editorial assistance in the preparation of this manuscript. Research reported in this publication was supported by the Pearson Center for Alcoholism and Addiction Research and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number P60AA06420. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E.P. Zorrilla is inventor on a patent for CRF1 antagonists (US20100249138).
References
- Abruzzo PM, Ghezzo A, Bolotta A, Ferreri C, Minguzzi R, Vignini A, Visconti P, Marini M. Perspective Biological Markers for Autism Spectrum Disorders: Advantages of the Use of Receiver Operating Characteristic Curves in Evaluating Marker Sensitivity and Specificity. Dis Markers. 2015;2015:329607. doi: 10.1155/2015/329607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA. Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice. Alcohol Clin Exp Res. 2015;39(9):1609–18. doi: 10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156(3):522–36. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Q, Shi D, Zhang Y, Liu H, Yao X. Exploration of the antagonist CP-376395 escape pathway for the corticotropin-releasing factor receptor 1 by random acceleration molecular dynamics simulations. Mol Biosyst. 2014;10:1958–1967. doi: 10.1039/c4mb00037d. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 2007;27(39):10568–77. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley D, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65(8):934–44. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C. Innovative drugs to treat depression: did animal models fail to be predictive or did clinical trials fail to detect effects? Neuropsychopharmacology. 2014;39:1041–1051. doi: 10.1038/npp.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W. Opioid neuroscience for addiction medicine: From animal models to FDA approval for alcohol addiction. Prog Brain Res. 2016;223:253–67. doi: 10.1016/bs.pbr.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Besheer J, Frisbee S, Randall PA, Jaramillo AA, Masciello M. Gabapentin potentiates sensitivity to the interoceptive effects of alcohol and increases alcohol self-administration in rats. Neuropharmacology. 2016;101:216–224. doi: 10.1016/j.neuropharm.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Bonfiglio JJ, Inda C, Senin S, Maccarrone G, Refojo D, Giacomini D, Turck CW, Holsboer F, Arzt E, Silberstein S. B-Raf and CRHR1 internalization mediate biphasic ERK1/2 activation by CRH in hippocampal HT22 Cells. Mol Endocrinol. 2013;27:491–510. doi: 10.1210/me.2012-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U, Specka M, Leweke FM, Nyhuis P, Banger M. Gabapentin’s acute effect on mood profile: a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:434–438. doi: 10.1016/j.pnpbp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Breen ME, Seifuddin F, Zandi PP, Potash JB, Willour VL. Investigating the role of early childhood abuse and HPA axis genes in suicide attempters with bipolar disorder. Psychiatr Genet. 2015;25:106–111. doi: 10.1097/YPG.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32:1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Buttenschøn HN, Krogh J, Nielsen MN, Kaerlev L, Nordentoft M, Mors O. Association analyses of depression and genes in the hypothalamus-pituitary-adrenal axis. Acta Neuropsychiatr. 2016;6:1–6. doi: 10.1017/neu.2016.26. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chang HS, Won E, Lee HY, Ham BJ, Lee MS. Association analysis for corticotropin releasing hormone polymorphisms with the risk of major depressive disorder and the response to antidepressants. Behav Brain Res. 2015;292:116–124. doi: 10.1016/j.bbr.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Ching-López A, Cervilla J, Rivera M, Molina E, McKenney K, Ruiz-Perez I, Rodríguez-Barranco M, Gutiérrez B. Epidemiological support for genetic variability at hypothalamic-pituitary-adrenal axis and serotonergic system as risk factors for major depression. Neuropsychiatr Dis Treat. 2015;11:2743–2754. doi: 10.2147/NDT.S90369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Crist RC, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, Ling W, Hillhouse MP, Bruce RD, Woody G, Berrettini WH. Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J. 2014;14(3):303–8. doi: 10.1038/tpj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang SP, Emison E, Delmonte T, D’Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, Ling W, Hillhouse MP, Bruce RD, Woody G, Berrettini WH. An intronic variant in OPRD1 predicts treatment outcome for opioid dependence in African-Americans. Neuropsychopharmacology. 2013;38(10):2003–10. doi: 10.1038/npp.2013.99. Erratum: Neuropsychopharmacology (2014) 39(4):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Doyle GA, Nelson EC, Degenhardt L, Martin NG, Montgomery GW, Saxon AJ, Ling W, Berrettini WH. A polymorphism in the OPRM1 3′-untranslated region is associated with methadone efficacy in treating opioid dependence. Pharmacogenomics J. 2016 doi: 10.1038/tpj.2016.89. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003a;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Gasparini F, van Heeke G, Markou A. Non-nicotinic neuropharmacological strategies for nicotine dependence: beyond bupropion. Drug Discov Today. 2003b;8:1025–1034. doi: 10.1016/s1359-6446(03)02890-3. [DOI] [PubMed] [Google Scholar]
- Dunn HA, Chahal HS, Caetano FA, Holmes KD, Yuan GY, Parikh R, Heit B, Ferguson SS. PSD-95 regulates CRFR1 localization, trafficking and β-arrestin2 recruitment. Cell Signal. 2016;28:531–540. doi: 10.1016/j.cellsig.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Fleck BA, Hoare SR, Pick RR, Bradbury MJ, Grigoriadis DE. Binding kinetics redefine the antagonist pharmacology of the corticotropin-releasing factor type 1 receptor. J Pharmacol Exp Ther. 2012;341:518–531. doi: 10.1124/jpet.111.188714. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 2004;145:59–70. doi: 10.1210/en.2003-0565. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell JJ, Simms J, Barwell J, Poyner DR, Watkins HA, Pioszak AA, Sexton PM, Hay DL. An allosteric role for receptor activity-modifying proteins in defining GPCR pharmacology. Cell Discov. 2016;2:16012. doi: 10.1038/celldisc.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, Lasek AW, Magill M, Schwandt ML, Momenan R, Hodgkinson CA, Bartlett SE, Swift RM, Bonci A, Leggio L. Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry. 2016;6(11):e953. doi: 10.1038/tp.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Aliczki M, Gyimesine Pelczer K. Classical and novel approaches to the preclinical testing of anxiolytics: a critical evaluation. Neurosci Biobehav Rev. 2013;37:2318–2330. doi: 10.1016/j.neubiorev.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Markou A. Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther. 2001;297:316–325. [PubMed] [Google Scholar]
- Hausch F. Structures of class B G protein-coupled receptors: prospects for drug discovery. Angew Chem Int Ed Engl. 2013;52:12783–12785. doi: 10.1002/anie.201307542. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670–84. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: Theragnostic biomarkers for personalized treatments. Prog Brain Res. 224:401–18. doi: 10.1016/bs.pbr.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Heilig M, Sommer WH, Spanagel R. The Need for Treatment Responsive Translational Biomarkers in Alcoholism Research. Curr Top Behav Neurosci. 2016;28:151–71. doi: 10.1007/7854_2015_5006. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Demirdjian L, Wu Y, Shoptaw S. Single nucleotide polymorphism near CREB1, rs7591784, is associated with pretreatment methamphetamine use frequency and outcome of outpatient treatment for methamphetamine use disorder. J Psychiatr Res. 2016;74:22–9. doi: 10.1016/j.jpsychires.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held K, Kunzel H, Ising M, Schmid DA, Zobel A, Murck H, Holsboer F, Steiger A. Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J Psychiatr Res. 2004;38:129–136. doi: 10.1016/s0022-3956(03)00076-1. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Hollenstein K, Kean J, Bortolato A, Cheng RK, Doré AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013;499:438–443. doi: 10.1038/nature12357. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 61:81–109. C1–C11. doi: 10.1146/annurev.psych.093008.100321. (201) [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp The. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Sci Transl Med. 2012 Oct;4(155):155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD. A review of pharmacogenetic studies of substance-related disorders. Drug Alcohol Depend. 2015;152:1–14. doi: 10.1016/j.drugalcdep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp do PM, Grabow BP, Olsen ME, Brodsky EK, McFarlin DR, Alexander AL, Emborg ME, Block WF, Fudge JL, Oler JA. Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol Psychiatry. 2016;80:345–355. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM. Nalmefene: a review of its use in the treatment of alcohol dependence. CNS Drugs. 2013;27:761–772. doi: 10.1007/s40263-013-0101-y. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Coverdale S, McCloskey TC, Hoffman DC, Cassella JV. Effects of the CRF1 receptor antagonist, CP 154,526, in the separation-induced vocalization anxiolytic test in rat pups. Neuropharmacology. 2000;39:1357–1367. doi: 10.1016/s0028-3908(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Ketchesin KD, Stinnett GS, Seasholtz AF. Binge Drinking Decreases Corticotropin-Releasing Factor-Binding Protein Expression in the Medial Prefrontal Cortex of Mice. Alcohol Clin Exp Res. 2016;40(8):1641–50. doi: 10.1111/acer.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilova Z, Zainullina A, Khusnutdinova E. Association analysis of CRHR1 gene, FKBP5 gene and YWHAE gene with suicidal behavior inindividuals from Russia. Eur Neuropsychopharmacol. 2014;24:S577. [Google Scholar]
- Komuro H, Sato N, Sasaki A, Suzuki N, Kano M, Tanaka Y, Yamaguchi-Kabata Y, Kanazawa M, Warita H, Aoki M, Fukudo S. Corticotropin-Releasing Hormone Receptor 2 Gene Variants in Irritable Bowel Syndrome. PLoS One. 2016;11(1):e0147817. doi: 10.1371/journal.pone.0147817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacol Rev. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12(8):1077–85. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027–1042. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Kreek MJ. Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet. 2014;78:290–28. doi: 10.1111/ahg.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, Lake S, Tantisira KG, Weiss ST, Wong ML. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004;9:1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat: interactions between the two drugs. Psychopharmacology. 1999;145:283–294. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Lodge NJ, Li YW, Chin FT, Dischino DD, Zoghbi SS, Deskus JA, Mattson RJ, Imaizumi M, Pieschl R, Molski TF, Fujita M, Dulac H, Zaczek R, Bronson JJ, Macor JE, Innis RB, Pike VW. Synthesis and evaluation of candidate PET radioligands for corticotropin-releasing factor type-1 receptors. Nucl Med Biol. 2014;41:524–535. doi: 10.1016/j.nucmedbio.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, de Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3:24–32. [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry. 2007;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Markou A, Cryan JF. Stress, anxiety and depression: toward new treatment strategies. Neuropharmacology. 2012;62:1–2. doi: 10.1016/j.neuropharm.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, McCammon JA. G-protein coupled receptors: advances in simulation and drug discovery. Curr Opin Struct Biol. 2016;41:83–89. doi: 10.1016/j.sbi.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan-Lobo L, Gsandtner I, Gaubitzer E, Rünzler D, Buchmayer F, Köhler G, Bonci A, Freissmuth M, Sitte HH. Subtype-specific differences in corticotropin-releasing factor receptor complexes detected by fluorescence spectroscopy. Mol Pharmacol. 2009;76(6):1196–210. doi: 10.1124/mol.109.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, Randall CL. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582–1588. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla C, Scidmore T, Jeong J, Everest M, Chidiac P, Poulter MO. A switch in G protein coupling for type 1 corticotropin-releasing factor receptors promotes excitability in epileptic brains. Sci Signal. 2016;9:ra60. doi: 10.1126/scisignal.aad8676. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;1:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oedegaard KJ, Alda M, Anand A, Andreassen OA, Balaraman Y, Berrettini WH, Bhattacharjee A, Brennand KJ, Burdick KE, Calabrese JR, Calkin CV, Claasen A, Coryell WH, Craig D, DeModena A, Frye M, Gage FH, Gao K, Garnham J, Gershon E, Jakobsen P, Leckband SG, McCarthy MJ, McInnis MG, Maihofer AX, Mertens J, Morken G, Nievergelt CM, Nurnberger J, Pham S, Schoeyen H, Shekhtman T, Shilling PD, Szelinger S, Tarwater B, Yao J, Zandi PP, Kelsoe JR. The Pharmacogenomics of Bipolar Disorder study (PGBD): identification of genes for lithium response in a prospective sample. BMC Psychiatry. 2016;16:129. doi: 10.1186/s12888-016-0732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orand A, Naliboff B, Gadd M, Shih W, Ju T, Presson AP, Mayer EA, Chang L. Corticotropin-releasing hormone receptor 1 (CRH-R1) polymorphisms are associated with irritable bowel syndrome and acoustic startle response. Psychoneuroendocrinology. 2016;73:133–141. doi: 10.1016/j.psyneuen.2016.07.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. HPA axis genetic variation, pubertal status, and sex interact to predict amygdala and hippocampus responses to negative emotional faces in school-age children. Neuroimage. 2015;109:1–11. doi: 10.1016/j.neuroimage.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpacuer C, Laviolle B, Boussageon R, Reymann JM, Bellissant E, Naudet F. Risks and benefits of nalmefene in the treatment of adult alcohol dependence: a systematic literature review and meta-analysis of published and unpublished double-blind randomized controlled trials. PLoS Med. 2015;12:e1001924. doi: 10.1371/journal.pmed.1001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008a;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Markou A. The effects of chronic versus acute desipramine on nicotine withdrawal and nicotine self-administration in the rat. Psychopharmacology. 2008b;198:351–362. doi: 10.1007/s00213-008-1144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosker GL. Acamprosate: a review of its use in alcohol dependence. Drugs. 2015;75:1255–1268. doi: 10.1007/s40265-015-0423-9. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Macedo GC, Domingues LP, Favoretto CA. An Update on CRF Mechanisms Underlying Alcohol Use Disorders and Dependence. Front Endocrinol (Lausanne) 2016;7:134. doi: 10.3389/fendo.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Ray B, Gaskins DL, Sajdyk TJ, Spence JP, Fitz SD, Shekhar A, Lahiri DK. Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-β precursor protein and amyloid-β peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience. 2011;184:139–150. doi: 10.1016/j.neuroscience.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 1867;12:CD00. doi: 10.1002/14651858.CD001867.pub3. (201) [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin DB. The counternull value of an effect size: a new statistic. Psychol Sci. 1994;5:329–334. [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, Cottone P. Pharmacological characterization of the 20% alcohol intermittent access model in alcohol-preferring rats: a model of binge-like drinking. Alcohol Clin Exp Res. 2013;37:635–643. doi: 10.1111/acer.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sanders J, Nemeroff C. The CRF system as a therapeutic target for neuropsychiatric disorders. Trends Pharmacol Sci. 2016;37:1045–1054. doi: 10.1016/j.tips.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Sato N, Suzuki N, Kano M, Tanaka Y, Kanazawa M, Aoki M, Fukudo S. Associations between single-nucleotide polymorphisms in corticotropin-releasing hormone-related genes and irritable bowel syndrome. PLoS One. 2016;11:e0149322. doi: 10.1371/journal.pone.0149322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ME, Andrews RD, van der Ark P, Brown T, Mannaert E, Steckler T, de Hoon J, Van Laere K. Dose-dependent effects of the CRF(1) receptor antagonist R317573 on regional brain activity in healthy male subjects. Psychopharmacology. 2010;208:109–119. doi: 10.1007/s00213-009-1714-1. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, de Wit H. Lost in translation: CRF1 ereceptor antagonists and addiction treatment. Neuropsychopharmacology. 2016;41:2795–2797. doi: 10.1038/npp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Nielsen CK, Li R, Bartlett SE. Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395. Addict Biol. 2014;19:606–611. doi: 10.1111/adb.12024. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Cerda CA, Pereira LA, Andrés ME, Gysling K. CRF binding protein facilitates the presence of CRF type 2α receptor on the cell surface. Proc Natl Acad Sci U S A. 2016;113(15):4075–80. doi: 10.1073/pnas.1523745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur J Neurosci. 2016;43(2):220–9. doi: 10.1111/ejn.13113. [DOI] [PubMed] [Google Scholar]
- Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM, 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer JS, Birnbaum MS, Voll RJ, Owens MJ, Plott SJ, Bourke CH, Wassef MA, Kilts CD, Goodman MM. Synthesis, F-18 radiolabeling, and microPET evaluation of 3-(2,4-dichlorophenyl)-N-alkyl-N-fluoroalkyl-2,5-dimethylpyrazolo[1,5-a]pyrimidin -7-amines as ligands of the corticotropin-releasing factor type-1 (CRF1) receptor. Bioorg Med Chem. 2015b;23:4286–4302. doi: 10.1016/j.bmc.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer JS, Bourke CH, Owens MJ, Voll RJ, Kilts CD, Goodman MM. Synthesis, binding affinity, radiolabeling, and microPET evaluation of 4-(2-substituted-4-substituted)-8-(dialkylamino)-6-methyl-1-substituted-3,4-dihyd ropyrido[2,3-b]pyrazin-2(1H)-ones as ligands for brain corticotropin-releasing factor type-1 (CRF1) receptors. Bioorg Med Chem Lett. 2015a;25:5111–5114. doi: 10.1016/j.bmcl.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M, Pandor A, Stevens JW, Rawdin A, Rice P, Thompson J, Morgan MY. Nalmefene for reducing alcohol consumption in people with alcohol dependence: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2015;33:833–847. doi: 10.1007/s40273-015-0272-0. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Nguyen M, Poudel MK, Warnick JE, Echevarria DJ, Beaton EA, Song C, Kalueff AV. The failure of anxiolytic therapies in early clinical trials: what needs to be done. Expert Opin Investig Drugs. 2015;24:543–556. doi: 10.1517/13543784.2015.1019063. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology. 2012;221:317–327. doi: 10.1007/s00213-011-2578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen JY, Cotterell A, Lohman RJ, Lim J, Han A, Yau MK, Liu L, Cooper MA, Vesey DA, Fairlie DP. Pathway-selective antagonism of proteinase activated receptor 2. Br J Pharmacol. 2014;171:4112–4124. doi: 10.1111/bph.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu Y, Yamamoto H, Hagino Y, Markou A, Ikeda K. The selective serotonin reuptake inhibitor paroxetine, but not fluvoxamine, decreases methamphetamine conditioned place preference in mice. Curr Neuropharmacol. 2011;9:68–72. doi: 10.2174/157015911795017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu Y, Yamamoto H, Ogai Y, Hagino Y, Markou A, Ikeda K. Fluoxetine as a potential pharmacotherapy for methamphetamine dependence: studies in mice. Ann N Y Acad Sci. 2006;1074:295–302. doi: 10.1196/annals.1369.026. [DOI] [PubMed] [Google Scholar]
- Tan EC, Chua TE, Lee TM, Tan HS, Ting JL, Chen HY. Case-control study of glucocorticoid receptor and corticotrophin-releasing hormone receptor gene variants and risk of perinatal depression. BMC Pregnancy Childbirth. 2015;15:283. doi: 10.1186/s12884-015-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kanazawa M, Kano M, Morishita J, Hamaguchi T, Van Oudenhove L, Ly HG, Dupont P, Tack J, Yamaguchi T, Yanai K, Tashiro M, Fukudo S. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLoS One. 2016;11:e0157347. doi: 10.1371/journal.pone.0157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellew JE, Lanier M, Moorjani M, Lin E, Luo Z, Slee DH, Zhang X, Hoare SR, Grigoriadis DE, St Denis Y, Di Fabio R, Di Modugno E, Saunders J, Williams JP. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20:7259–7264. doi: 10.1016/j.bmcl.2010.10.095. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Turcu AF, Spencer-Segal JL, Farber RH, Luo R, Grigoriadis DE, Ramm CA, Madrigal D, Muth T, O’Brien CF, Auchus RJ. Single-dose study of a corticotropin-releasing factor receptor-1 antagonist in women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2016;101:1174–1180. doi: 10.1210/jc.2015-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013a;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci. 2013b;34:437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield T, Jr, Logrip ML, Rivier CL, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Juncá R, Symon A, López P, Fiedler JL, Rojas G, Heskia C, Lara P, Marín F, Guajardo V, Araya AV, Sasso J, Herrera L. Relationship of cortisol levels and genetic polymorphisms to antidepressant response to placebo and fluoxetine in patients with major depressive disorder: a prospective study. BMC Psychiatry. 2014;14:220. doi: 10.1186/s12888-014-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Caetano FA, Dunn HA, Ferguson SS. PDZK1/NHERF3 differentially regulates corticotropin-releasing factor receptor 1 and serotonin 2A receptor signaling and endocytosis. Cell Signal. 2015;27:519–531. doi: 10.1016/j.cellsig.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193(2):283–94. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Watson WP, Robinson E, Little HJ. The novel anticonvulsant, gabapentin, protects against both convulsant and anxiogenic aspects of the ethanol withdrawal syndrome. Neuropharmacology. 1997;36:1369–1375. doi: 10.1016/s0028-3908(97)00118-4. [DOI] [PubMed] [Google Scholar]
- Weber H, Richter J, Straube B, Lueken U, Domschke K, Schartner C, Klauke B, Baumann C, Pané-Farré C, Jacob CP, Scholz CJ, Zwanzger P, Lang T, Fehm L, Jansen A, Konrad C, Fydrich T, Wittmann A, Pfleiderer B, Ströhle A, Gerlach AL, Alpers GW, Arolt V, Pauli P, Wittchen HU, Kent L, Hamm A, Kircher T, Deckert J, Reif A. Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol Psychiatry. 2016;21:813–822. doi: 10.1038/mp.2015.125. [DOI] [PubMed] [Google Scholar]
- Weston C, Winfield I, Harris M, Hodgson R, Shah A, Dowell SJ, Mobarec JC, Woodlock DA, Reynolds CA, Poyner DR, Watkins HA, Ladds G. Receptor activity-modifying protein-directed G protein signaling specificity for the calcitonin gene-related peptide family of receptors. J Biol Chem. 2016;291:21925–21944. doi: 10.1074/jbc.M116.751362. [erratum: 291(49):25763] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–91. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- Wootten D, Lindmark H, Kadmiel M, Willcockson H, Caron KM, Barwell J, Drmota T, Poyner DR. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br J Pharmacol. 2013;168:822–834. doi: 10.1111/j.1476-5381.2012.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang Z, Liu P, Li D, Lin J. An insight into antagonist binding and induced conformational dynamics of class B GPCR corticotropin-releasing factor receptor 1. Mol Biosyst. 2015;11:2042–2050. doi: 10.1039/c5mb00159e. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang S, Rice KC, Munro CA, Wand GS. Restraint stress and ethanol consumption in two mouse strains. Alcohol Clin Exp Res. 2008;32:840–852. doi: 10.1111/j.1530-0277.2008.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Williams DA, Zaidi SA, Yuan Y, Braithwaite A, Bilsky EJ, Dewey WL, Akbarali HI, Streicher JM, Selley DE. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-(4′-pyridylcarboxamido)morphin an (NAP) Modulating the Mu Opioid Receptor in a Biased Fashion. ACS Chem Neurosci. 2016;7:297–304. doi: 10.1021/acschemneuro.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Qi XR, Gao SF, Lu J, van Wamelen DJ, Kamphuis W, Bao AM, Swaab DF. Different stress-related gene expression in depression and suicide. J Psychiatr Res. 2015;68:176–185. doi: 10.1016/j.jpsychires.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Qin L, Zacarías V, de Vries H, Han GW, Gustavsson M, Dabros M, Zhao C, Cherney RJ, Carter P, Stamos D, Abagyan R, Cherezov V, Stevens RC, IJzerman AP, Heitman LH, Tebben A, Kufareva I, Handel TM. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature. 2016 Dec 7; doi: 10.1038/nature20605. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57:1–13. [PMC free article] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Invest Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]


