Abstract
Background
Preeclampsia is a prevalent and enigmatic disease, in part characterized by poor remodeling of the spiral arteries. However, preeclampsia does not always clinically present, even when remodeling has failed to occur. Hypotheses surrounding the ‘second hit’ that is necessary for the clinical presentation of the disease focus on maternal inflammation and oxidative stress. Yet, the studies to date that have investigated these factors have utilized cross-sectional study designs or small study populations.
Objective
In the present study we sought to explore longitudinal trajectories, beginning early in pregnancy, of a panel of inflammation and oxidative stress markers in women who went on to have preeclamptic or normotensive pregnancies.
Study design
We examined 441 subjects from the ongoing LIFECODES prospective birth cohort, including 50 mothers who developed preeclampsia and 391 mothers with normotensive pregnancies. Participants provided urine and plasma samples at four time points during gestation (median 10, 18, 26, and 35 weeks) that were analyzed for a panel of oxidative stress and inflammation markers. Oxidative stress biomarkers included 8-isoprostane and 8-hydroxydeoxyguanosine (8-OHdG). Inflammation biomarkers included C-reactive protein (CRP) as well as the cytokines IL-1β, IL-6, IL-10, and TNF-α. We created Cox proportional hazard models to calculate hazard ratios based on time of preeclampsia diagnosis in association with biomarker concentrations at each of the four study visits.
Results
In adjusted models, hazard ratios (HRs) of preeclampsia were significantly (p<0.01) elevated in association with all inflammation biomarkers measured at visit 2 (median 18 weeks, HRs 1.31–1.83 in association with an interquartile range increase in biomarker). HRs at this time point were the most elevated for CRP, IL-1β, IL-6, IL-10, and also for the oxidative stress biomarker 8-isoprostane (HR=1.68, 95% confidence interval=1.14, 2.48). HRs for TNF-α were consistently elevated at all four of the study visits (HRs 1.49–1.63, p<0.01). In sensitivity analyses, we observed that these associations were attenuated within groups typically at higher risk of developing preeclampsia, including African American mothers, mothers with higher BMI at the beginning of gestation, and in pregnancies that ended preterm.
Conclusions
This study provides the most robust data to date on repeated measures of inflammation and oxidative stress in preeclamptic compared to normotensive pregnancies. Within these groups, inflammation and oxidative stress biomarkers show different patterns across gestation, beginning as early as 10 weeks. The start of the second trimester appears to be a particularly important time point for measurement of these biomarkers. While biomarkers alone do not appear to be useful in the prediction of preeclampsia, these data are useful in understanding the maternal inflammatory profile in pregnancy prior to development of the disease, and may be used to further develop understanding of potentially preventative measures.
Keywords: biomarkers, circulation, cytokines, hypertension, inflammation, isoprostane, longitudinal, oxidative stress, preeclampsia
Condensation
Using repeated biomarkers from 4 time points across pregnancy, we demonstrated significant associations between biomarkers of inflammation and oxidative stress and preeclampsia.
Introduction
Preeclampsia is a hypertensive disorder of pregnancy characterized by high blood pressure in combination with proteinuria after 20 weeks gestation.1 While some risk factors are known, understanding of the underlying biological mechanisms remains limited. Some evidence, however, points toward a role of maternal inflammation and oxidative stress. For example, some have hypothesized that an important difference between preeclampsia vs. normal or growth restricted pregnancy is due at least in part to an overwhelming inflammatory stimulus or hyper-responsive state in the mother.4 Oxidative stress could also play a role in the etiology of preeclampsia through multiple pathways.5, 6 First, reactive oxygen species (ROS) can cause apoptosis of the syncytiotrophoblast during the placentation process and impair the normal arteriolar remodeling.7, 8 Second, oxidative stress could cause, conflate, or be the consequence of the altered inflammatory response in preeclamptic pregnancies. Finally, oxidative stress has been hypothesized to activate maternal endothelial cells as a precursor to preeclampsia.9
How, why, and when these conditions develop remain important and unanswered questions. It is unclear whether the inflammatory or oxidative stress responses are local to the intrauterine compartment or are systemic in nature. A number of studies have examined circulating or excreted biomarkers in attempt to answer these questions. In the present analysis we focus on the question of when. By examining repeated measures of oxidative stress and inflammation biomarkers at four study visits we explore trajectories in these levels over time in preeclamptic compared to normotensive pregnancies. Additionally, we estimate hazard ratios in association with levels of these biomarkers at each time point to quantify the risk of preeclampsia associated with these levels in certain windows of pregnancy. While we hypothesized that inflammation and oxidative stress biomarkers would be elevated in mothers who went on to develop preeclampsia, our investigation of timing was exploratory in nature.
Materials and Methods
Study population
The ongoing LIFECODES birth cohort began recruitment in 2006 at Brigham and Women’s Hospital (BWH) in Boston, MA. Under the study design, women are recruited early in pregnancy (<15 weeks gestation) at tertiary care centers in the Boston area and plan to deliver at BWH.10 At baseline (median 10 weeks gestation), women provide questionnaire data, urine and blood samples, and informed consent. Gestational age is assessed according to guidelines of the American College of Obstetricians and Gynecologists (ACOG),11 with last menstrual period verified by first trimester ultrasound. Additional questionnaire data and samples are collected at three subsequent study visits (median 18, 26, and 35 weeks gestation). Following delivery, details of the pregnancy progression and any complications are abstracted from medical records. The birth cohort study received IRB approval from BWH.
The present analysis utilizes subjects from a case-control study of preterm birth that included participants from LIFECODES recruited between 2006 and 2008. This study captured all women who delivered prior to 37 weeks gestation (N=130) as well as roughly 3:1 unmatched controls (delivery >=37 weeks gestation; N=352). The primary objective of this study was to examine the relationship between environmental contaminants and preterm birth. In the present analysis, as a secondary objective, we sought to examine the role of oxidative stress and inflammation in preeclampsia within these participants.
Diagnosis of preeclampsia and diagnosis date were abstracted from medical records. Preeclampsia was defined according to ACOG clinical guidelines, by elevated maternal blood pressure after 20 weeks gestation (≥ 140mmHg systolic or ≥ 90mmHg diastolic) in combination with proteinuria (>300mg/24 hours or protein/creatinine ratio > 0.20).1 All cases of preterm preeclampsia received 24-hour proteinuria tests, while other cases were diagnosed according to the ratio. All potential cases of preeclampsia were verified by two maternal-fetal medicine specialists, and if diagnoses disagreed a third specialist reviewed the case. We identified 50 cases of preeclampsia, 31 of whom delivered preterm. We included cases of superimposed preeclampsia (n=9) but excluded one case of postpartum preeclampsia. For the present analysis, we excluded participants with other gestational hypertensive disorders so as to provide a clean comparison of inflammation and oxidative stress profiles in preeclamptic vs. normotensive pregnancies (n=24 with gestational hypertension; n=16 with chronic hypertension who did not develop preeclampsia). This allowed for a final samples size of 441 (n=50 preeclamptics, n=391 normotensives).
Biomarker measurement
Plasma from each of the four study visits was stored at −80 degrees Celsius until shipment to the University of Michigan for analysis of inflammation biomarkers at the Cancer Center Immunology Core. C-reactive protein (CRP) was measured using enzyme-linked immunosorbent assay and was detectable to 10 ug/mL. A panel of cytokines, including IL-1β, IL-6, IL-10, and TNF-α was assayed using the Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel (EMD Millipore Corp., St. Charles, MO, USA). The limit of detection was 0.128 pg/mL for all cytokines.
Urine samples were similarly stored at −80 degrees Celsius until analysis. Cayman Chemical Company (Ann Arbor, MI, USA) measured 8-hydroxydeoxyguanosine (8-OHdG) and total 8-isoprostane via enzyme immunoassay. For 8-isoprostane, urine samples first underwent affinity purification. In order to account for urine dilution, we measured specific gravity using a digital handheld refractometer (Atago Co, Ltd, Tokyo, Japan). For presenting distributions of urinary biomarkers, we created corrected 8-OHdG and 8-isoprostane concentrations as described elsewhere.12 For statistical models, we included uncorrected oxidative stress biomarker concentrations and treated specific gravity as a covariate. We excluded implausible 8-OHdG concentrations (n=3 observations). All laboratory analyses were blinded to the cases status of the participants. Inflammation and oxidative stress measures below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.13
Statistical analysis
All statistical analyses were performed using R version 3.2.3, and were weighted using inverse probability weightings based on the probability of preterm vs. term selection for the case-control study so that the results would be generalizable to the base BWH population. Population characteristics were tabulated for preeclamptic and normotensive pregnancies and differences in distributions were tested using a Chi square test for independence. Distributions of inflammation and oxidative stress biomarkers in preeclamptics compared to normotensives at visit 1 were examined using selected percentiles. Additionally, we created generalized additive mixed models (GAMM) with a random intercept for each subject to model each biomarker as predicted by splined gestational age at sample collection, with an interaction term for preeclampsia. From these models we created predicted value plots of each biomarker across gestation for preeclamptics and normotensives.
To quantify the association between the biomarkers measured and preeclampsia we performed time-to-event analyses using Cox proportional hazards models, estimating hazards ratios (HRs) of preeclampsia. As above, these analyses were weighted so that the distribution of preeclampsia in each model was comparable to that in the base BWH population. Each model included one ln-transformed biomarker either from one study visit or a geometric average created from levels measured at visits 1–3 modeled in relation to gestational age at diagnosis of preeclampsia or gestational age at delivery. The average measured was created as a more stabilized metric of subject-specific inflammation or oxidative stress biomarkers over pregnancy, as individual biomarkers demonstrate moderate variability over time.12, 14 We created unadjusted models and models adjusted for covariates that were: 1) selected a priori (maternal age and race/ethnicity); or 2) associated with both preeclampsia and the biomarker of interest in bivariate analyses, and influenced effect estimates by >10%. For oxidative stress markers we additionally created a model adjusting for specific gravity alone for comparison. We tested the proportional hazards assumption using the survival package in R.15 In sensitivity analyses, we examined effect modification by maternal demographic or behavioral factors that have previously demonstrated differential susceptibility to preeclampsia.
Results
In the present study population, women who developed preeclampsia were more likely to be obese (BMI >30 kg/m2) at the first study visit, to have used assisted reproductive technology (ART) to get pregnant, to deliver preterm, and to be carrying a male fetus (Table 1). Of these variables, we previously observed that urinary 8-isoprostane and plasma CRP, IL-6, and TNF-α concentrations were higher in obese compared to normal weight subjects.14, 16 For ART, both oxidative stress biomarkers were slightly lower in mothers who conceived using ART compared to those who conceived naturally. No differences in any of the biomarkers were observed by fetal sex. Thus, we considered maternal age, race/ethnicity, visit 1 BMI, and ART as covariates in Cox proportional hazards models.
Table 1.
Demographic characteristics of mothers with normotensive and preeclamptic pregnancies: n (weighted %) and p-value for weighted Chi square test of independence.
| Characteristic | Normotensive pregnancy (n=391, 92%) |
Preeclamptic pregnancy (n=50, 8%) |
p-value for independence |
|
|---|---|---|---|---|
| Age category | <25 years | 48 (13%) | 4 (11%) | |
| 25-<30 years | 72 (19%) | 13 (26%) | 0.74 | |
| 30-<35 years | 151 (38%) | 19 (35%) | ||
| ≥35 years | 120 (30%) | 14 (27%) | ||
| Race/ethnicity | White | 229 (59%) | 31 (65%) | |
| African American | 57 (15%) | 12 (23%) | 0.13 | |
| Other | 105 (26%) | 7 (13%) | ||
| Health insurance | Private | 214 (82%) | 40 (83%) | 0.88 |
| Public | 68 (18%) | 9 (17%) | ||
| Education | High school/technical | 113 (29%) | 19 (33%) | |
| Junior or some college | 106 (28%) | 18 (34%) | 0.45 | |
| College degree+ | 163 (43%) | 13 (32%) | ||
| Body mass index | < 25 kg/m2 | 218 (56%) | 13 (28%) | |
| 25–30 kg/m2 | 107 (28%) | 12 (25%) | <0.01 | |
| >30 kg/m2 | 63 (16%) | 25 (47%) | ||
| Tobacco use in pregnancy | No | 370 (94%) | 45 (94%) | 0.88 |
| Yes | 21 (6%) | 5 (6%) | ||
| Alcohol use in pregnancy | No | 363 (94%) | 49 (97%) | 0.55 |
| Yes | 19 (6%) | 1 (3%) | ||
| Parity | Nulliparous | 169 (44%) | 26 (52%) | 0.35 |
| Parous | 222 (56%) | 24 (48%) | ||
| Use of ART | No | 360 (92%) | 41 (79%) | <0.01 |
| Yes | 31 (8%) | 9 (21%) | ||
| Preterm birth | No | 301 (90%) | 19 (62%) | <0.01 |
| Yes | 90 (10%) | 31 (38%) | ||
| Fetal sex | Male | 170 (44%) | 31 (69%) | <0.01 |
| Female | 221 (55%) | 19 (31%) |
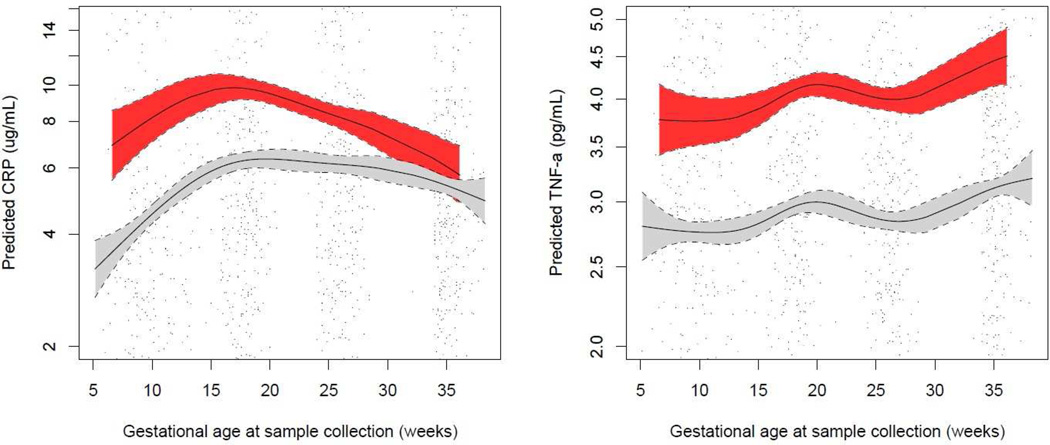
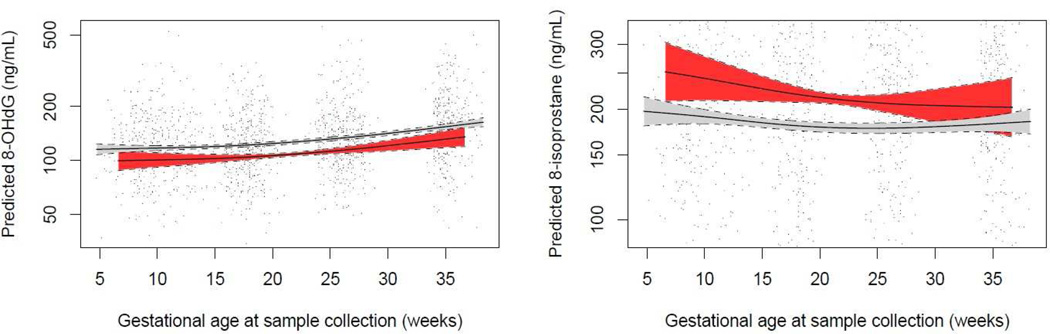
Mothers who went on to develop preeclampsia had higher levels of CRP, TNF-α, and 8-isoprostane early in pregnancy, at median 10 weeks gestation (Table 2). GAMM showed that an interaction term between preeclampsia and gestational age at sample collection was significant in models predicting CRP, TNF-α, and specific gravity corrected 8-OHdG, but not specific gravity corrected 8-isoprostane (Figures 1 and 2 for inflammation and oxidative stress biomarkers, respectively). CRP levels appeared to be most disparate in preeclamptics and normotensives early in pregnancy, but were more similar toward the end of gestation. TNF-α concentrations were consistently higher in cases of preeclampsia across pregnancy. In regard to oxidative stress biomarkers, urinary 8-OHdG concentrations were uniformly lower across pregnancy in preeclamptics, while 8-isoprostane concentrations showed a greater difference between preeclamptics and normotensives early in gestation.
Table 2.
Median (25th, 75th percentile) weighted concentrations of inflammation and oxidative stress biomarkers and specific gravity at visit 1 of pregnancy (median gestational age=10 weeks).
| Normotensive pregnancy (n=391) | Preeclamptic pregnancy (n=50) | ||||
|---|---|---|---|---|---|
| Plasma inflammation biomarkers | N | Median (25th, 75th percentile) | N | Median (25th, 75th percentile) | p-value |
| C-reactive protein (µg/mL) | 336 | 3.91 (2.13, 8.44) | 44 | 7.85 (3.82, 16.8) | 0.05 |
| IL-1β (pg/mL) | 336 | 0.29 (0.17, 0.52) | 44 | 0.28 (0.14, 0.61) | 0.44 |
| IL-6 (pg/mL) | 336 | 1.31 (0.78, 2.42) | 44 | 1.33 (0.76, 3.38) | 0.70 |
| IL-10 (pg/mL) | 336 | 12.5 (8.62, 19.3) | 44 | 14.6 (9.68, 21.8) | 0.29 |
| TNF-α (pg/mL) | 336 | 2.85 (2.04, 3.87) | 44 | 3.35 (2.68, 4.22) | <0.01 |
| Urinary oxidative stress biomarkers | |||||
| 8-hydroxydeoxyguanosine (ng/mL) | 381 | 128 (64.0, 206) | 50 | 113 (79.8, 216) | 0.85 |
| 8-isoprostane (ng/mL) | 383 | 211 (99.5, 372) | 50 | 369 (240, 522) | 0.04 |
| Specific gravity (no units) | 390 | 1.016 (1.010, 1.023) | 50 | 1.019 (1.012, 1.025) | 0.07 |
Figure 1.
Predicted plasma inflammation biomarker concentrations and 95% confidence intervals by gestational age at sample collection in preeclamptic (red) vs. normotensive (gray) pregnancies.
Predicted values from generalized additive mixed models of ln-transformed inflammation biomarker concentrations in relation to Loess-smoothed gestational age at sample collection with an interaction term for preeclampsia. Models include a random intercept for subject ID and are weighted for generalizability to the base cohort population. Solid lines represent predicted values and dashed lines represent lower and upper 95% confidence limits for each group. Points represent actual biomarker concentrations measured in mothers who developed preeclampsia (red) vs. mothers who had normotensive pregnancies (black).
Figure 2.
Predicted urinary oxidative stress biomarker concentrations and 95% confidence intervals by gestational age at sample collection in preeclamptic (red) vs. normotensive (gray) pregnancies.
Predicted values from generalized additive mixed models of ln-transformed specific gravity corrected oxidative stress biomarker concentrations in relation to Loess-smoothed gestational age at sample collection with an interaction term for preeclampsia. Models include a random intercept for subject ID and are weighted for generalizability to the base cohort population. Solid lines represent predicted values and dashed lines represent lower and upper 95% confidence limits for each group. Points represent actual biomarker concentrations measured in mothers who developed preeclampsia (red) vs. mothers who had normotensive pregnancies (black).
Results for unadjusted models (Model 1) and models adjusted for maternal race/ethnicity and BMI at visit 1 (Model 2) were similar for inflammation biomarkers, with some attenuation of effect estimates in adjusted models (Table 3). CRP at visit 2 (median=18 weeks, 25th percentile=17 weeks, 75th percentile=19 weeks) showed the strongest association with preeclampsia (adjusted HR=1.83, 95% CI=1.33, 2.51). TNF-α was most consistently associated with increased HRs across gestation. Other cytokines, as with CRP, showed strongest associations at Visit 2.
Table 3.
Hazard ratios (HR) of preeclampsia and 95% confidence intervals (CIs) in association with an interquartile range difference in inflammation biomarker.
| Average of visits 1–3 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| CRP | 2.01 (1.52, 2.65) | <0.01 | 1.45 (1.15, 1.83) | <0.01 | 2.15 (1.64, 2.83) | <0.01 | 1.50 (1.12, 2.01) | 0.01 | 1.01 (0.74, 1.37) | 0.96 |
| IL-1β | 1.22 (1.02, 1.45) | 0.03 | 1.13 (0.93, 1.37) | 0.21 | 1.33 (1.13, 1.57) | <0.01 | 1.23 (1.02, 1.48) | 0.03 | 1.28 (1.07, 1.53) | 0.01 |
| IL-6 | 1.26 (1.07, 1.48) | 0.01 | 1.08 (0.91, 1.28) | 0.38 | 1.33 (1.14, 1.56) | <0.01 | 1.41 (1.15, 1.71) | <0.01 | 1.23 (1.01, 1.50) | 0.04 |
| IL-10 | 1.23 (1.03, 1.46) | 0.02 | 1.16 (0.98, 1.38) | 0.08 | 1.31 (1.11, 1.55) | <0.01 | 1.33 (1.08, 1.64) | 0.01 | 1.16 (0.95, 1.42) | 0.14 |
| TNF-α | 1.70 (1.43, 2.02) | <0.01 | 1.61 (1.34, 1.93) | <0.01 | 1.60 (1.33, 1.91) | <0.01 | 1.71 (1.42, 2.06) | <0.01 | 1.74 (1.42, 2.12) | <0.01 |
| Model 2 | ||||||||||
| CRP | 1.43 (1.04, 1.96) | 0.03 | 1.08 (0.83, 1.42) | 0.56 | 1.83 (1.33, 2.51) | <0.01 | 1.25 (0.90, 1.73) | 0.18 | 0.79 (0.59, 1.06) | 0.11 |
| IL-1β | 1.16 (0.98, 1.37) | 0.09 | 1.10 (0.91, 1.32) | 0.34 | 1.31 (1.12, 1.54) | <0.01 | 1.17 (0.97, 1.42) | 0.10 | 1.20 (1.00, 1.44) | 0.05 |
| IL-6 | 1.19 (0.97, 1.46) | 0.09 | 0.94 (0.76, 1.18) | 0.62 | 1.32 (1.09, 1.59) | <0.01 | 1.36 (1.09, 1.69) | 0.01 | 1.21 (0.95, 1.55) | 0.13 |
| IL-10 | 1.30 (1.06, 1.60) | 0.01 | 1.21 (0.99, 1.48) | 0.06 | 1.40 (1.16, 1.68) | <0.01 | 1.35 (1.08, 1.70) | 0.01 | 1.20 (0.94, 1.53) | 0.14 |
| TNF-α | 1.59 (1.30, 1.94) | <0.01 | 1.49 (1.22, 1.83) | <0.01 | 1.50 (1.23, 1.84) | <0.01 | 1.65 (1.33, 2.04) | <0.01 | 1.63 (1.3, 2.04) | <0.01 |
Model 1 unadjusted for covariates. Model 2 adjusted for maternal race/ethnicity and body mass index at visit 1.Sample sizes for model 1 (preeclamptic and normotensive pregnancies): Average (49, 390); Visit 1 (44, 336); Visit 2 (43, 324); Visit 3 (40, 313); Visit 4 (32, 312). Sample sizes for model 2 (preeclamptic and normotensive pregnancies): Average (49, 387); Visit 1 (44, 333); Visit 2 (43, 323); Visit 3 (40, 312); Visit 4 (32, 309).
In sensitivity analyses for inflammation markers, we examined effect modification by maternal smoking, visit 1 BMI, and race/ethnicity as well as fetal sex and preterm birth. The greatest differences were observed by maternal smoking status during pregnancy. Mothers who smoked had significantly larger HRs for the association between all inflammation biomarkers except for IL-1β and preeclampsia, despite the low number of smokers in this population (n=5 with preeclampsia, n=21 with normotensive pregnancy). For preterm birth, HR for CRP, IL-1β, and IL-6 were lower in cases compared controls but interaction terms were imprecise.
HRs for associations between 8-OHdG and preeclampsia were consistently inverse across models that were unadjusted (Model 1), adjusted for specific gravity only (Model 2), and additionally adjusted for BMI at visit 1 and use of ART (Model 3; see Table 4). In fully adjusted models, effect estimates were similar in magnitude and statistically significant for 8-OHdG levels from each of the four study visits.
Table 4.
Hazard ratios (HR) of preeclampsia and 95% confidence intervals (CIs) in association with an interquartile range difference in oxidative stress biomarker.
| Average of visits 1–3 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| 8-OHdG | 0.84 (0.64, 1.09) | 0.19 | 1.03 (0.83, 1.27) | 0.81 | 0.88 (0.71, 1.10) | 0.26 | 0.73 (0.59, 0.91) | 0.01 | 0.80 (0.61, 1.04) | 0.09 |
| 8-iso | 1.62 (1.22, 2.15) | <0.01 | 1.69 (1.27, 2.25) | <0.01 | 1.57 (1.20, 2.05) | <0.01 | 1.09 (0.87, 1.38) | 0.44 | 1.19 (0.90, 1.57) | 0.23 |
| Model 2 | ||||||||||
| 8-OHdG | 0.46 (0.32, 0.65) | <0.01 | 0.44 (0.32, 0.61) | <0.01 | 0.34 (0.23, 0.51) | <0.01 | 0.54 (0.36, 0.81) | <0.01 | 0.64 (0.41, 1.00) | 0.05 |
| 8-iso | 1.89 (1.27, 2.81) | <0.01 | 1.55 (1.06, 2.25) | 0.02 | 1.91 (1.28, 2.86) | <0.01 | 1.49 (1.06, 2.11) | 0.02 | 1.64 (1.07, 2.52) | 0.02 |
| Model 3 | ||||||||||
| 8-OHdG | 0.47 (0.33, 0.66) | <0.01 | 0.40 (0.28, 0.56) | <0.01 | 0.32 (0.21, 0.49) | <0.01 | 0.53 (0.35, 0.81) | <0.01 | 0.59 (0.37, 0.95) | 0.03 |
| 8-iso | 1.52 (1.04, 2.23) | 0.03 | 1.35 (0.96, 1.91) | 0.09 | 1.68 (1.14, 2.48) | 0.01 | 1.23 (0.89, 1.69) | 0.22 | 1.57 (1.04, 2.35) | 0.03 |
Model 1 unadjusted for covariates. Model 2 adjusted for specific gravity (average or visit specific) only. Model 3 adjusted for specific gravity (average or visit specific) as well as maternal race/ethnicity and body mass index at visit 1. Sample sizes for Model 1 and Model 2 (preeclamptic and normotensive pregnancies) for 8-OHdG: Average (50, 391); Visit 1 (50, 381); Visit 2 (43, 341); Visit 3 (44, 327), Visit 4 (30, 312); and for 8-iso: Average (50, 391); Visit 1 (50, 383); Visit 2 (43, 341); Visit 3 (44, 328); Visit 4 (30, 312). Sample sizes for Model 3 (preeclamptic and normotensive pregnancies for 8-OHdG: Average (50, 388); Visit 1 (50, 378); Visit 2 (43, 340); Visit 3 (44, 326); Visit 4 (30, 309); and for 8-iso: Average (50, 388); Visit 1 (50, 380); Visit 2 (43, 340); Visit 3 (44, 327); Visit 4 (30, 309).
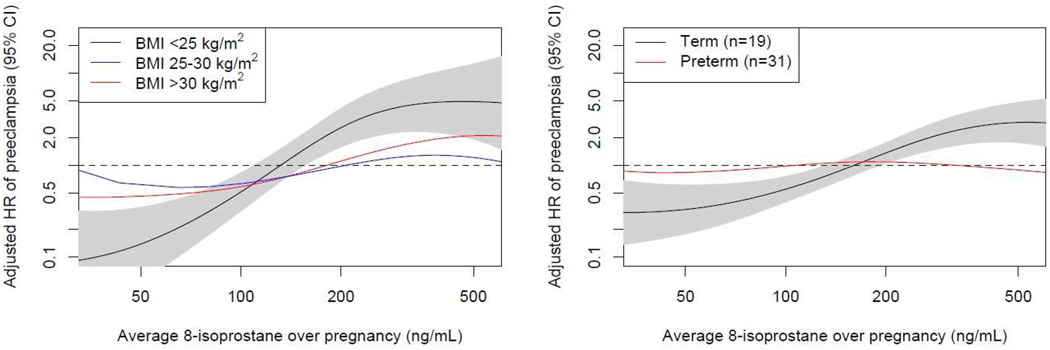
Contrary to the findings for 8-OHdG, 8-isoprostane was consistently associated with an increase in HR of preeclampsia (Table 4). HRs were elevated across pregnancy, but the highest HR was observed in association with levels measured at visit 2. In sensitivity analyses we observed that demographic categories typically associated with higher 8-isoprostane levels (including obese, black, other race/ethnicity, preterm, and smokers) had significantly lower HR (i.e., closer to the null) for the association between 8-isoprostane and preeclampsia. As examples, smoothed plots of HR in association with average 8-isoprostane over pregnancy by preterm birth and BMI are displayed in Figure 3.
Figure 3.
Predicted hazard ratios of preeclampsia in association with average 8-isoprostane concentration over pregnancy by early pregnancy body mass index (panel 1) or preterm delivery (panel 2).
Predicted values from cox proportional hazards models of preeclampsia in relation to ln-transformed geometric average of 8-isoprostane over pregnancy by categories of visit 1 BMI (panel 1) or preterm delivery (panel 2). Model includes spline term with 3 degrees of freedom for ln-transformed geometric average of 8-isoprostane. Gray polygon represents 95% confidence limits for reference category (BMI <25 kg/m2 or term delivery).
Comment
We examined the relationship between a panel of inflammation and oxidative stress biomarkers measured at up to four time points during pregnancy in relation to risk of developing preeclampsia. We observed consistent positive associations between inflammation markers and hazard ratios (HRs), with the strongest associations observed at approximately 18 weeks of pregnancy for most biomarkers; however, for TNF-α, HRs were consistently elevated at all four time points in pregnancy. Similarly, 8-isoprostane, a biomarker of lipid peroxidation and oxidative stress, was associated with elevated HRs consistently across pregnancy. Finally, 8-OHdG, also thought to be indicative of oxidative stress but more specifically of oxidative DNA damage, was found to be protective against PE with consistent findings over gestation.
A number of studies have examined inflammation markers during pregnancy in relation to preeclampsia. Those measuring inflammatory biomarkers at one time during pregnancy have largely supported our findings that mothers with preeclampsia have higher serum or plasma levels of CRP, IL-6, IL-10, and TNF-α during gestation.17–29 These have been primarily small case-control studies with blood samples collected later in gestation or at delivery, after the disease status is known. Studies with contrary findings had major design differences, such as measurement of cytokines in placentae30 or examination of preeclampsia only without severe features.31 However, a recent analysis of a multiplex panel of inflammation biomarkers analyzed in serum samples from ~16 weeks gestation was largely null.32 One other study to date has examined longitudinal profiles of cytokines in preeclamptic compared to normotensive pregnancies (n=32 cases of preeclampsia, 67 controls) and observed the greatest effect estimates for TNF-α and IL-6 later in pregancy.33
As with inflammation, several studies have examined oxidative stress biomarkers in the context of preeclampsia.34–36 For urinary 8-isoprostane, one small case-control study examined three repeated measures during gestation and observed no associations with measures from any of the points.36 However, a more recent cross-sectional study with a larger sample size and urine samples collected at ~12 weeks gestation demonstrated that the proportion of subjects with preeclampsia increased in a dose-dependent manner with increasing urinary 8-isoprostane quintile.35 Our study suggests that both timing of sample collection, characteristics of the mothers, and severity of preeclampsia may contribute to these differences in previous findings. These findings could be particularly important for understanding the impact of antioxidant supplementation in preventing preeclampsia, which has strong biologic plausibility6 but has not been demonstrated in clinical trials.37 Most trials focus on recurrent preeclampsia to obtain larger numbers of cases, but these mothers are more likely to have preterm preeclampsia 38 which was not associated with oxidative stress levels in our study.
Previous work examining maternal urinary 8-OHdG in relation to preeclampsia have been null;35, 39 however, one study observed a suggestive but non-significant trend of higher proportions of participants with preeclampsia in lower quintiles of urinary 8-OHdG concentrations, which is in accordance with our findings.35 This surprising may be explained by impaired DNA excision repair processes in mothers with these preeclampsia.35 Alternatively, decreased levels of 8-OHdG in urine from preeclamptic mothers may be due to higher levels of accumulation in the placenta, which has been reported in multiple studies.40, 41
Our sensitivity analyses provided several novel insights in the relationship between inflammation and oxidative stress biomarkers and the development of preeclampsia. We observed that individuals who delivered preterm had HRs that were closer to the null for 8-isoprostane as well as several inflammation markers. This could suggest that inflammation and oxidative stress may play a more important role in the etiology of less severe preeclampsia, and that other mechanisms are at play in the more severe cases that result in preterm delivery. This finding is consistent with our other analyses in this population, where we observed null associations between these biomarkers and preterm birth that was placentally mediated.12, 14 This is supportive of different phenotypes for preeclampsia, which should be considered carefully when studying the etiology of this complex disease.
Demographic and behavioral factors impacted associations in the present analysis as well. African Americans had attenuated associations between CRP, IL-6, and 8-isoprostane and preeclampsia compared to whites (data not shown). Also, subjects with higher BMI had lower associations between 8-isoprostane and preeclampsia compared to subjects with normal BMI. This could suggest a buffering effect, as individuals in these categories tend to have higher background levels of inflammation and oxidative stress as indicated by levels of these biomarkers in this and other study populations.12, 14
The strengths of this study were the availability of repeated measures of oxidative stress and inflammation across gestation, use of high sensitivity assays, and the ability to generalize our findings to the pregnant population in the Boston area. We were limited by sample size and power, particularly in sensitivity analyses, because of our use of a preexisting case-control study of preterm birth. We also were unable to examine potentially relevant confounders such as diet. Nevertheless, this is one of the strongest studies to date to examine biomarkers of inflammation and oxidative stress in pregnancy prospectively in relation to the development of preeclampsia.
In conclusion, inflammation and oxidative stress biomarkers show different patterns across pregnancy, beginning as early as 10 weeks gestation, in mothers who go on to develop preeclampsia compared to those who have a normotensive pregnancy. The start of the second trimester shows the greatest differences in these biomarkers between the two groups. Furthermore, elevated levels of systemic inflammation and oxidative stress are most apparent in mothers who have term rather than preterm preeclampsia, and it would behoove future research studies to further explore these groups separately when attempting to understand mechanisms. While biomarkers alone do not appear to predict preeclampsia,42 these data are useful in understanding the maternal inflammatory profile in pregnancy prior to development of the disease, and may be used to further develop potentially preventative measures.
Acknowledgments
We thank: Elizabeth Hurst and colleagues for analysis of urinary oxidative stress biomarkers; and Joel Whitfield for analysis of plasma inflammation biomarkers.
Source of Funding: This research was funded by the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health, grant numbers: R01ES018872, P42ES017198, and P30ES017885, and by the Intramural Research Program of NIEHS. The original cohort was supported by an unrestricted grant from Abbott Diagnostics Division (9MZ-04-06N03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the authors report any conflict of interest.
This research was presented in abstract form at the 28th Conference of the International Society for Environmental Epidemiology, Rome, Italy, September 2016.
References
- 1.Obstetricians ACo, Gynecologists. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstetrics and gynecology. 2013;122:1122. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Bmj. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coonrod DV, Hickok DE, Zhu K, Easterling TR, Daling JR. Risk factors for preeclampsia in twin pregnancies: a population-based cohort study. Obstetrics & Gynecology. 1995;85:645–650. doi: 10.1016/0029-7844(95)00049-w. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. American journal of obstetrics and gynecology. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 5.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 6.Roberts JM, Hubel CA. Oxidative stress in preeclampsia. American journal of obstetrics and gynecology. 2004;190:1177–1178. doi: 10.1016/j.ajog.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Burton G, Yung H-W, Cindrova-Davies T, Charnock-Jones D. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30:43–48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heazell A, Moll S, Jones C, Baker P, Crocker I. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28:S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? The Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 10.Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environmental health perspectives. 2016 doi: 10.1289/EHP188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(ACOG). ACoOaG. Method for estimating due date. Committee Opinion No. 611. Obstetrics & Gynecology. 2014;124:863–866. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson KK, McElrath TF, Chen Y-H, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American journal of obstetrics and gynecology. 2015;212:208. doi: 10.1016/j.ajog.2014.08.007. e1-08. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990;5:46–51. [Google Scholar]
- 14.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. American journal of reproductive immunology. 2014;72:326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer Science & Business Media; Number of pages. [Google Scholar]
- 16.Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. 2014 doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szarka A, Rigó J, Lázár L, Bekő G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunology. 2010;11:1. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie C, Yao MZ, Liu JB, Xiong LK. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56:550–559. doi: 10.1016/j.cyto.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioğlu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-α in normal pregnancies and in pregnancies complicated by preeclampsia. The Journal of Maternal-Fetal & Neonatal Medicine. 2010;23:880–886. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and Inflammatory Markers (TNF-α, IL-6 and IL-8) in Pre-Eclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. American Journal of Reproductive Immunology. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharma D, Singh A, Trivedi SS, Bhattacharjee J. Intergenotypic variation of nitric oxide and inflammatory markers in preeclampsia: a pilot study in a North Indian population. Human immunology. 2011;72:436–439. doi: 10.1016/j.humimm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Kalinderis M, Papanikolaou A, Kalinderi K, et al. Elevated Serum Levels of Interleukin-6, Interleukin-1β and Human Chorionic Gonadotropin in Pre-eclampsia. American Journal of Reproductive Immunology. 2011;66:468–475. doi: 10.1111/j.1600-0897.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 23.Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008;140:21–26. doi: 10.1016/j.ejogrb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson Y, Rubèr M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. Journal of reproductive immunology. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Montagnana M, Lippi G, Albiero A, Salvagno GL, Franchi M, Guidi GC. Serum pro-inflammatory cytokines in physiological and pre-eclamptic pregnancies. Gynecological Endocrinology. 2008;24:113–116. doi: 10.1080/09513590801895575. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. American journal of epidemiology. 2005;162:1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. American journal of hypertension. 2004;17:154–160. doi: 10.1016/j.amjhyper.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Tjoa M, Van Vugt J, Go A, Blankenstein M, Oudejans C, Van Wijk I. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. Journal of reproductive immunology. 2003;59:29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 29.de Jonge LL, Steegers EA, Ernst GD, et al. C-reactive protein levels, blood pressure and the risks of gestational hypertensive complications: the Generation R Study. Journal of hypertension. 2011;29:2413–2421. doi: 10.1097/HJH.0b013e32834c58e5. [DOI] [PubMed] [Google Scholar]
- 30.Marusic J, Prusac IK, Tomas SZ, Karara JR, Roje D. Expression of inflammatory cytokines in placentas from pregnancies complicated with preeclampsia and HELLP syndrome. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;26:680–685. doi: 10.3109/14767058.2012.746301. [DOI] [PubMed] [Google Scholar]
- 31.Alanbay I, Coksuer H, Ercan CM, et al. Chitotriosidase, interleukin-1 beta and tumor necrosis factor alpha levels in mild preeclampsia. Archives of gynecology and obstetrics. 2012;285:1505–1511. doi: 10.1007/s00404-011-2157-6. [DOI] [PubMed] [Google Scholar]
- 32.Taylor BD, Tang G, Ness RB, et al. Mid-pregnancy circulating immune biomarkers in women with preeclampsia and normotensive controls. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2016;6:72–78. doi: 10.1016/j.preghy.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK, Allen J, Knudsen UB. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta obstetricia et gynecologica Scandinavica. 2011;90:791–796. doi: 10.1111/j.1600-0412.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 34.Barden A, Beilin LJ, Ritchie J, Croft KD, Walters BN, Michael CA. Plasma and urinary 8-iso-prostane as an indicator of lipid peroxidation in pre-eclampsia and normal pregnancy. Clinical Science. 1996;91:711–718. doi: 10.1042/cs0910711. [DOI] [PubMed] [Google Scholar]
- 35.Peter Stein T, Scholl TO, Schluter MD, et al. Oxidative stress early in pregnancy and pregnancy outcome. Free radical research. 2008;42:841–848. doi: 10.1080/10715760802510069. [DOI] [PubMed] [Google Scholar]
- 36.Regan CL, Levine RJ, Baird DD, et al. No evidence for lipid peroxidation in severe preeclampsia. American journal of obstetrics and gynecology. 2001;185:572–578. doi: 10.1067/mob.2001.116754. [DOI] [PubMed] [Google Scholar]
- 37.Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia (Review) The Cochrane Collaboration. 2008 doi: 10.1002/14651858.CD004227.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hnat MD, Sibai BM, Caritis S, et al. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparas. American journal of obstetrics and gynecology. 2002;186:422–426. doi: 10.1067/mob.2002.120280. [DOI] [PubMed] [Google Scholar]
- 39.Chen S-F, Lo L-M, Li M-J, Yeh Y-L, Hung T-H. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reproductive sciences. 2012;19:505–512. doi: 10.1177/1933719111426601. [DOI] [PubMed] [Google Scholar]
- 40.Fujimaki A, Watanabe K, Mori T, Kimura C, Shinohara K, Wakatsuki A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta. 2011;32:367–372. doi: 10.1016/j.placenta.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Kimura C, Watanabe K, Iwasaki A, et al. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;26:491–496. doi: 10.3109/14767058.2012.733766. [DOI] [PubMed] [Google Scholar]
- 42.Conde-Agudelo A, Villar J, Lindheimer M. World Health Organization systematic review of screening tests for preeclampsia. Obstetrics & Gynecology. 2004;104:1367–1391. doi: 10.1097/01.AOG.0000147599.47713.5d. [DOI] [PubMed] [Google Scholar]