Abstract
Background
Gestational opioid use/misuse is escalating in the United States, however, little is understood about the fetal effects of medications used to treat maternal opioid use disorders.
Objective
The purpose of this study was to determine the effect of maternal buprenorphine administration on longitudinal fetal neurobehavioral development.
Study Design
Forty-nine buprenorphine-maintained women attending a substance use disorder treatment facility with generally uncomplicated pregnancies underwent fetal monitoring for 60 minutes at times of trough and peak maternal buprenorphine levels. Data were collected at 24, 28, 32, and 36 weeks gestation. Fetal neurobehavioral indicators (i.e., heart rate, motor activity and their integration (fetal movement-fetal heart rate coupling)) were collected via an actocardiograph, digitized and quantified. Longitudinal data analysis relied on hierarchical linear modeling.
Results
Fetal heart rate, heart rate variability and heart rate accelerations were significantly reduced at peak versus trough maternal buprenorphine levels. Effects were significant either by or after 28 weeks of gestation, and tended to intensify with advancing gestation. Fetal motor activity and fetal movement-fetal heart rate coupling were depressed from peak to trough at 36 weeks of gestation. Polysubstance-exposure did not significantly affect fetal neurobehavioral parameters, with the exception that fetuses of heavier smokers moved significantly less than those of lighter smokers at 36 weeks. By the end of gestation, higher maternal buprenorphine dose was related to depression of baseline fetal cardiac measures at trough.
Conclusions
Maternal buprenorphine administration has acute suppressive effects on fetal heart rate and movement, and the magnitude of these effects increases as gestation progresses. Higher dose (≥ 13 mg) appears to exert greater depressive effects on measures of fetal heart rate and variability. These findings should be balanced against comparisons to gestational methadone effects, relatively good outcomes of buprenorphine-exposed infants, and recognition of the benefits of medication assisted treatment for pregnant women with opioid use disorders in optimizing pregnancy outcomes.
Keywords: Buprenorphine, fetus, fetal neurobehavior, fetal heart rate, fetal movement, maternal substance abuse, maternal substance abuse treatment
Introduction
Problems resulting from consumption of licit and illicit opioids constitute a continually escalating national health crisis in the United States1,2 as is the concurrent rise in opioid use disorders(OUDs).3 Affected populations include pregnant women, resulting in an increase in the prevalence of neonatal abstinence syndrome (NAS).4 Due to the dual risk to mother and fetus, treatment, particularly opioid agonist treatment (OAT), is an increasing priority for this group. Until recently, the standard of care has been maternal treatment with methadone, a full mu-receptor agonist. However, buprenorphine – a partial agonist/antagonist – has become an increasingly popular5 WHO6 and ASAM7 recommended medication choice since the publication of the findings from the MOTHER study, which reported amelioration of NAS severity in buprenorphine-exposed infants as compared to methadone.8 Assessing the fetus provides a way to evaluate the in situ and gestational effects of substances on the developing nervous system.9 Two published reports compare buprenorphine to methadone-exposure on aspects of fetal neurobehavior. In a pilot study, buprenorphine-exposed fetuses (n = 6) displayed indicators of greater fetal well-being at peak levels, assessed by measures of fetal heart rate and motor activity in early and late gestation respectively, compared to methadone-exposed fetuses.10 In the other, buprenorphine treatment (n = 33) generated fewer changes in fetal heart rate parameters from trough to peak levels as compared to methadone treatment, evaluated at a single gestational period.11
Objectives
The primary aim of this prospective, longitudinal study is to more comprehensively document the neurobehavioral development of the buprenorphine-exposed fetus over time through measurement of fetal cardiac patterning, fetal motor activity, and their integration. The acute effects of medication on fetal functioning were determined by comparing fetal neurobehavioral parameters at times of maternal medication trough and peak during the second half of gestation and examining the potential effect of buprenorphine dose. Given that the study relies on a clinical sample of women with substance use disorders, secondary analyses were conducted to determine whether other licit and illicit substance exposure affected results.
Materials and Methods
Participants
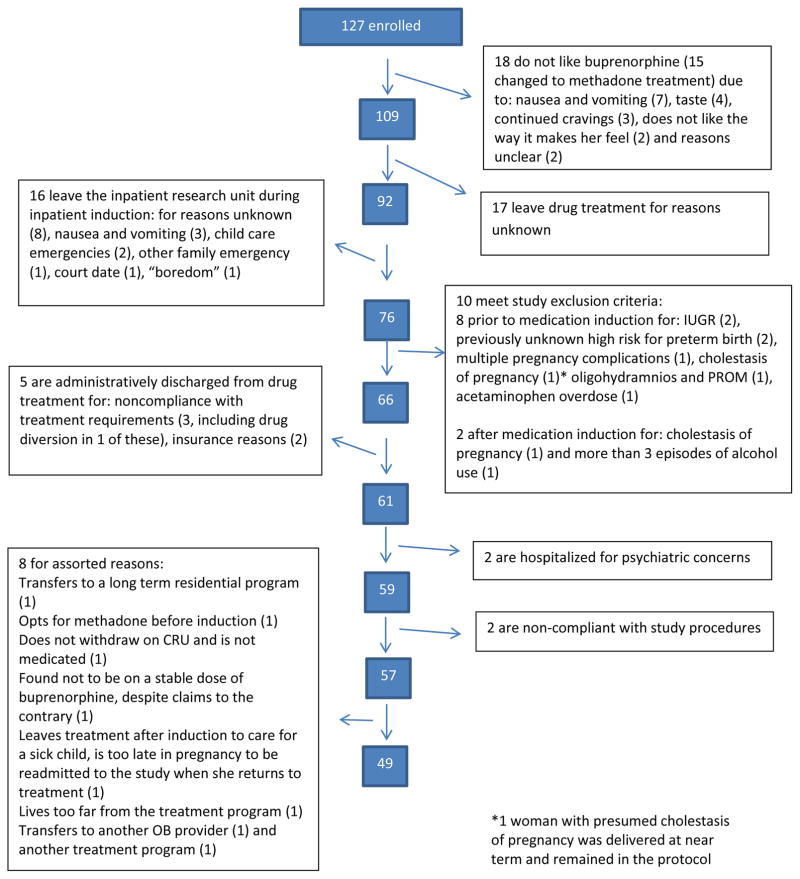
Eligibility was restricted to pregnant women with OUD and singleton, generally uncomplicated pregnancies. Significant maternal conditions that could independently affect fetal functioning, including HIV infection, hypertension and diabetes, were exclusionary. Due to the nature of the population, use of other licit (e.g. cigarettes) and illicit (e.g. heroin) substances was not exclusionary, with the exception of current alcohol dependence to avoid potential teratogenic effects and/or heavy benzodiazepine use when the potential for withdrawal during the inpatient buprenorphine induction period was considered unsafe. A total of 127 women consented to participate. As seen in Figure 1, 78 women left the protocol for a variety of reasons. Of those, 39 (50%) remained in treatment but either switched to methadone or chose not to receive OAT, and 39 (50%) left the treatment program altogether. The final sample was comprised of 49 women attending a substance use disorder treatment facility, previously described.12 Approval was granted by the governing institutional review board, and signed consent was obtained from all participants.
Figure 1.
Study Enrollment flowchart
Procedures
At program intake, women requiring OAT were offered participation in an open label clinical trial that provided buprenorphine as an alternative to methadone for treatment of OUD. Participants included both clinically referred women who became pregnant while stable on buprenorphine (n = 11) and those who underwent a three-day induction on our inpatient research unit. The induction protocol includes a period of opioid washout and attendant withdrawal due to buprenorphine’s pharmacological characteristics. Stable methadone-maintained women were not offered study participation. Because participants were pregnant, no more than mild withdrawal based on frequent scoring using the Clinical Opioid Withdrawal Scale13 was tolerated prior to initial buprenorphine dosing. A maximal dose of 24 mg could be achieved by the third day of the induction protocol, after which participants returned to the treatment program as outpatients. Participants who entered the study as stable buprenorphine-maintained patients were dosed with their maintenance dose and entered the treatment program as outpatients.
Participants received a single daily, observed (through tablet dissolving), sublingual dose of mono-buprenorphine at approximately the same time each morning, with take-home dosing only when necessary and approved. All women received obstetric care at the treatment program from intake through delivery. Participants provided weekly urine specimens that were dipstick-screened for ten substances; cut-off values are included in Table 1.
Table 1.
Maternal treatment and substance exposure characteristics (n=49)
| Mean | SD | Range | |
|---|---|---|---|
| Maternal substance history: | |||
| Age at first substance use (years) | 19.8 | 4.8 | (9–31) |
| Age at regular (3x/week or more), substance use (years) | 21.0 | 4.6 | (14–32) |
| Duration regular substance use upon treatment entry (years) | 6.5 | 4.4 | (1–22) |
| Treatment measures: | |||
| Gestational age upon treatment entry (weeks) | 21.3 | 7.0 | (5.3–32.5) |
| Length of time in treatment prior to delivery (days) | 121.1 | 56.7 | (29–241) |
| Buprenorphine dose (mg) | |||
| At 24 weeks gestation (n=20) | 12.0 | 7.1 | (2–28) |
| At 28 weeks gestation (n=30) | 12.3 | 6.0 | (2–24) |
| At 32 weeks gestation (n=42) | 12.7 | 6.3 | (2–24) |
| At 36 weeks gestation (n=40) | 12.8 | 6.5 | (2–24) |
| Urine toxicology screenings during treatment | 16.4 | 8.0 | (3–33) |
| Positive research urine toxicology screenings* at delivery (%/ SD) | 19.7 | 25.6 | (1–100) |
| Participants with 1 or more positive screenings (#/%) | 29 | (59%) | |
substance screened for include (#/%): opiates (12;41%), THC (10; 34%), benzodiazepines (11;38%), cocaine (8;28%), opioid containing pain relievers (6;21%), methadone (4;14%), methamphetamine (2;7%), amphetamines (1;3%), barbiturates (0;0%) and buprenorphine (49; 100%)
Cut off values for urine toxicology screenings: opiates (morphine) (300 ng/mL); THC (50 ng/mL); benzodiazepines (300 ng/mL); cocaine (300 ng/mL); opioid containing pain relievers (100 ng/mL); methadone (300 ng/mL); methamphetamine (500 ng/mL); amphetamines (1000 ng/mL); barbiturates (300ng/mL), and buprenorphine (10 ng/mL)
Maternal-fetal monitoring procedure
Data were collected during two 60-minute fetal monitoring sessions on one day during the 24th, 28th, 32nd, and 36th week of gestation based on dating ultrasound. The sessions were timed at pharmacological trough (just pre-daily dose) and peak (2 ½ hours post-dose) maternal buprenorphine levels.
Fetal data were recorded with a Toitu (MT320) fetal actocardiograph utilizing a transabdominal Doppler transducer. Fetal data were collected from the output port of the monitor and digitized at 1000 Hz through an internal A/D board using streaming software. Offline processing of fetal data was accomplished through customized software (GESTATE; James Long Company).
Quantification of fetal neurobehavioral measures
Fetal heart rate (FHR)
FHR underwent error rejection procedures based on moving averages of acceptable values as needed. FHR variability (FHRV) was computed continuously (i.e., root mean square of each 1-min epoch of FHR averaged over the recording period) and episodically (i.e., count of accelerations defined as excursions in FHR ≥ 10 bpm for ≥ 15 seconds).
Fetal movement (FM)
The actograph reliably detects fetal motor activity by preserving the remaining signal after band-passing frequency components of the Doppler signal that are associated with FHR and maternal somatic activity.14 Movement bouts were identified each time the signal attained amplitude of 15 units until there was a cessation of signal for at least 10 seconds. Motor activity reflects the number of bouts multiplied by their mean duration yielding a measure of the amount of time the fetus spent moving, in seconds. The integration between fetal movements and heart rate (FM-FHR coupling) was quantified as the proportion of time individual movements were associated with a change in FHR, using previously developed criteria.15 FM-FHR coupling reflects coactivation of the sympathetic and parasympathetic components of the autonomic nervous system.
Data analysis
Not all participants were available for the fetal assessment at each gestational age. Within the 49 participants, visits were distributed as follows: n = 20 (24 weeks); n = 30 (28 weeks); n = 42 (32 weeks); and n = 40 (36 weeks). The majority of women participated on two or more occasions (n=41, 83.6%). Distributions for each fetal variable were examined for outliers. To examine the change in fetal neurobehavior from trough to peak, within and across gestational periods, data analysis relied on hierarchical linear modeling (HLM) via the Mixed procedure in SAS (version 9.4), which accounts for dependency in data measured repeatedly on the same individuals. An advantage of the HLM approach is that it utilizes all available data by employing restricted maximum likelihood estimation (REML) within the model framework, eliminating list-wise deletion. Five models (one for each fetal measure) were constructed, with buprenorphine level at peak or trough, gestational age (4 points), and an interaction term (level X gestational age) as predictors. Dose effects were examined using a similar approach for fetal neurobehaviors measured at peak and trough. Mean buprenorphine dose was dichotomized via median split and entered as a categorical predictor of change in fetal parameters from trough to peak; dose effects were estimated separately at each gestational age. Given the small sample size, poly-substance exposures (other drugs and cigarette smoking) were not evaluated as covariates or modifying variables; rather dichotomous variables and change scores from peak to trough (see results section) were created and group differences evaluated by t-tests.
Results
On average, participants were 27.6 years old (SD = 4.4) with 11.5 years of education (SD= 1.9) and primarily Caucasian (43(87.8%); 6(12.2%) African American). Most were multigravida (41, 83.7%). As expected with a clinical sample of women with OUD, there was evidence of illicit drug use and licit drug misuse during treatment; Table 1 presents information regarding prior and current participant drug use. Nearly all women (45, 91.8%) smoked cigarettes. Approximately half (42%) of the fetuses was male.
Fetal neurobehavioral development
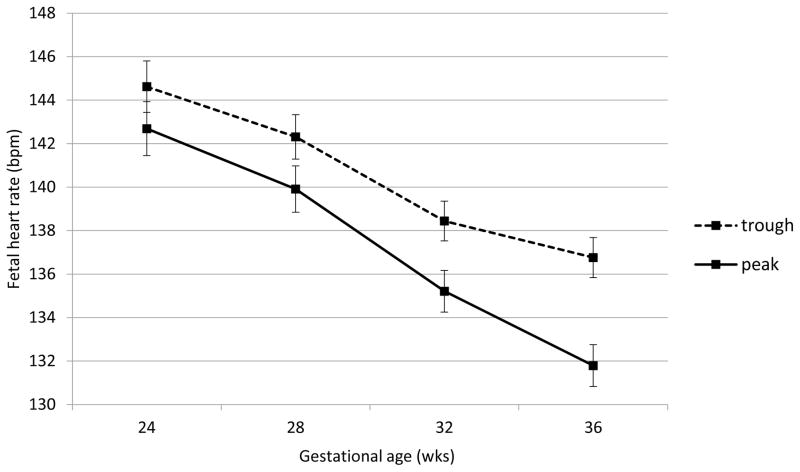
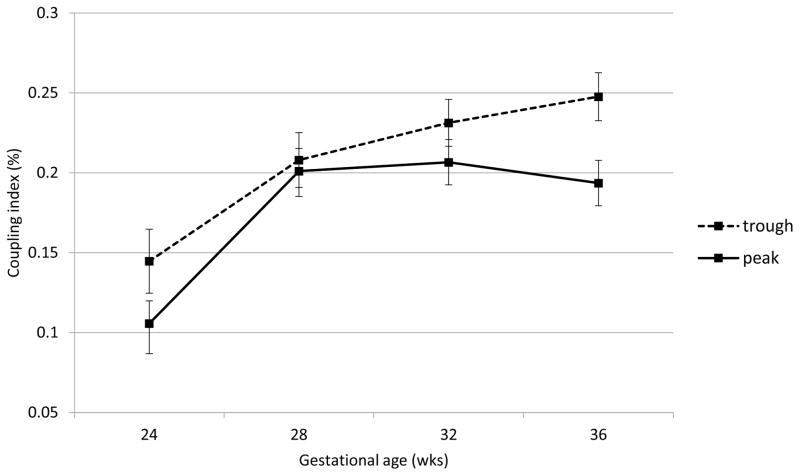
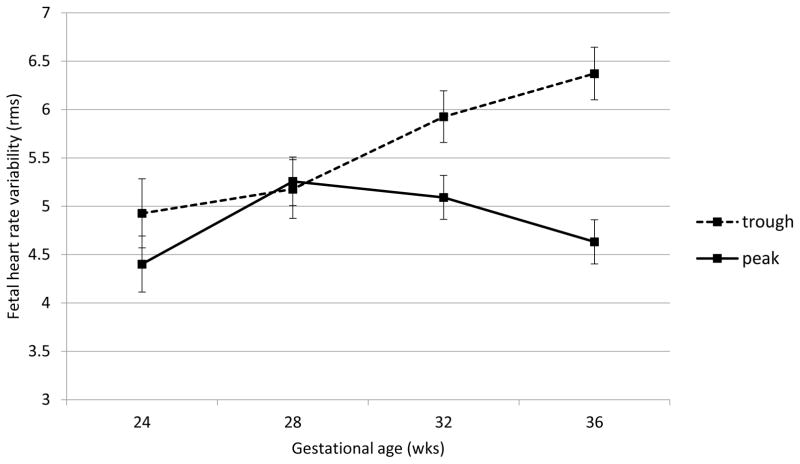
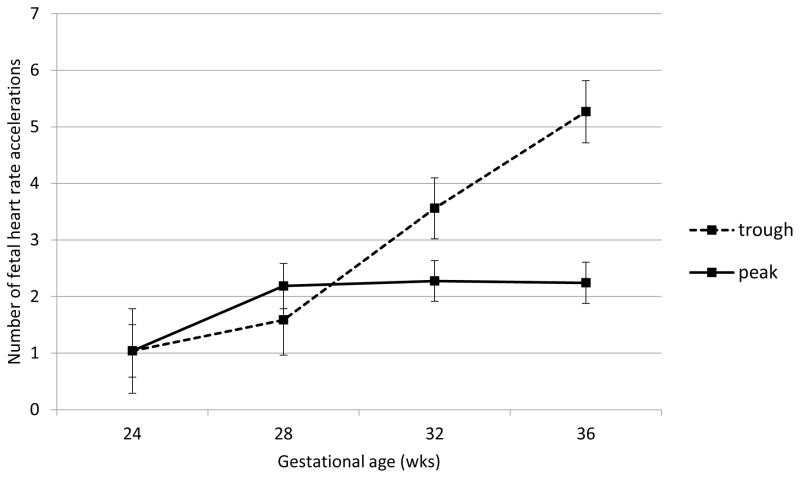
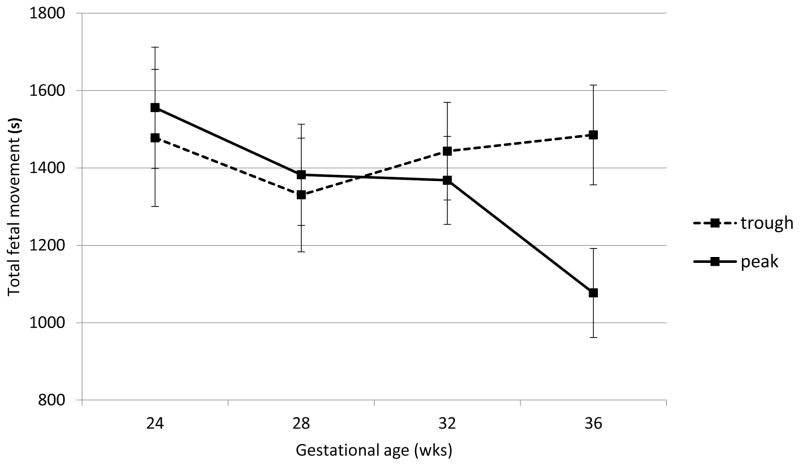
Fetal heart rate, motor activity and their integration were all significantly affected by acute buprenorphine exposure. Data are presented in Figures 2 through 6. The expected decline in FHR and concomitant increase in measures of variability, heart rate accelerations, and FM-FHR coupling with advancing gestation are apparent at trough in Figures 2, 3, 4 and 6 (ps < .0001). Buprenorphine depresses FHR (Figure 2); significant differences commence at 28 weeks (FHR 28 weeks: β = 2.45, SE = 1.02, p = .04, FHR 32 weeks: β = 3.29, SE = 0.88, p = .001, FHR 36 weeks: β = 5.10, SE = 0.89, p < .0001). Developmental patterns for FHRV (Figure 3) and the number of accelerations (Figure 4) exhibited during the 60 minute recordings are similar and reveal an increasing influence of buprenorphine over time; divergence begins at 32 weeks (FHRV 32 weeks: β = 0.86, SE = 0.27, p = .002, FHRV 36 weeks: β = 1.74, SE = 0.27, p < .0001). Accelerations 32 weeks: β = 1.34, SE = 0.55, p = .018, Accelerations 36 weeks: β = 3.02, SE = 0.56, p < .0001). Buprenorphine did not affect overall fetal motor activity (Figure 5) with the exception of a suppressive effect at 36 weeks (β = 408.71, SE = 144.20, p = .008). Similarly, the effect on FM-FHR coupling (Figure 6) was limited to the final assessment period (β = 0.05, SE = 0.02, p = .002), and reflects a reduction in this parameter following buprenorphine administration.
Figure 2.
Fetal heart rate. Buprenorphine administration was associated with significantly slower fetal heart rate at 28, 32, and 36 weeks gestation; no difference was observed at 24 weeks gestation.
Figure 6.
Fetal movement-fetal heart rate coupling. Buprenorphine administration was associated with significantly less FM-FHR coupling at 36 weeks gestation; but not earlier.
Figure 3.
Fetal heart rate variability. Buprenorphine administration was associated with significantly reduced fetal heart rate variability at 32 and 36 weeks gestation no difference was observed at earlier gestational ages.
Figure 4.
Fetal heart rate accelerations. Buprenorphine administration was associated with significantly fewer fetal heart rate accelerations at 32 and 36 weeks gestation. No difference was observed at earlier gestational ages.
Figure 5.
Fetal motor activity. Buprenorphine administration was associated with significantly reduced fetal motor activity at 36 weeks gestation, but not earlier.
Contribution of dose
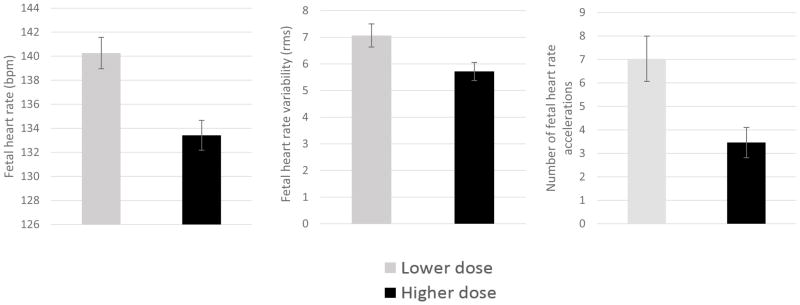
There was variation in the daily buprenorphine treatment dosage, ranging from a single 2 mg tablet to 28 mg (Table 1). Within individuals, buprenorphine dose was highly stable over time (M correlation coefficient between visits, r = 0.94). Moreover, it was uncommon for dosing to change during treatment; dose for only 3 participants (6%) varied by > 4 mg. As a result, a dichotomous variable was created based on median dose (<13 mg, n = 24, vs ≥ 13 mg, n = 25) to examine associations with fetal measures at trough, peak and the degree of change from trough to peak. Fetuses exposed to chronically higher buprenorphine dose showed reduced FHR at trough across gestational ages (F (1, 49) = 10.30, p = .002). Higher dose was also associated with reduced FHRV and fewer accelerations at trough at 32 and 36 weeks (p = .038 and p = .017, respectively). Figure 7 provides a visual representation of 36 week results. FM-FHR coupling was also depressed at trough in the higher dosage group, but only at 32 weeks (p =.006); motor activity was unaffected.
Figure 7.
At buprenorphine trough, 36 week fetuses of women treated with higher buprenorphine dose (≥13 mg) presented with slower fetal heart rate, lower fetal heart rate variability, and approximately half the number of fetal heart rate accelerations as compared to fetuses of women treated with a lower dose.
Dose associations with fetal measures were largely absent at peak. However, and somewhat paradoxically, by 36 weeks, and at times earlier in gestation, computation of change scores of fetal parameters from trough to peak revealed that fetuses exposed to lower treatment doses reacted with greater suppression in FHR (p =.020) and reduction in accelerations, (p =.014), in response to buprenorphine administration, most likely the result of a floor effect of already diminished cardiac variability in the higher dose group.
Polysubstance exposure
On average, participants had 16 toxicology screenings during the protocol (Table 1). Twenty (41%) had no positive findings through delivery; of the remaining 59%, evidence of use was detected a third of the time; detected substances are presented in Table 1. Due to limitations in sample size, the potential confounding influence of specific substances could not be evaluated; instead comparisons were made between fetuses of participants who were abstinent (18;45%) versus non-abstinent (22;55%) through 36 weeks gestation. None of the fetal neurobehavioral parameters at either trough or peak, nor change scores from trough to peak, were significantly different in abstinent versus non-abstinent groups (ps range from .10 to .98). Buprenorphine treatment dose was unrelated to abstinence, X2 = 1.61, p = .204.
Since nearly all participants smoked, and there was high stability over time in number of cigarettes smoked per day over gestation (r’s range from 0.75 to 0.92), mean values were computed and dichotomized as < 9 (n = 20) versus ≥10 (n = 29) cigarettes smoked per day. At 36 weeks, smoking dosage was not significantly associated with fetal behaviors at trough or change from trough to peak with one exception: fetuses of heavier smokers moved significantly less than those of lighter smokers (M = 1980.9 and 1051.8, respectively; t (38) = 3.33, p = .002. Acute buprenorphine exposure generated less reduction in fetal motor activity in the heavier smokers, t (38) = 3.11, p = .004, likely a result of the chronic reduction in motor activity evident at trough in this group.
Comment
Participant attrition in research involving substance using populations is common, as was the case in this study. However, although attrition was high, dissatisfaction with buprenorphine per se was only a stated factor in 21 (27%) cases. Complaints of nausea and vomiting, which are common pregnancy symptoms, accounted for another 10 (13%). Anecdotally, some women who left the protocol to switch medications had a better clinical response to methadone, emphasizing the importance of medication choice for women with OUDs and the value of research in this arena. Three women on higher doses developed cholestasis of pregnancy. All three had concurrent hepatitis C virus (HCV) infection, and there is evidence to suggest that the risk of developing cholestasis of pregnancy is increased in women infected with HCV.16–19 We could find no reports of increased risk for this condition associated with buprenorphine administration; however a potential association with dose should be evaluated in future studies.
With respect to the main study objectives, we consistently found that when comparing fetal neurobehavioral expression between pharmacological trough and peak, buprenorphine exposure depressed fetal heart rate and both measures of variability in heart rate. Effects increased in magnitude as gestation progressed, particularly at and after 28 weeks gestation. Fetal motor activity and FM-FHR coupling were also depressed, but only at 36 weeks. In general, this pattern reveals that fetal neurobehavioral parameters are most affected as they mature. From a methodological perspective, the lack of acute effects prior to 28 weeks highlights the value of assessing the fetus at multiple points in gestation; collecting data only at 24 weeks, for example, would have generated inaccurate conclusions about buprenorphine’s effects.
The current findings are consistent with two earlier reports of maternal buprenorphine and fetal neurobehavior that found some suppression of fetal functioning (fetal heart rate, heart rate variability, accelerations)10.11 and movement10 with maternal buprenorphine maintenance. The degree to which buprenorphine confers greater or lesser effects on the developing fetus than methadone cannot be addressed by this study since there was no direct comparison to a methadone-exposed group. However, comparison to findings from an earlier study using the same methods and design applied to a methadone-maintained population (n = 40)20 tentatively suggests that buprenorphine affects fetal functioning less than methadone. For example, at 36 weeks gestation methadone-exposed fetuses showed a 37% reduction in FHR variability and 46% decrease in motor activity at peak as compared to the comparable 27% reduction in FHR variability and 28% reduction in motor activity for buprenorphine, and appeared to exhibit fewer accelerations at trough (3.6 v 5.3). However, that study was conducted between 2000 and 2005, and changing drug use patterns and the sociodemographic composition of the clinic’s participants during the ensuing years may diminish the utility of this comparison. Nonetheless, these comparisons are consistent with the results of two other reports that suggest buprenorphine is somewhat less disruptive than methadone to fetal functioning.10,11
Developmental trends in fetal neurobehaviors are apparent in Figures 2 through 6, and are somewhat attenuated compared to non-opioid dependent populations.9,21 However, trough levels were associated with maternal dose, such that higher doses were significantly associated with slower fetal heart rate and reduced variability, despite medication levels being at nadir. This relation was present at 32 weeks and beyond, and suggests a lingering and perhaps cumulative medication effect at higher doses (i.e., ≥ 13 mg). This finding resonates with a recent report utilizing the same sample that found that maternal buprenorphine dose and concentrations of buprenorphine in plasma of recently post-partum women are correlated,22 a relationship not found with methadone.23
The unexpected dose effect generated a paradoxical finding of less suppression of fetal reactivity to higher doses of buprenorphine. For example, the mean change in accelerations at 36 weeks from trough to peak was 4.6 per 60 minutes in the lower dose group but only 1.4 in the higher dose. However, the lower dose exhibited twice as many accelerations at trough (7.0 to 3.5), suggesting that fetal functioning in the higher dose group was already at somewhat of a floor.
In contrast to other studies which impart strict controls on non-OAT other substance use to better isolate treatment medication effects, the current study reflects the real world situations encountered in standard drug treatment centers, which includes participant relapse and multiple licit and illicit exposures. Thus, evaluating buprenorphine effects in this group more closely approximates typical implementation. We did not find continued misuse of other substances in addition to buprenorphine treatment to be associated with alterations in fetal neurobehaviors at trough. Lack of abstinence also did not exert a differential effect on the acute response to buprenorphine with the exception of nicotine. Heavier smoking was associated with suppression of fetal motor activity, confirming prior observations.24,25 Caution is needed in concluding that other substances do not potentiate or interfere with the fetal response to buprenorphine, as the small sample coupled with variation in exposures limits our ability to conduct more detailed analyses and use other substances as either covariates or modifiers. In particular, this study may be under-powered to detect significant effects of other substances on either fetal parameters or in relation to buprenorphine effects.
OAT for pregnant women with OUDs is often necessary enable them to seek prenatal care, prepare for childbirth, address trauma, find employment and reduce other substance use/misuse. In many women able to tolerate it, buprenorphine may be a better option than methadone based on findings of reduced severity of NAS alone. Nonetheless, current results indicate that buprenorphine does have consequences for fetal neurobehavioral development and these effects intensify as gestation advances. From a clinical perspective, the changes to fetal heart rate and behavior noted here may not significantly affect the interpretation of a non-stress test or pregnancy management. The exception is at or near term, when the depressive effects of buprenorphine on fetal heart rate and variability may confound the evaluation of fetal well-being in women with OUD on medication therapy. Long-term consequences of these alterations are unknown. This information should be balanced against the relatively good outcomes of buprenorphine-exposed infants, careful consideration of maternal dose, and the value of OAT in optimizing pregnancy outcomes with OUD.
Acknowledgments
This study was funded by NIH/NIDA RO1 DA013689. Study medication was provided by Indivior, who had no role in study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
The authors thank the participants, without whom this work would be impossible, and staff at the Center for Addiction and Pregnancy.
Abbreviations
- NAS
Neonatal abstinence syndrome
- OAT
Opioid agonist treatment
- MOTHER
Maternal opioid treatment, Human experimental research study
- OUD
Opioid use disorder
- FHR
Fetal heart rate
- FHRV
Fetal heart rate variability
- FM
Fetal movement
- HLM
Hierarchical linear modeling
- REML
Restricted maximum likelihood estimation
Footnotes
This research was conducted in Baltimore, Maryland, USA
Lauren Jansson is a paid consultant for Chiesi, Inc.
Clinical Trials Registry name and registration number: Fetal and Infant Effects of Maternal Buprenorphine Treatment NCT15671079 https://clinicaltrials.gov/ct2/show/NCT01561079?term=jansson&rank=12
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAlllister JM, Davis MM. Neonatal abstinence syndrome an associated health care expenditures United States, 2000–2009. JAMA. 2012;307(18) doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 2.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. Treatment Episode Data Set (TEDS): 2000–2010. DASIS Series S-61, HHS Publication No. (SMA) 12-4701. [Google Scholar]
- 4.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of neonatal abstinence syndrome – 28 states, 1999–2013. Morbidity and Mortality Weekly Report. 2016;65(31):799–802. doi: 10.15585/mmwr.mm6531a2. [DOI] [PubMed] [Google Scholar]
- 5.Krans EE, Bogan D, Richardson G, Park SY, Dunn SL, Day N. Factors associated with buprenorphine versus methadone use in pregnancy. Substance Abuse. 2016 doi: 10.1080/08897707.2016.1146649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Geneva, Switzerland: World Health Organization; 2014. [PubMed] [Google Scholar]
- 7.American Society of Addiction Medicine (ASAM) The ASAM National Practice Guideline for the use of medications in the treatment of addiction. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiPietro JA, Kivlighan KT, Costigan KA, et al. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81(1):115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansson LM, DiPietro JA, Velez M, et al. Fetal neurobehavioral effects of exposure to methadone or buprenorphine. Neurotoxicology and Teratology. 2011;33(2):240–243. doi: 10.1016/j.ntt.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salisbury AL, Coyle MG, O’Grady KE, et al. Fetal assessment before or after dosing with buprenorphine or methadone. Addiction. 2012;107(0 1):36–44. doi: 10.1111/j.1360-0443.2012.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson LM, Svikis D, Lee J, Paluzzi P, Rutigliano P, Hackerman F. Pregnancy and Addiction: A Comprehensive Care Model. Journal of Substance Abuse Treatment. 1996;13(4):321–329. doi: 10.1016/s0740-5472(96)00070-0. [DOI] [PubMed] [Google Scholar]
- 13.Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105(1–2):154–159. doi: 10.1016/j.drugalcdep.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besinger R, Johnson T. Doppler recordings of fetal movement: Clinical correlation with real-time ultrasound. Obstet Gynecol. 1989;74:277–280. [PubMed] [Google Scholar]
- 15.DiPietro J, Hodgson D, Costigan K, Hilton S, Johnson T. Development of fetal movement-fetal heart rate coupling from 20 weeks through term. Early Hum Dev. 1996;44:139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 16.Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. British Journal of Obstetrics and Gynaecology. 1999;106:498–500. doi: 10.1111/j.1471-0528.1999.tb08305.x. [DOI] [PubMed] [Google Scholar]
- 17.Paternoster D, Fabris F, Palu G, et al. Intra-hepatic cholestasis of pregnancy in hepatitis C virus infection. Acta Obstet Gynecol Scand. 2002;81:99–103. [PubMed] [Google Scholar]
- 18.Ropponen A, Sund R, Ylikorkala O, Aittomaki K. Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: a population based study. American Association for the study of liver diseases. doi: 10.1002/hep.21111. [DOI] [PubMed] [Google Scholar]
- 19.Wiiarnpreecha K, Thongprayoon C, Sanguankeo A, UpalaS Ungprasert P, Cheungpastiporn W. Hepatitis C infection and intrahepatic cholestasis of pregnancy: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2016 doi: 10.1016/j.clinre.2016.07.004. pii: S2210-7401(16)30103-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Jansson LM, Elko A, DiPietro J. Fetal response to maternal methadone administration. Obstet Gynecol. 2005;193:611–617. doi: 10.1016/j.ajog.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 21.DiPietro JA, Costigan KA, Voegtline KM. Studies in fetal behavior: Revisited, renewed and reimagined. Monogr Soc Res Child Dev. 2015;80(3):vii, 1–94. doi: 10.1111/mono.v80.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson LM, Spencer N, McConnell K, et al. Maternal buprenorphine maintenance and lactation. Journal of Human Lactation. 2016 doi: 10.1177/0890334416663198. pii: 0890334416663198 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Jansson LM, Choo R, Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121(1):106–114. doi: 10.1542/peds.2007-1182. [DOI] [PubMed] [Google Scholar]
- 24.Habek D. Effects of smoking and fetal hypokinesia in early pregnancy. Arch Med Res. 2007;38(8):864–867. doi: 10.1016/j.arcmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Kelly J, Mathews KA, O’Conor M. Smoking in pregnancy: effects on mother and fetus. Br J Obstet Gynecol. 1984;91(2):111–117. doi: 10.1111/j.1471-0528.1984.tb05892.x. [DOI] [PubMed] [Google Scholar]