Abstract
Introduction
Erythropoeitin (EPO) has been identified as a neuroregenerative agent. We hypothesize that it may accelerate recovery after crush injury and may vary with crush severity.
Methods
Mice were randomized to mild, moderate, or severe crush of the sciatic nerve and were treated with EPO or control after injury. Sciatic function index (SFI) was monitored over the first week. Microstructural changes were analyzed by immunofluorescence for neurofilament (NF) and myelin (P0), and electron microscopy was used to assess ultrastructural changes.
Results
In moderate crush injuries, EPO significantly improved SFI 7 days post-injury, an effect not observed in other severities. Increases in the ratio of P0 to NF were observed after EPO treatment in moderate crush injuries. Electron microscopy demonstrated endothelial cell hypertrophy in the EPO group.
Conclusions
EPO accelerates recovery in moderately crushed nerves, which may be through effects on myelination and vascularization. Injury severity may influence the efficacy of EPO.
Keywords: nerve crush injury, peripheral nerve injury, erythropoietin, neuroregeneration, electron microscopy
Introduction
Peripheral nerve injuries, in particular, traumatic injuries, are a significant source of morbidity and pose a challenge for treating physicians faced with limited options for improving outcomes.1,2 There is an expanding body of evidence which suggests that erythropoietin (EPO), originally described based on its function as a hematopoietic cytokine, has additional effects as a mediator of neurotrophic and neuroregenerative processes.3-12 These encouraging findings have led to multiple reports of EPO improving or accelerating nerve recovery in both the central11,13-15 and peripheral nervous systems.16-20 Evidence suggests this may in part be attributable to EPO inhibiting apoptosis, protecting against oxidative damage, and stimulating axonal outgrowth after injury through its effects on Schwann cells,10 in addition to its promotion of angiogenesis. EPO is an FDA-approved drug with a limited side-effect profile, making it an attractive agent for translational studies of peripheral nerve injury.
Current clinical classification systems of peripheral nerve injury apply characteristics of individual neurons to entire nerves, with the axon either intact or severed, and the epineureum either preserved or compromised.21-23 In reality, with thousands of individual neurons comprising a complex peripheral nerve, crush injury may result in subpopulations of intact axons adjacent to subpopulations of severed axons with the potential for drastic variability of structural compromise and functional impairment. The complex and varied nature of peripheral nerve injuries demands an experimental model that can evaluate treatments across a range of injury severities in order to determine which injury patterns are most likely to benefit from an intervention.
Patients with nerve injuries in the setting of limb trauma can present with identical findings and vastly different capacities to recover. As a candidate treatment for peripheral nerve trauma, it is important to evaluate the effects of EPO on peripheral nerve injuries of different severities. We studied a murine model of sciatic nerve crush injury with 3 distinct injuries of increasing force termed mild, moderate, and severe crush. We have previously found that EPO speeds functional recovery of animals after crush injury over a time course faster than would be observed through nerve regeneration.19 This strongly suggests that EPO may be providing supportive protection of function to neurons which remain intact and unsevered but somehow nonfunctional. We therefore hypothesize that certain nerve crush injuries are amenable to EPO-mediated functional recovery, while others may be so severe that EPO would not be helpful. Still others would be so mild that they would not cause a functional deficit for which EPO may be helpful. Moreover, if EPO is effective at speeding recovery before neurons can regenerate, then we further hypothesize that there may be measurable differences in the amount of myelin in EPO treated injuries. Since no current method for evaluating the severity of nerve injury in the sciatic nerve crush model is available, we developed a method of measuring the relative force delivered to the nerve and then measured the capacity of EPO to accelerate functional recovery with different severities of crush as a means to test our hypothesis.
Materials and Methods
Murine model of peripheral nerve crush injury
All animal procedures were approved by the University Committee on Animal Research (UCAR) at the University of Rochester. Ten-week-old female C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) weighing between 20 and 25 g were anesthesized with intraperitoneal injection of ketamine (60 mg/kg) and xylazine (4 mg/kg). The crush injury was induced as follows. Briefly, the left hindlimb was shaved, washed, and prepped with betadine (povidone-iodine), then incisions were made along the lateral length of the femur and through the iliotibial band to expose the sciatic nerve. Once exposed, either mild, moderate, or severe crush injury was induced using diagonal jawed forceps (Miltex 18-1107 Swiss Cilia Forceps; Integra Miltex; York, PA; mild and moderate crush) or a stainless steel needle holder (Webster needle holders RH2560; V. Mueller, CareFusion Corp., San Diego, CA; severe crush), at a point proximal to the sciatic nerve trifurcation into the common fibular, tibial, and sural nerves. The crush was held for 30 seconds in all cases.
To quantify the force of the crush injury, pressure sensitive film (Sensor Products Inc., Madison, NJ) was used to determine the pressure generated by the surgical instruments. Using known forces, the film was calibrated to provide a linear curve of pixel density versus pressure (Pascals), with an R2=0.98883. ImageJ (http://imagej.nih.gov) was used to analyze all images. The 3 injury intensities, mild, moderate, and severe, were then recreated with the film resting between the forceps, and the post-crush changes to the film were analyzed for pixel density within the region of the forceps corresponding to the placement of the sciatic nerve. These densities were fit to the known curve in order to determine each pressure. Pressures are reported in megapascals (MPa); as a reference, 1 MPa is equal to approximately 145 pounds per square inch (PSI). Each intensitiy was tested 3 times, and the averages of the groups were compared. These injuries correspond to 2.5 MPa (±0.25 MPa), 4.9 MPa (±0.18 MPa), and 9.5 MPa (±1.03 MPa) for mild, moderate, and severe crush, respectively.
After the injury, a single 5-0 nylon suture (Ethicon, Inc.) was used to close the fascia, and 3 interrupted 5-0 sutures were used to close the skin incision. Mice were immediately returned to their cages and allowed free active motion and weight-bearing under the supervision of the university's veterinary staff. Subcutaneous buprenorphine (0.05 mg/kg) was given at the time of surgery and every 12 hours over the next 3 days for analgesia.
Erythropoeitin treatment
EPO (Epoetin Alfa (PROCRIT); Janssen Products, Horsham, PA; National Drug Code 59676-310-00) was randomly administered to half of the animals in each injury group immediately after the crush injury. The surgeon who administered EPO or saline control to animals was blinded at the time of administration by having EPO in 1 of 2 syringes and saline control in the other. Half of the animals received treatment from 1 syringe and were marked using ear tagging methods as part of the university animal protocol. The other half received saline injection and were marked accordingly. Randomization was only resolved after data were analyzed, and the researchers were blinded to the status of the animals during the entire experiment as part of this protocol. Group sizes are reported below for functional and histologic analysis. EPO was given at a dose of 5,000 Units/kg based on previous studies of EPO in animals and humans.19,20,24,25 The supply of EPO was refrigerated and then suspended in a 100μL solution of sterile saline at the time of injection. The treatment was delivered by intraperitoneal injection in accordance with pharmacy and veterinary recommendations. Control animals were given intraperitoneal injections of 100 μL of sterile saline at equivalent time points.
Functional alaysis using sciatic function index (SFI)
Walking track analysis was performed according to a previously published model that uses the sciatic function index (SFI) as a noninvasive means to monitor recovery after scatic nerve injury.26 Briefly, mice were trained to walk along a confined corridor prior to injury, with different colored paint thinly applied to the injured and uninjured hindpaws. Before injury, and on days 1, 3, 7, 14, 21, and 28 post-injury, animals from the 3 injury groups were walked along the corridor until clear prints were made (minimum n=5 per severity per time point); this often required multiple attempts. This group was used to characterize the natural course of recovery after the 3 crush severities. In addition, separate cohorts of mice from the EPO and control groups were walked at days 1, 2, and 7 post-injury (minimum n=5 per severity per treatment per time point). Our previous work showed effects of EPO treatment at time points earlier than could be expected under classical models of nerve regeneration.19 As such, we wanted to focus on the effects of EPO that occurred early without subjecting to biases associated with handling fragile nerves soon after crush injury. Day 7 was chosen, given the most significant differences between groups in the untreated cohorts, with inclusion of days 1 and 2 post-injury to evaluate whether any changes occurred in the days immediately following injury and after treatment. We confined this portion of the study to early effects. Using digital calipers, the following measurements were made from the prints: paw length (PL, the distance from the heel to the third toe) and toe spread (TS, the distance from the first to the fifth toe). Two measurements were made from separate prints on both the uninjured and injured side, and averages were used to calculate the SFI. SFI was calculated using a previously described formula:27
Where ETS is experimental toe spread, NTS is normal toe spread, EPL is experimental paw length, and NPL is normal paw length.
Histology and immunohistochemistry
Mice were sacrificed on post-injury day 7 for histologic analysis of the injury site (n=3 per severity). Immediately following sacrifice, sections of the sciatic nerve containing areas proximal and distal to the crush site were removed carefully, washed in cold PBS, and fixed in 4% paraformaldehyde for 4 h at 4°C. Nerves were then embedded in paraffin and serial 5μm-thick cross-sections were taken 2 mm proximal to the crush site (proximal), at the crush site (crush), and 2 mm distal to the crush site (distal).
Immunofluorescence was performed on post-injury day (PID) 7 using antibodies to myelin protein zero (P0; Aves Lab, Cat# PZ0, 1:1000) or neurofilament (NF; Aves Lab, Cat# NFH, 1:1000). The slides were pretreated with 0.01M citrate buffer (pH 6.0) for antigen retrieval. Nonspecific blocking was performed with 1:20 diluted serum for 30 minutes. Following overnight incubation at 4°C with the primary antibody, the fluorescent-labeled secondary antibody was incubated for 1 hour. Staining for myelin protein zero (P0), a major constituent of myelin, was used to evaluate the myelination status of neurons within different areas around the crush site, while NF staining allowed for evaluation of neuron continuity. Computational image analysis was performed using ImageJ (http://imagej.nih.gov) with the cell counter plug-in to quantify the number of P0 and NF positive axons at PID 7. A minimum of 3 slides per level (proximal, crush, or distal) per treatment was analyzed, using sections from different animals. Reviewers were blinded to the groups.
Electron Microscopy
On PID 3, the sciatic nerves were surgically exposed, gently excised, and picked up with tweezers at excision ends to avoid inducing ultrastructural artifact. The nerves were immediately immersed into a fixative containing 2.5% glutaraldehyde/4.0% paraformaldehyde in 0.1M sodium cacodylate buffer, fixed for 24 hours at 4°C and post-fixed 2.0 hours in a combination of 1.5% potassium ferrocyanide/1.0% osmium tetroxide in 0.1M sodium cacodylate. Before processing, both ends of each nerve were trimmed away, and the remaining length of each nerve was dehydrated in a graded series of ethanol to 100%, transitioned into propylene oxide, infiltrated with EPON/araldite resin overnight, embedded longitudinally in a mold containing fresh resin, and polymerized for 48 hours at 60°C. Each nerve was cut at 1μm and stained with Toluidine Blue to ascertain the specific region to be thin sectioned at 70nm using a diamond knife and an ultramicrotome. The thin sections were placed onto 150 mesh carbon/formvar nickel grids and examined using a Hitachi 7650 transmission electron microscope (TEM) with attached Gatan Erlangshen digital camera and DigitalMicrograph software.
Statistics
Results are presented as the mean ± standard deviation (SD). Comparison between SFI in the 3 injury groups without any treatment was done using 2-way ANOVA with Bonferonni correction for multiple comparisons. Differences in SFI between EPO- and vehicle-treated groups were analyzed using multiple t-tests with the Holm-Sidak method to correct for multiple comparisons. For all analyses, statistical significance was set at alpha=0.05. A power analysis was performed based on the differences in SFI found between groups with and without EPO in our previous work.19 This analysis revealed a 35% difference between treatment groups. Based on this difference, and a desired power level of greater than 80%, we arrived at a minimum group size of 5 mice per group for functional outcomes. Our previous work had suggested that a group size of 3 would be sufficient for immunohistochemical studies.
Results
Natural Course of Functional Recovery
There were immediate differences in the degree of functional impairment imparted by the 3 different crush intensities (Figure 1; n=3-5 mice per severity per time point). A higher SFI indicates worse function. At PID 1, the mild crush group performed significantly better than either moderate (mild: 67.4 ± 25.4; moderate: 100.7 ± 4.7, P<0.05) or severe crush (mild: 67.4 ± 25.4; severe: 102.5 ± 11.1, P<0.01). Significant difference was maintained at PID 3 for mild versus moderate (mild: 39.0 ± 20.7; moderate: 98.4 ± 8.0, P<0.01) and mild versus severe (mild: 39.0 ± 20.7; severe: 95.7 ± 14.9, p<0.01), at which point the mild crush group was already beginning to show functional improvement. There were no significant differences between the moderate and severe groups at these early time points.
Figure 1. Functional Recovery Varies According to Crush Severity.
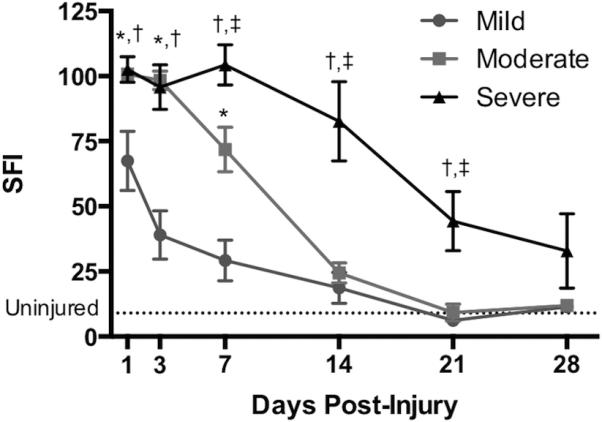
In untreated mice, walking track analysis was performed at various time points after crush injury to track functional recovery, as indicated by the sciatic function index (SFI). A higher SFI indicates worse function. All 3 groups recover function by 28 days post-injury, and the severity of injury affected the time to recovery. (*P<0.05 between mild and moderate; †P<0.05 between mild and severe; ‡P<0.05 between moderate and severe).
By PID 7, the moderate crush group was beginning to show functional improvement and performed significantly better than severe crush (moderate: 71.8 ± 17.3; severe: 104.3 ± 15.6, P<0.05), though it remained significantly worse functionally than the mild crush group (mild: 29.2 ± 17.6; moderate: 71.8 ± 17.3, P<0.01). By PID 14 there were no significant differences between the functional status of the mild and moderate groups, though the severe crush group remained worse than either mild (mild: 18.7 ± 12.0; severe: 82.7 ± 30.5, P<0.01) or moderate crush (moderate: 24.4 ± 7.7; severe: 82.7 ± 30.5, P<0.01). At PID 21 the severe crush group was beginning to show functional recovery, however it was still significantly worse than either mild (mild: 6.2 ± 0.7; severe: 44.4 ± 25.5, P<0.05) or moderate crush (moderate: 9.2 ± 6.5; severe: 44.4 ± 25.5, P<0.05). There were no significant differences between any of the groups at PID 28.
EPO accelerates functional recovery in moderately crush nerves
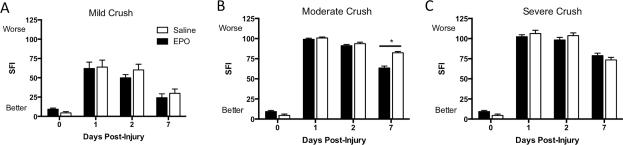
Walking track analysis was performed before crush injury, and at PIDs 1, 2, and 7 to assess the effects of EPO on early nerve regeneration after 3 severities of crush injury (minimum n=5 per severity per treatment per time point) (Figure 2). A higher SFI indicates worse function, while lower SFI values correspond with better function. Significant improvement in SFI was seen at PID 7 in the moderately crushed group treated with EPO, compared to vehicle treated controls (Saline: 82.3 ± 1.7; EPO: 63.5 ± 2.5, P<0.05) (Figure 2B). No significant differences in SFI were seen after EPO treatment at PID 7 in either the mild (Saline: 29.9 ± 5.6; EPO: 24.2 ± 5.0) (Figure 2A) or severe (Saline: 73.5 ± 3.3; EPO: 78.8 ± 3.1) (Figure 2C) crush groups.
Figure 2. EPO Treatment Improves Functional Recovery in Moderately Crushed Nerves.
Sciatic function index (SFI) was determined at post-injury days 1, 2, and 7 in the 3 crush severities for both EPO and saline treated groups. Higher SFIs suggest worse function, while lower SFIs indicate better function. A: No significant differences were seen between the 2 treatment groups after mild crush injury. By 7 days post-injury, both groups have achieved almost full functional recovery. B: EPO treatment resulted in significant improvement in SFI at 7 days post-injury in the moderate crush group, suggesting accerated functional recovery. C: No significant differences in SFI were seen in the severe crush groups after either EPO or vehicle treatment. (Saline=white bars, circles; EPO = black bars. *P<0.05).
EPO increases myelin protein zero and neurofilament counts in moderately crushed nerves
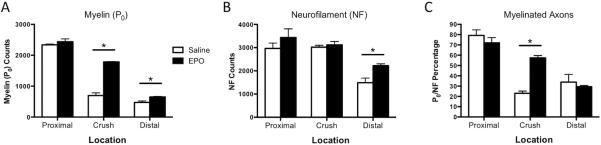
Given the functional benefits of EPO at PID 7 in moderately crushed nerves, histologic sections proximal to the crush site, at the crush site, and distal to the crush site were stained and quantified from moderately crushed nerves 7 days after injury (n=3 per severity per time point) (Figure 3). Antibodies to P0 and NF were used to evaluate the underlying changes in myelination status and axon continuity in the moderately crushed group after either saline or EPO treatment. Myelin protein zero (P0) was assessed as a measure of the amount of intact myelin surrounding fibers in the crush-injured nerve. Treatment with EPO led to significant increases in the number of fibers positive for P0 at the crush site (Saline: 696 fibers ± 49; EPO: 1784 fibers ± 16, P<0.05), and distal to the crush site (Saline: 473 fibers ± 49; EPO: 652 fibers ± 17, P<0.05) (Figure 3A). Changes in NF staining were seen only distal to the crush site (Saline: 1487 fibers ± 201; EPO: 2221 fibers ± 80, P<0.05), consistent with distal axon degeneration after injury (Figure 3B). The ratio of P0 to NF staining was used as a proxy for the number of myelinated nerve fibers, and significant increases in this ratio were seen at the crush site in EPO-treated nerves (Saline: 23% ± 2%; EPO: 57% ± 2%, P<0.05).
Figure 3. Immunofluorescent Analysis of Moderately Crushed Nerves Revealed Increases in the Ratio of P0 to NF after EPO Treatment.
Quantitative histomorphometry was performed in cross-sections taken from the moderate crush group at 7 days post-injury. Sections were taken from sites proximal to the crush (proxima), at the crush site (crush), and distal to the crush site (distal), then stained for myelin protein zero (P0) or neurofilament (NF). A: Significant increases in the number of P0 positive axons were observed at the crush site and distal to the crush site in EPO-treated nerves, suggesting EPO-mediated increases in myelination. B: Significant increases in neurofilament were seen only distal to the crush site in EPO-treated nerves. C: The ratio of P0 to NF positive fibers was calculated as a proxy for the percentage of myelinated fibers. Significant increases in this ratio were seen at the crush site in the EPO treatment group, suggesting that EPO may act through increased myelination of axons around the crush site. (White bars = saline treated; black bars = EPO treated. *P<0.05).
EPO Increases Vascularization of Damaged Nerves
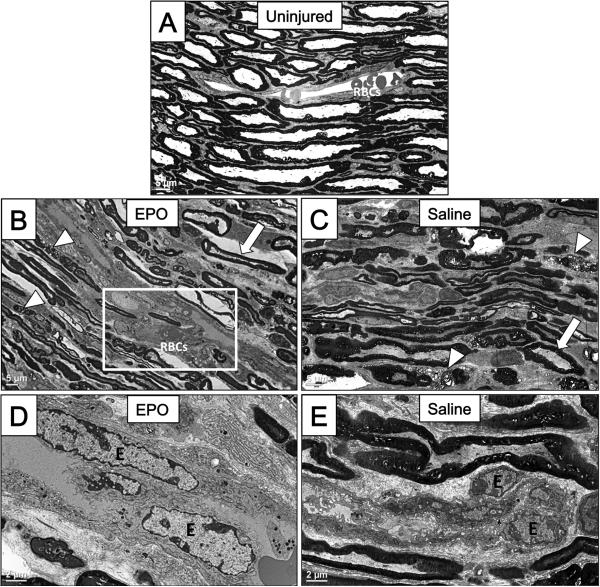
Electron microscopy was performed on sections from moderately crushed nerves treated with EPO and saline at PID 3 to visualize differences in early ultrastructural changes following injury (Figure 4). In uninjured control nerves, normal sized endothelial cells and red blood cells (RBCs) can be appreciated in the vascular lumen, surrounded by organized layers of myelinated axons (Figure 4A). Fragments of damaged myelin are appreciated after crush injury in the EPO-treated group (arrowheads), which line up adjacent to a branching blood vessel (Figure 4B). The vessel in the EPO treatment group displayed increased diameter along with hypertrophied endothelial nuclei (Figure 4B and D). Within the same specimen, there was also evidence of preserved myelin in some areas (arrow). Similarly, saline-treated nerves had mixed evidence of myelin damage (arrowheads) and myelin preservation (arrow), suggesting a mixed injury (Figure 4C). However, there was a notable absence of any branching or dilated vasculature. Comparisons between saline- (Figure 4E) and EPO-treated nerves (Figure 4D) at the same magnification demonstrate the drastic effect of EPO on endothelial nuclei (labelled “E”), which are notably enlarged following crush injury and EPO treatment.
Figure 4. EPO Increases Vascular Proliferation in Crush Injuries Following Treatment.
Transmission electron microscopy was performed to evaluate differences in ultrastructural changes after crush injury. A: Longitudinal section of an uninjured sciatic nerve. Nerve fibers seen above and below a single non-branching blood vessel contain normal sized endothelial cells and red blood cells (RBCs) in the vascular lumen. (Scale bar = 5μm). B: Crushed sciatic nerve 3 days after injury and EPO treatment shows evidence of damage to myelin with the presence of fragmented small myelin sheath ovoids (arrowheads) lining up adjacent to a branching blood vessel (boxed area) with increased vessel diameter. Within the frame there is evidence of undamaged myelin (arrow). (Scale bar = 5μm). C: Crushed sciatic nerve 3 days after injury and saline treatment. These specimens similarly had evidence of both damaged myelin ovoids (arrowheads) and undamaged areas (arrow). In saline-treated specimens, there was a notable absence of branching or expanding vasculature. (Scale bar = 5μm). D: Higher magnification of EPO-treated nerves shows notable hypertrophy of vascular endothelial cells (“E”), suggesting vascular proliferation. (Scale bar = 2μm). E: At the same magnification as D, saline treated sciatic nerves have notable absence of vascular endothelial cell hypertrophy (“E”). (Scale bar = 2μm).
Discussion
We report the variable effects of a single, early dose of EPO on nerve regeneration after different severities of sciatic crush injury. The results suggest that in moderately crushed nerves, EPO accelerates functional recovery, which may be through local effects on myelination and vascular proliferation. These positive effects were not observed in either mild or severely injured nerves. These findings suggest that there may be a critical injury severity for which EPO exerts beneficial effects on nerve oregeneration, and that either too little damage or too much damage renders treatment ineffective.
Evaluating the effects of EPO on a murine model of variable nerve damage is critical to guiding its translation into clinical use. In practice, partially injured peripheral nerves, which have a variable capacity to heal, are some of the most common injuries encountered. Large and predictable functional benefits of EPO treatment for peripheral nerve injuries have been described.16,18,19,28 This study expands upon the current literature by evaluating whether the degree of injury has an effect on the response to EPO, particularly at early time points after injury. Reports of the use of the sciatic nerve crush injury model do not rigorously define the amount of force, and therefore, the amount of injury imparted on the nerve. This is important in the scenario that partial nerve damage disrupts a variable number of nerve fibers depending on the intensity of injury. An appropriate translation to clinical applicability depends chiefly on an accurate characterization of the most suitable injury type for EPO treatment.
To address the need for an injury model of varying severity, we have described crushes of increasing severity termed mild, moderate, and severe. These injuries have distinct patterns of recovery (Figure 1), and all groups regain normal function by 28 days after injury. Predictable functional recovery allows for investigations of interventions that may influence the time course of recovery in different injury severities. Importantly, the short time course in which full fuctional recovery can be achieved makes this an attractive model for laboratory use in which month- and year-long recoveries may burden limited resources. Further, the choice of animal species should be intentional in peripheral nerve experimentation. The C57BL6/J mouse species was chosen in this study due to the potential for genetic manipulation in future experiments, and what that can reveal about underlying mechanisms. With genetic variants selectively missing EPO receptors in Schwann cells and neurons, experiments can be recreated to see if the positive effects from this study are lost without the receptor.
Much of the current literature on peripheral nerve injuries in humans relies on classifications that fail to distinguish between subpopulations of neurons that may sustain differential degrees of injury. Instead, clinical classifications provide a picture of a uniformly injured nerve with each portion equally affected by the injury.21-23 In actuality, crush injuries often induce non-uniform patterns of injury, with varying degrees of myelin disruption and differences between subpopulations of neurons. It is therefore possible that subpopulations of neurons may be amenable to pharmacologic treatments while others are not. As shown in this study, EPO has the strongest effect on moderately injured nerves. It may be possible that mildly injured nerves have not suffered sufficient injury for the effects of EPO to be functionally significant, while severely crushed nerves have too few salvageable fibers for EPO to demonstrate a significant effect. The injury for which EPO is likely to contribute the most functional benefit is therefore confined by factors not currently assessed in our clinical classification schemes.
Along with the functional benefits of EPO seen 7 days after injury in moderately crushed nerves (Figure 2B), there was an associated increase in the ratio of P0 to NF seen on immunohistochemistry (Figure 3). These findings are consistent with previously reported effects of EPO on Schwann cells and myelination. Schwann cells express a receptor for EPO, which may be responsible for the beneficial effects of EPO administration.5 EPO stimulates Schwann cell migration, and its administration has been shown to have a positive effect on myelination.11,13 These beneficial effects of EPO on myelination have already been translated into clinical trials for demyelinating diseases such as multiple sclerosis15 and amyotrophic lateral sclerosis.14 Results from studies on EPO administration after peripheral nerve injury suggest that there may be a role for its use in these types of injuries as well.
High magnification imaging using TEM allowed for ultrastructural comparisons between the EPO and control groups 3 days after moderate crush injury (Figure 4). These higher magnification images suggest the mixed injury pattern described earlier, in which areas of damaged and degraded myelin (arrowheads) are near areas of myelin preservation (arrows). The most notable observations were the increased diameter of capillary lumens infiltrating the crushed nerve (Figure 4B), along with hypertrophied endothelial nuclei relative to control treated nerves (Figure 4D). Endothelial cell nuclei increase in size during periods of proliferation. In a study of sciatic nerve crush injury in rats, Podhajsky and Myers29 observed endothelial nuclei swelling 1 week after crush injury. In our study, the same changes were observed, though at a much earlier time point, suggesting that EPO may accelerate the normal physiologic response to crush injury.
In our model of differential degrees of sciatic nerve crush injury, all animals recover function by 28 days, and temporal patterns of recovery vary according to injury severity (Figure 1). We chose early time points for evaluation in this study, since the most significant differences in recovery occur within the first week after injury. The effects, therefore, appear to be partly through increased myelination of neurons that are temporarily disrupted but still maintain the capacity to regain function. This is further supported by the absence of any differences in neurofilament counts between the 2 treatment groups proximal to and at the crush site 7 days after injury (Figure 3B). The ability for EPO to promote axonal regrowth after nerve injury has been previously described,3,4,20 however, such a mechanism is unlikely to be responsible for the early effects seen in our study.
This study is limited, because it is difficult to quantify the effect of different crush intensities on the degree of disruption of myelination and of neurofilament continuity. Moreover, it is difficult to generalize the findings in this murine model to any or all of the traumatic nerve injuries encountered in humans. Specifically, the findings presented here do not address the effects of EPO in promoting recovery after nerve transection, the injury pattern that is perhaps most in need of treatments that will improve regeneration. A limitation of this study is the use of SFI. Although this method is often used, there is a natural selection for the very best possible footprints, which is part of this method. Our study is no exception. Our animals are typically walked down the track 2-3 times per trial. This is done prior to any selection of footprints for analysis. We believe that this means that we have given each animal the best opportunity the walk on its foot as normally as it can prior to analysis. We avoid systematic bias in this process by performing our analyses in blinded fashion. However, the standard method of SFI assessment naturally allows for a bias toward the best possible walking that an animal can perform on any given day. Another limitation of this study is the selection of time points for functional evaluation in the first week. We selected to have functional evaluations at posttreatment and operative days 1, 2, and 7, primarily because our preliminary work showed a treatment effect in the first week after injury. This effect was greatest in animals treated on the day of injury, though it was significant for animals treated the day before and after injury. We recognize that it would have been optimal to evaluate animals daily, with cohorts sacrificed and analyzed histologically every single day. However, our functional evaluation was confined to the first 48 hours after injury, and then measurements at 1 week to compare results to our previous work. An additional limitation is the absence of NF and P0 counts from uninjured nerves, which would have provided an additional point of useful comparison. Little can be said about the functional status of myelin simply based on the positive staining with P0. Simple P0 positivity does not guarantee that the myelin is functional. A functional myelin sheath encircles a single neurofilament, and EM would be required to ensure that more neurofilaments are myelinated in the setting of EPO treatment. We do not have these definitive data, rather we have only a suggestion that EPO increases myelin content in the nerve. Nonetheless, this model is a standard method for comparison and evaluation of treatments in the literature and has the benefit of offering histopathological correlates of function in a relatively simple surgical system.
Crush injuries of the sciatic nerve have been used to study functional impairment and recovery in the past. Little is known about the relative effects that crush injury has on myelin at the site of crush, or on neurons proximal or distal to it. We attempted to vary the severity of crush injury in this study, but we realize that without an absolute measure of myelin content and NF staining, our relative assessments only pertain to the injury site and the areas proximal and distal to it. We cannot make absolute assessments about myelin and its natural recovery after injury, as we have not studied those effects. However, our results suggest that a study of myelin over time, in the hours after injury using this model, is warranted. Severity of injury, even when measured, may not be strictly reproducible. We have found in our own experiments that severely injured animals treated with saline in 1 experiment fared differently than the severely injured animals evaluated at a different time of the year. We can say that, even our mildest injury causes functional deficits, and those are highly reproducible in our hands. Our severest crush injuries are more variable, and we have attributed this to the relatively few fibers left intact after injury. If we suppose there are injuries where only 6-10% of fibers are intact to function afterwards, then a 1% difference in this amount between animals represents an 18% change in neuronal fibers traversing the injury site. We believe that our severest injuries sever all but about 5% of fibers, and this may explain the higher variability in these experiments. Without a single standard for injury severity in crush models, we believe that severity is a limitation in any experiment on sciatic nerve crush injury, and our results should be interpreted with caution.
In conclusion, the results from this study suggest that EPO accelerates functional recovery after moderate crush injury and might act primarily on intact fibers with damaged but not fully compromised myelin through a myelin protective mechanism. Future research is indicated to further describe the mechanisms responsible for the early changes in moderately injured nerves, as well as the long-term effects of EPO on functional recovery.
Acknowledgements
MBG was funded in part by an award from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (TL1 TR000096). JCE was funded in part through the NIH K08 Clinical Investigator Award (K08 AR060164-01A), as well the American Society for Surgery of the Hand Clinician Scientist Award.
Abbreviations
- DPI
Days post-injury
- EPO
Erythropoetin
- H&E
Hematoxylin and eosin
- MPa
Mega pascals
- NF
Neurofilament
- P0
Myelin protein zero
- PID
Post-injury day
- SD
Standard deviation
- SFI
Sciatic function index
- TEM
Transmission electron microscopy
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Chen LE, Seaber AV, Glisson RR, et al. The functional recovery of peripheral nerves following defined acute crush injuries. J Orthop Res. 1992;10:657–664. doi: 10.1002/jor.1100100508. [DOI] [PubMed] [Google Scholar]
- 2.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–873. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. FASEB J. 2001;15:1804–1806. doi: 10.1096/fj.00-0857fje. [DOI] [PubMed] [Google Scholar]
- 4.Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18:1497–1506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue G, Gaultier A, Li X, et al. Erythropoietin promotes Schwann cell migration and assembly of the provisional extracellular matrix by recruiting beta1 integrin to the cell surface. Glia. 2010;58:399–409. doi: 10.1002/glia.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda S, Nagao M, Takahata K, et al. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- 7.Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 8.Keswani SC, Buldanlioglu U, Fischer A, et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- 9.Campana WM, Li X, Shubayev VI, Angert M, Cai K, Myers RR. Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur J Neurosci. 2006;23:617–626. doi: 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- 10.Byts N, Siren AL. Erythropoietin: a multimodal neuroprotective agent. Exp Transl Stroke Med. 2009;1:4. doi: 10.1186/2040-7378-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YK, Kim G, Park S, et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem Biophys Res Commun. 2012;417:753–759. doi: 10.1016/j.bbrc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Berkingali N, Warnecke A, Gomes P, et al. Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin. Hear Res. 2008;243:121–126. doi: 10.1016/j.heares.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Cervellini I, Annenkov A, Brenton T, Chernajovsky Y, Ghezzi P, Mengozzi M. Erythropoietin (EPO) increases myelin gene expression in CG4 oligodendrocyte cells through the classical EPO receptor. Mol Med. 2013;19:223–229. doi: 10.2119/molmed.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HY, Moon C, Kim KS, et al. Recombinant human erythropoietin in amyotrophic lateral sclerosis: a pilot study of safety and feasibility. J Clin Neurol. 2014;10:342–347. doi: 10.3988/jcn.2014.10.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenreich H, Fischer B, Norra C, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–2588. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li D, Li Q, et al. Erythropoietin promotes peripheral nerve regeneration in rats by upregulating expression of insulin-like growth factor-1. Arch Med Sci. 2015;11:433–437. doi: 10.5114/aoms.2015.50976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Gao Y, Zhou Y, et al. Localized and sustained delivery of erythropoietin from PLGA microspheres promotes functional recovery and nerve regeneration in peripheral nerve injury. Biomed Res Int. 2015;2015:478103. doi: 10.1155/2015/478103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin ZS, Zhang H, Bo W, Gao W. Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. AJNR Am J Neuroradiol. 2010;31:509–515. doi: 10.3174/ajnr.A1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ. Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am. 2008;90:1644–1653. doi: 10.2106/JBJS.G.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi R, Buyukakilli B, Brines M, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 22.Sunderland S. Nerves and Nerve Injuries. 2 ed. Churchill Livingstone; Edinburgh: 1978. [Google Scholar]
- 23.Seddon HJ. A Classification of Nerve Injuries. Br Med J. 1942;2:237–239. doi: 10.1136/bmj.2.4260.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 25.Erbayraktar S, Grasso G, Sfacteria A, et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci U S A. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varejao AS, Meek MF, Ferreira AJ, Patricio JA, Cabrita AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J Neurosci Methods. 2001;108:1–9. doi: 10.1016/s0165-0270(01)00378-8. [DOI] [PubMed] [Google Scholar]
- 27.Inserra MM, Bloch DA, Terris DJ. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery. 1998;18:119–124. doi: 10.1002/(sici)1098-2752(1998)18:2<119::aid-micr10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Sun B, Yu Z, An J, Liu Q, Ren T. High dose erythropoietin promotes functional recovery of rats following facial nerve crush. J Clin Neurosci. 2009;16:554–556. doi: 10.1016/j.jocn.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Podhajsky RJ, Myers RR. The vascular response to nerve crush: relationship to Wallerian degeneration and regeneration. Brain Res. 1993;623:117–123. doi: 10.1016/0006-8993(93)90018-i. [DOI] [PubMed] [Google Scholar]