Abstract
Rationale
Whereas reward-modulatory opioid actions have been intensively studied in subcortical sites such as the nucleus accumbens (Acb), the role of cortical opioid transmission has received comparatively little attention.
Objectives
To describe recent findings on the motivational actions of opioids in the prefrontal cortex (PFC), emphasizing studies of food motivation and ingestion. PFC-based opioid effects will be compared/contrasted to those elicited from the Acb, to glean possible common functional principles. Finally, the motivational effects of opioids will be placed within a network context involving the PFC, Acb, and hypothalamus.
Results
Mu-opioid receptor (μ-OR) stimulation in both the Acb and PFC induces eating and enhances food-seeking instrumental behaviors; μ-OR signaling also enhances taste reactivity within a highly circumscribed zone of medial Acb shell. In both the Acb and PFC, opioid-sensitive zones are aligned topographically with the sectors that project to feeding-modulatory zones of the hypothalamus, and intact glutamate transmission in the lateral/perifornical (LH-PeF) hypothalamic areas is required for both Acb- and PFC-driven feeding. Conversely, opioid-mediated feeding responses elicited from the PFC are negatively modulated by AMPA signaling in the Acb shell.
Conclusions
Opioid signaling in the PFC engages functionally opposed PFC→hypothalamus and PFC→Acb circuits, which respectively drive and limit non-homeostatic feeding, producing a disorganized and ‘fragmented’ pattern of impulsive food-seeking behaviors and hyperactivity. In addition, opioids act directly in the Acb to facilitate food motivation and taste hedonics. Further study of this cortico-striato-hypothalamic circuit, and incorporation of additional opioid-responsive telencephalic structures, could yield insights with translational relevance for eating disorders and obesity.
Introduction
In the spirit of this Special Issue, we discuss a topic that featured prominently in the acclaimed and highly impactful scientific career of the late Dr. Athina Markou: the neural substrates underlying reward function. Although Dr. Markou’s interests cut across multiple domains of biological psychiatry, her work was unified by the idea that studying central reward function (often using the highly adaptable and informative brain stimulation-reward technique) could provide a ‘window’ into challenging questions regarding the affective components of drug reward, withdrawal, or psychiatric conditions such as schizophrenia. Here, we explore the network mechanisms underlying the feeding-modulatory actions of telencephalic opioids. Neither of these subjects (feeding or opioids) were particular foci of Dr. Markou’s research. Nevertheless, we hope that this discussion can enhance understanding of general principles by which telencephalic networks modulate motivational function, which may have broad relevance to the issues that Dr. Markou studied so productively and successfully in her career.
Feeding-Modulatory Opioid Actions in the Acb
It has long been hypothesized that endogenous opioid function modulates some aspect of food reward, possibly the hedonic experience of eating preferred foods. Early studies showed that opioid receptor blockade reduces the perceived pleasantness of palatable foods, without significantly altering feelings of hunger, basic taste perception, or taste intensity (Drewnowski et al. 1992; Fantino et al. 1986; Yeomans and Gray 1996). Relatedly, systemic treatment with opioid agonists or antagonists in rats selectively increase, or decrease, respectively, consumption of palatable solutions and diets preferentially over standard chow (Apfelbaum and Mandenoff 1981; Cooper 1983; Giraudo et al. 1993; Levine et al. 1982), and systemic opioid antagonist administration was found to prevent the formation and expression of taste preferences (Cooper 1983; Cooper and Turkish 1989; Evans and Vaccarino 1990; Lynch 1986). Accordingly, Levine and colleagues demonstrated that the efficacy of naloxone at reducing food intake (1) is inversely related to the level of food deprivation the animal is subjected to (Levine et al. 1995; Rudski et al. 1994; Weldon et al. 1996), and (2) is dependent upon individual dietary preferences (Glass et al. 1996). Because food deprivation enhances palatability and food-reward valuation (Berridge 1991; Cabanac and Duclaux 1973; Cameron et al. 2014), both findings could be interpreted as indicating that endogenous opioid function modulates of the rewarding impact of the food.
Attempts to localize feeding-modulatory opioid actions in the brain have revealed opioid-responsive sites at neural levels ranging from the cortex to the brainstem (Giraudo et al. 1998; Kim et al. 2004; Leibowitz and Hor 1982; Mena et al. 2011; Wilson et al. 2003; Woods and Leibowitz 1985; Zhang and Kelley 2000). Among the most extensively studied site of feeding-modulatory opioid actions is the Acb, and drug manipulations in this structure have shed some light on the distinct motivational processes that contribute to the opioid modulation of feeding. Early studies found that intra-Acb morphine infusions (injections were located in the medial core) increased food intake, and that naloxone reversed this effect (Majeed et al. 1986; 1986). Furthermore, intra-Acb μ-OR stimulation increased intake of palatable solutions, regardless of taste modality or caloric content (sucrose, saccharin, or saline solutions) (Zhang and Kelley 2002), and a study employing specially formulated diets in which flavor varied but macronutrient content was held constant showed that intra-Acb μ-OR blockade reduced intake of the flavor preferred at baseline (Woolley et al. 2006). These findings converge on the interpretation that endogenous opioid function (at least in the Acb) modulates the rewarding impact of intrinsically preferred foods or tastes, rather than selectively affecting a particular taste, orosensory characteristic, or post-ingestive consequence.
In this context, is important to note that ‘reward’ is the emergent property of several inter-related yet partly dissociable processes: the learning and subsequent assignment of motivational significance to cues and goal-objects in the environment, ‘energizing’ of approach behaviors and instrumental acts directed at those cues, and generation of a positively valenced affective state during commerce with those cues and goal objects (Baldo et al. 2013; Berridge 2004; Salamone et al. 2007). Incentive-motivation theory, for example, posits that the feeding central motivational state (CMS) consists of multiple parallel process working in tandem to produce coherent behavioral sequences; these include ‘instrumental’ seeking-type processes that increase the likelihood of goal attainment, and ‘transactional’ processes (e.g., commerce with food) (Bindra 1974; Konorski 1967; Toates 1986). Relatedly, ethologically based frameworks propose distinctions between ‘preparatory/approach’ vs. ‘consummatory’ behaviors (Ball and Balthazart 2008; Craig 1917; Ikemoto and Panksepp 1999). These functions are highly interrelated, and they function cooperatively in the healthy brain to enable reward learning, generate adaptive goal-directed behavior, and facillitate the expression of basic consummatory action patterns when and where appropriate. Yet, evidence has accrued that these functional domains are mediated by partly dissociable neuromodulator systems and pathways. Some of this evidence has emerged from the analysis of similarities and differences in Acb-based opioid and dopaminergic actions upon various indices of food-reinforced behavior and unconditioned reactions to tasteants (Baldo et al. 2013; Barbano and Cador 2006; 2007). Intra-Acb dopamine manipulations, for example, are less effective at altering unconditioned, low-effort responses (e.g., simple ingestive behaviors or taste reactions), relative to conditioned food anticipation, cue-driven approach, hyperactivity, or effortful food-seeking actions (Baldo and Kelley 2007; Berridge 2007; Salamone et al. 2007). Rats treated with dopamine receptor antagonists or dopamine lesions in the Acb will display markedly diminished general activity in the presence of food and less switching among competing behaviors, while total food intake itself is unaffected (Bakshi and Kelley 1991; Baldo et al. 2002). Relatedly, Salamone and colleagues have shown in a variety of tasks that dopamine depletion in the Acb produces shifts in choice towards less effortful food-seeking behaviors, although overall food intake is unchanged (Aberman and Salamone 1999; Nowend et al. 2001; Salamone et al. 1994). The fact that intake itself is unchanged suggests that the ‘consummatory’ motivational component (involving commerce with the food) remains relatively intact. This conclusion is further supported by the observation that Acb dopamine depletion does not eliminate orofacial taste reactions to passively infused sucrose solutions, an index of the hedonic evaluation of foods and tasteants (Berridge et al. 1989).
Acb-based opioid systems, on the other hand, appear to play a role not only in invigorating instrumental behaviors, but also enhancing the primary rewarding aspects of eating. Systemic morphine increases and naloxone decreases the number of evoked hedonic taste responses to sweet sucrose solutions (Doyle et al. 1993; Parker et al. 1992; Pecina and Berridge 1995; Rideout and Parker 1996) and suppresses aversive reactions to bitter quinine solutions (Clarke and Parker 1995; Parker et al. 1992). It has been argued that these stereotyped, cross-species reactions to pleasant and unpleasant tastes are the manifestation of an internal evaluation of the hedonic quality of a taste stimulus (Grill and Norgren 1978). Meticulous mapping studies employing local μ-OR agonist injections coupled to the analysis of resultant ‘plumes’ of Fos expression have revealed a highly circumscribed area in the medial AcbSh where μ-OR stimulation augments hedonic-like taste reactions (Pecina and Berridge 2005). Nevertheless, a wider zone, extending into the medial core, was found to mediate μ-OR-driven hyperphagia but not the enhancement of hedonic taste reactions. Furthermore, intra-Acb core μ-OR stimulation augments progressive-ratio (PR) responding for sucrose (a schedule in which progressively more responses are required for each successive reinforcer) (Zhang et al. 2003), and opioid stimulation of either the Acb core or shell facilitates sucrose-associated Pavlovian-to-Instrumental Transfer (PIT), a reflection of the underlying process by which Pavlovian learning invigorates goal-seeking actions, (Pecina and Berridge 2013). Opioids influence reward-seeking behavior through interactions with the mesolimbic dopamine system (Fields and Margolis 2015; Zheng et al. 2007); however, unlike dopamine manipulations, μ-OR stimulation also facilitates hedonic reactions to taste. In the incentive-salience framework proposed by Berridge and Robinson (Berridge 2009; Berridge and Kringelbach 2015; Robinson and Berridge 2001), dopamine facilitates the ‘wanting’ of rewards, whereas opioids facilitate both ‘wanting’ and ‘liking’, although the ‘liking’ function is highly restricted to the anterior medial AcbSh.
This anatomical heterogeneity across sectors of the Acb agrees with the more general observation that there are gradients of opioid sensitivity spanning the entire striatal complex. Early morphine microinfusion mapping studies revealed an anatomical gradient of opioid-induced feeding, with strong hyperphagia evoked by infusion of D -Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin (DAMGO), a specific μ-OR-subtype agonist, into the Acb and weaker responses in more dorsal, lateral, and posterior infusion sites (Bakshi and Kelley 1993). The most effective site for eliciting feeding was an area spanning the lateral aspects of the Acb core and the medial aspects of the shell. Nevertheless, opioid-driven feeding effects were not restricted to the Acb. Morphine infusions into the ventral aspects of dorsal and medial striatum also elicited feeding, albeit to a lesser degree compared to the Acb. A later microinfusion-mapping study using DAMGO confirmed that the strongest opioid-driven food-intake effects were elicited from the Acb core (Zhang and Kelley 2000). Significant effects were also observed with placements in the ventrolateral striatum and in the lateral core, and even in dorsal striatum (although opioid effects in this latter site were less consistent across subjects). Accordingly, a recent peptide-microdialysis study confirmed that enkephalins are released into the extracellular space of the anterior medial dorsal striatum during palatable feeding, and that DAMGO infusion into this striatal zone elicits palatable feeding (DiFeliceantonio et al. 2012).
To summarize, the studies reviewed above suggest a sensitivity gradient for opioid-modulated behavioral functions spanning the anterior medial AcbSh to the dorsal striatum. This gradient is characterized by several features. First, food ingestion itself can be elicited by μ-OR agonist injections within a wide zone centered on the Acb core/shell boundary, but including lateral aspects of the core, shell, and the ventrolateral striatum. Opioid-driven feeding responses can also be elicited from select areas in dorsal striatum; these effects are more inconsistent and less robust relative to Acb-mediated effects. As injection placements move caudally away from ventromedial and ventrolateral striatum, the magnitude of opioid-driven feeding effects diminishes. Second, there appears to be an anatomical segregation of function with regard to opioid-driven pursuit of food goals and modulation of incentive salience (e.g., enhancement of progressive ratio responding and PIT) vs. opioid modulation of taste reactivity. The former can be elicited from sites both in the Acb core and medial shell, whereas the latter appears to be tightly restricted to the anterior medial shell. To the authors’ knowledge, there have been no mapping studies of opioid-modulated operant responding, PIT, or taste reactivity focusing on dorsal striatum; this represents an interesting direction for future research, particularly considering the convergent microinfusion and microdialysis data indicating that there may be an important opioid-sensitive feeding zone in the anterior medial dorsal striatum.
Feeding-Modulatory Opioid Actions in the PFC
Compared to the Acb, far less is known regarding the behavioral mechanisms underlying feeding-modulatory opioid actions in the PFC. Evidence thus far indicates that μ-OR stimulation in ventromedial sectors of frontal cortex induces a robust feeding central motivational state (CMS), although the mechanistic details of this state are not fully understood. μ-OR stimulation in the ventromedial prefrontal cortex (vmPFC), mainly the infralimbic region, engenders feeding both in food-deprived and ad libitum-maintained rats (Mena et al. 2011); the organization of this feeding response consists of brief feeding bouts, and abrupt switching between food-directed responses and responses directed away from food (e.g., ‘exploratory-like’ ambulatory or rearing behaviors). This pattern is essentially the opposite of that engendered by GABA-mediated inactivation of the vmPFC (i.e., longer feeding bouts and less ambulation and rearing) (Baldo et al. 2016; Mena et al. 2011), suggesting that the net behaviorally relevant effect of μ-OR stimulation is to activate or disinhibit cortical output. Presently, the role of μ-ORs in regulating pyramidal neuron activity (and thus cortical output) is not well understood. It is interesting to note that μ-ORs mediate hippocampal pyramidal neuron disinhibition by suppressing local inhibitory interneurons (McQuiston and Saggau 2003; Zieglgansberger et al. 1979). These hippocampal interneurons are similar to μ-OR-bearing interneurons in cortex (Curley and Lewis 2012; Ferezou et al. 2007; Krook-Magnuson et al. 2011; Taki et al. 2000), lending some plausibility to the idea that cortical μ-OR stimulation may have net disinhibitory or activational effects upon cortical output.
In addition to driving feeding itself, μ-OR stimulation in vmPFC also robustly amplifies responding in a sucrose-reinforced progressive ratio (PR) task and promotes ‘impulsiveness-like’ deficits in differential reinforcement of low response rates task (DRL) (Selleck et al. 2015). Together, these effects indicate that cortical μ-OR stimulation increases the motivational value of food and energizes food-seeking repertoires, as well as disrupting inhibitory control over food-seeking. The precise mechanisms underlying these effects are unclear, but a number of possibilities come to mind. For example, because electrophysiological studies have suggested that units in vmPFC encode information regarding food-associated taste characteristics (including taste hedonics) and reward valuation (Jezzini et al. 2013; Parent et al. 2015), the intra-PFC μ-OR-mediated increase in food intake could reflect an enhancement of the hedonic properties of the food. Nevertheless, the μ-OR stimulation-induced pattern of bout initiation coupled with the shortening of individual bouts indicates that commerce with the food does not sustain the relatively longer periods of consumption that might be expected with increased gustatory reward (Davis and Smith 1992; Ostlund et al. 2013; Spector et al. 1998). This could be interpreted as enhanced salience of the sucrose incentive in the absence of hedonic taste facilitation (‘wanting’ in the absence of increased ‘liking’). In this regard, an important question for future research is whether intra-vmPFC μ-OR stimulation modulates taste reactions to sucrose.
Alternatively, the changes in feeding-bout microstructure described above could reflect opioid-mediated perturbation of underlying response-selection functions of the PFC. In a general sense, medial PFC plays a prominent role in modulating ongoing behavior, and inhibiting disadvantageous behavior, to match prevailing (and often changing) contingencies; examples include the regulation of set-shifting (Birrell and Brown 2000; Dalley et al. 2004; Floresco et al. 2008; Ragozzino et al. 1999) and the expression of extinction learning (Eddy et al. 2016; Peters et al. 2008; Quirk et al. 2006; Rhodes and Killcross 2007). With regard to ingestive behaviors, recent findings that inhibition of ventromedial PFC disturbs the temporal distribution of licking bouts in an incentive-contrast paradigm, ‘misaligning’ licking bout durations to high and low concentrations of sucrose (Parent et al. 2015). This finding suggests a PFC-based operation that matches the temporal duration of licking bouts with the reward value of the food. It is interesting to hypothesize that, in a free-feeding context, this ‘supervisory’ operation aligns the temporal duration of consummatory responses with contingencies of taste-reward valuation and the need for periodic environmental reconnaissance, enabling flexible, adaptive switching between the two competing response sets. Such a function would serve to keep food-directed and non-food-directed repertoires in balance, thereby optimizing intake while minimizing risk (Blanchard and Blanchard 1989; Dukas 2002; Krebs et al. 1996; Krebs et al. 1997; Onuki and Makino 2005). This purported switching function, combined with a facilitation of the incentive value of food (as reflected in the abovementioned effects on PR and DRL performance), could produce the observed μ-OR stimulation-induced changes in feeding approach and bout duration. As will be discussed below, it is possible that the modulation of and switching between food-directed vs. non-food-directed activity by PFC-based μ-ORs reflects the recruitment of distinct PFC efferent pathways.
From an anatomical perspective, recent studies have begun to map the effects of intra-tissue infusions of a μ-OR agonist in order to determine whether, as in striatum, there are heterogeneities in opioid sensitivity across different regions of frontal cortex. First, to define the basic effect, the μ-OR agonist, DAMGO, was infused directly into vmPFC. In this initial experiment, injections were placed near the dorsal border of infralimbic cortex, which some sites crossing into the ventral aspect of prelimbic cortex (Mena et al. 2011). These infusions dose-dependently enhanced food intake in both food-deprived and ad libitum-maintained rats. A subsequent mapping experiment revealed that DAMGO infusions in the ad libitum condition also enhanced food intake when injections were placed in medial aspects of subgenual orbitofrontal cortex. DAMGO effects were weaker when infusions with infusions sited more dorsally in the medial wall (i.e., anterior cingulate cortex), dorsolaterally in anterior somatosensory cortex, or laterally in orbitofrontal cortex (i.e., lateral subgenual orbital cortex, anterior aspects of insular cortex)(Mena et al. 2011). Hence, a gradient of μ-opioid sensitivity is apparent in frontal cortex. The strongest feeding-modulatory sites are located in a ventromedial zone comprising parts of medial PFC and orbitofrontal cortex; as infusions move dorsally and laterally from this zone, progressively weaker effects are observed. Presently, it is unknown whether opioid modulation of PR or DRL can be elicited from frontal territories beyond the infralimbic cortex. This represents an important area for future research.
Motivational effects of telencephalic opioids: a cortico-striato-hypothalamic network model
Consideration of the topographic organization of cortical and striatal gradients of opioid sensitivity suggest possible efferent pathways through which telencephalic opioids modulate appetitive motivation. First, the frontal cortical sites from which the strongest μ-OR-induced feeding responses can be elicited are clustered in a ‘ventromedial corridor’ consisting of sites both in medial and orbitofrontal cortex that innervate opioid-responsive zones in the Acb and dorsal striatum (Heilbronner et al. 2016; Schilman et al. 2008; Thompson and Swanson 2010; Vertes 2004). In particular, the infralimbic area projects strongly to the Acb shell (Heilbronner et al. 2016; Thompson and Swanson 2010), including the anteromedial zone that plays a specialized role in mediating taste hedonics (Pecina and Berridge 1995). Opioid-sensitive sites in frontal cortex have also been shown to project to the hypothalamus, including lateral and perifornical areas of tuberal hypothalamus from which intense feeding responses can be elicited by local infusions of glutamate agonists or neuropeptide Y (Floyd et al. 2001; Gabbott et al. 2005; Reppucci and Petrovich 2016; Vertes 2004). Similarly to the ventromedial PFC, the medial AcbSh projects to feeding-modulatory areas of hypothalamus both directly and indirectly via the ventral pallidum (Groenewegen et al. 1993; Haber et al. 1985; Heimer et al. 1991; Mogenson et al. 1983).
The anatomical relationships described above suggest a circuit for higher-order control of feeding behavior, consisting of telencephalic nodes in ventromedial frontal cortex and medial Acb shell outputting to a dienephalic node in tuberal hypothalamus. The functional relevance of this circuit has been confirmed in studies employing drug microinfusions and the analysis of immediate-early gene expression to ‘dissect’ distinct pathways among those sites. Early studies focused on the functional relationship between the medial AcbSh ‘feeding hotspot’ and the lateral hypothalamus in the control of food intake. Kelley and colleagues performed a series of dual-site microinfusion studies in rats in which the AcbSh and hypothalamus (in a zone spanning lateral and perifornical areas of tuberal hypothalamus; LH-PeF) were jointly targeted with infusion cannulae. Feeding responses were elicited either by AMPA receptor blockade, GABA receptor stimulation, or μ-OR stimulation in the Acb; in the same animal, a GABA agonist or glutamate antagonist was concurrently infused into the LH-PeF (Maldonado-Irizarry et al. 1995; Stratford and Kelley 1999; Will et al. 2003). For each orexigenic manipulation of the Acb, it was found that reducing neural activity in the hypothalamus (via either glutamate blockade or GABA stimulation) eliminated the Acb-mediated hyperphagia, indicating that intact hypothalamic function is necessary for the expression of Acb-driven hyperphagia. This conclusion is further bolstered by recent optogenetic studies showing that silencing the Acb shell increases consumption, stimulating that region decreases consumption, and that these effects are mediated through projections of D1-bearing Acb neurons projecting to the hypothalamus (O’Connor et al. 2015; Parent et al. 2015). Further work indicated that either GABA receptor or μ-OR stimulation in the Acb shell provoked expression of the immediate-early gene, Fos, in several hypothalamic regions, including the LH-PeF (Baldo et al. 2004; Stratford and Kelley 1999; Zhang and Kelley 2000). Immunohistochemical co-labeling studies indicated that intra-Acb GABA-ergic or μ-OR manipulations provoke Fos expression in orexigenic neuronal populations including hypocretin/orexin (H/O)-containing cells in the LH-PeF (Baldo et al. 2004; Zheng et al. 2003). It is important to note that, in the abovementioned Fos mapping study, a number of sites in addition to the hypothalamus were activated by intra-Acb DAMGO. These included the ventral tegmental area (VTA) and nucleus of the solitary tract (NTS) (Zhang and Kelley 2000). Accordingly, local GABA-mediated inactivation of the VTA or NTS blocked intra-Acb DAMGO-induced amplification of sweetened-fat intake (Will et al. 2003). It is unknown whether Acb interactions with these mesencephalic and brainstem sites are enacted mainly through direct projections or through a hypothalamic relay. One study has identified a serial relationship among the Acb, to H/O-expressing hypothalamic neurons, to the VTA mediating intra-Acb DAMGO-driven feeding (Zheng et al. 2007). It is certainly possible that both serial and parallel projections are involved; pathway-specific optogenetic or chemogenetic manipulations could shed further light on this issue.
Recent findings have also demonstrated a role for a functional interaction between PFC and hypothalamus in the control of feeding. Infusions of the μ-OR agonist, DAMGO, directly into the vmPFC (infralimbic and ventral prelimbic territories) induced Fos expression in the LH-PeF, including within a group of medially-localized H/O-containing cells (Mena et al. 2013). This finding suggests that intra-vmPFC DAMGO activates neurons in this hypothalamic area, possibly via glutamatergic afferents arriving from the vmPFC. Evidence for glutamate involvement in this functional relationship between the PFC and hypothalamus was provided by the finding that hyperphagia induced by intra-vmPFC DAMGO was reversed by intra-LH-PeF infusions of low doses of the glutamate NMDA receptor subtype antagonist, AP-5 (Mena et al. 2013). The LH-PeF subregion targeted in this study is similar to the zone from which strong neuropeptide-Y-induced feeding responses have been reported (Stanley et al. 1993), and also to the area where local inactivation or glutamate receptor blockade reduces hyperphagia induced by intra-Acb μ-OR stimulation (Maldonado-Irizarry et al. 1995; Stratford and Kelley 1999). Further evidence for a PFC-hypothalamus functional relationship is provided by studies examining the control of Pavlovian conditioned cues over food consumption. Displaying a stimulus previously paired with hunger-driven hyperphagia causes a subsequent increase in food intake in sated rats. Examination of activity-dependent gene expression during cue-induced overeating revealed activation in PFC and amygdalar inputs to the hypothalamus (as defined by labeling from a retrograde tracer placed in the hypothalamus) (Petrovich et al. 2005). Interestingly, the AcbSh→hypothalamus projection did not seem to be involved. A lesion study confirmed that the PFC is required for the expression of cue-induced overeating (Petrovich et al. 2007). Hence, the hypothalamus appears to be an output node not only for drug-induced hyperphagia elicited from the Acb or PFC, but also for higher-order computations relevant for the modulation of intake by learned cues.
The vmPFC→AcbSh projection also modulates feeding, but in the opposite direction. Thus, bicuculline-induced disinhibition in infralimbic cortex limits feeding responses engendered by intra-AcbSh AMPA blockade (Richard and Berridge 2013), and intra-Acb shell neural activation, including that engendered by AMPA receptor stimulation (Stratford et al. 1998), electrical stimulation (Krause et al. 2010), or optogenetic activation (O’Connor et al. 2015) arrests feeding and provokes competing behaviors such as intense motor activity (Ikeda et al. 2003). These results suggest a complex, glutamate-coded functional relationship among the PFC, Acb, and LH-PeF, whereby PFC-driven feeding is mediated by glutamate transmission in the LH-PeF, but negatively modulated by AMPA signaling in the Acb. These results can be interpreted as reflecting the activity of a cortico-striato-hypothalamic circuit, consisting of functionally opposed PFC and AcbSh efferents converging on a hypothalamus-based output node. It has been hypothesized that these putative PFC→hypothalamus ‘feeding driver’ and PFC→AcbSh ‘feeding limiter’ pathways counterbalance one another to maintain food-directed activity within adaptive limits. It is not yet known, however, to what extent these functional relationships are driven by monosynaptic glutamatergic projections originating from the PFC vs. polysynaptic routes of control; such a determination awaits the application of optogenetic or chemogenetic manipulations to dissect the individual pathways.
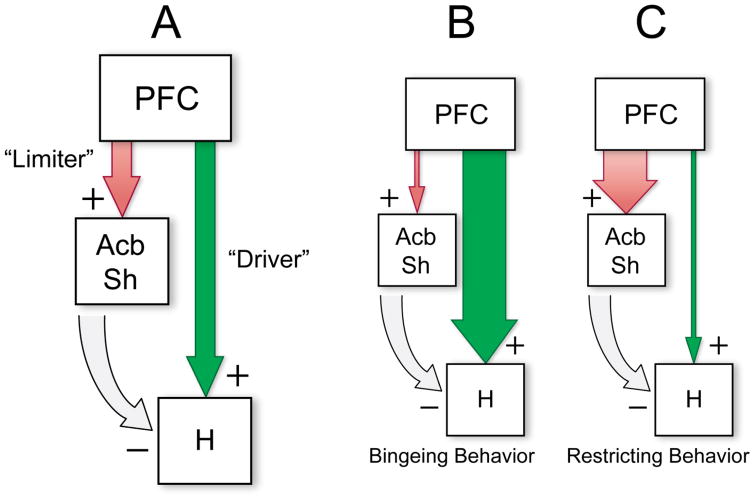
To summarize the major tenets of the network model, μ-OR activity in the PFC or Acb appears to drive feeding via an obligatory output node in the PeF-LH, albeit by distinct mechanisms. Glutamatergic PFC projections stimulate hypothalamic feeding systems, while the inhibition of GABA-ergic Acb efferents disinhibits those systems. Conversely, activation of the Acb via AMPA receptor stimulation (possibly arising from glutamatergic PFC projections) inhibits feeding, partly through descending inhibitory control over the hypothalamus, but also through the recruitment of non-food-directed behaviors. The incoherent engagement of these parallel ‘feeding-driver’ and ‘feeding-limiter’ pathways by opioid signaling could result in disorganized, impulsive food-seeking behaviors, such as those engendered by μ-OR stimulation in ventromedial PFC (Mena et al. 2011; Selleck et al. 2015). Fig. 1 shows a schematic summarizing this model, along with possible feeding pathologies arising from different types of network dysfunction.
Fig. 1.
Schematic depicting the proposed cortico-striato-hypothalamic feeding-modulatory network, along with possible feeding pathologies arising from different types of network dysfunction. Panel (A) shows the underlying organization of prefrontal cortical (PFC) projections to the nucleus accumbens shell (AcbSh) and feeding circuits in the hypothalamus (H). Excitatory PFC→AcbSh glutamatergic projections (indicated by ‘+’ signs) acting through AMPA-type receptors act as a “limiter circuit”, restraining bouts of consummatory activity. PFC→H projections elicit feeding, acting as a “driver circuit” that can be engaged by frontal activation, including that associated with local opioid release. The AcbSh, in turn, sends an inhibitory GABA-ergic projection (‘−’ sign) to the H. Panel (B) displays possible network alterations that would be predicted to cause bingeing behavior. These alterations include increased activity in the ‘driver’ pathway, and/or diminished function of the ‘limiter’ pathway. Influence of these pathways over their respective terminal fields is depicted by the width of the arrows. The opposite changes (i.e., overactive ‘limiter’ and/or underactive ‘driver’ pathways) would be expected to result in abnormal restriction of feeding behavior, as depicted in panel (C).
Finally, it is important to consider how additional opioid-responsive telencephalic sites can be incorporated into this cortico-striato-hypothalamic network hypothesis. Based upon hodological considerations and functional evidence, the amygdala is a prime candidate. This structure sends robust projections to Acb, PFC, and hypothalamus, and hence is positioned to modulate PFC and Acb directly as well as to enact parallel regulation of Acb- and PFC-innervated zones of hypothalamus (Reppucci and Petrovich 2016). Sites within the amygdaloid complex support μ-OR-induced effects on feeding and food motivation; for example, μ-OR stimulation in the central amygdaloid region (CeA) causes hyperphagia (Kim et al. 2004) and strongly amplifies the activational effects of Pavlovian cues over food approach and other instrumental actions (Mahler and Berridge 2012). Furthermore, intra-CeA μ-OR stimulation-induced hyperphagia appears to interact in a complex, reciprocal way with opioid function in the Acb, as evidenced by the finding that opioid receptor blockade in amygdalar sites blocks opioid-driven feeding elicited from the Acb, and vice-versa (Kim et al. 2004). Along with the CeA, the basolateral area of the amygdala (BLA) plays an obligatory role in the amplification of palatable feeding induced by intra-Acb μ-OR stimulation (Parker et al. 2015; Will et al. 2004). Thus, pharmacological inactivation of either BLA or CeA eliminated the increase in sweetened-fat intake induced by intra-Acb infusions of DAMGO, without suppressing baseline levels of intake (Will et al. 2004). Finally, interactions between the PFC and amygdala may also participate in the regulation of food motivation. For example, a recent study demonstrated that optogenetic activation of PFC projections to the basolateral amygdala enhances feeding (Land et al. 2014). Finally, Pavlovian cue-induced overeating recruits both BLA→ hypothalamus and PFC→hypothalamus pathways (Petrovich et al. 2005), suggesting a route through which BLA and PFC processing can converge on a common hypothalamic output node.
Together, the studies discussed above suggest multiple pathways through which amygdalar processing can integrate with the feeding ‘driver’ and ‘limiter’ circuits described above, including but not limited to: (1) parallel convergence onto a common hypothalamic effector node; (2) regulation of opioid responses at the level of the Acb and/or PFC, either via monosynaptic projections or multi-step pathways through intermediaries such as orexin neurons or VTA dopamine neurons; (3) descending control of amygdala function by the PFC. Teasing these network interactions apart using contemporary optogenetic and chemogenetic approaches represents an exciting direction for future research.
Clinical Implications
Aberrant activity prefrontal cortex and nucleus accumbens contributes to deficits in impulse control in a number of psychiatric disorders characterized by excessive appetitive motivation, including disorders of food intake such as binge eating disorder (BED) (Dong et al. 2016; Karhunen et al. 2000; Schienle et al. 2009; Seo et al. 2013; Uher et al. 2004). Several studies have suggested that these deficits arise from supernormal opioid transmission (Blasio et al. 2014; Gorelick et al. 2008; Love et al. 2009; Mitchell et al. 2012; Morganstern et al. 2012; Selleck et al. 2015; Zubieta et al. 1996), and these studies are supported by clinical findings that opioid antagonists have at least some degree of efficacy across several disorders characterized by loss of control over goal-seeking behavior (Cambridge et al. 2013; Kim et al. 2001; Mitchell et al. 2007; Volpicelli et al. 1992). However, there is variability in the reports of opiate antagonist clinical efficacy (McElroy et al. 2013; Ziauddeen et al. 2013), suggesting that further studies are needed to more thoroughly delineate opioid actions within the brain and how normal brain function is influenced by opioid antagonists. The network model outlined in this article suggest that using poly-drug approaches may enhance the efficacy of opiate antagonists in treating disorders such as BED, as well as other conditions such as alcohol dependence, for which opioid antagonists represent one of the only FDA-approved treatments (Pettinati et al. 2006; Soyka and Rosner 2008; Volpicelli 1995). Because nodes in the network have been specified neurochemically (i.e.,as described previously, μ-ORs in the PFC and Acb, as well as other sites including the CeA; orexin systems in the LH-PeF, AMPA receptors in the Acb shell), it is possible to identify combinations of treatments that together could have an additive or super-additive effect on network function. Specifically, co-administering opioid antagonists with treatments that target downstream nodes of the network (for example, orexin systems in the hypothalamus) may prove more effective than opioid antagonists alone. It has been suggested, for example, that orexin manipulations could represent an effective treatment for conditions such as drug addiction or relapse (Plaza-Zabala et al. 2012; Zhou et al. 2011) (Picetti et al. 2013). More generally, future studies aimed at enhancing our understanding of the neural networks through which opioids exert reward-modulatory effects will be crucial for developing better treatments for a wide variety of disorders, including drug dependence and withdrawal, psychiatric conditions that were the focus of Dr. Athina Markou’s career.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–52. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Apfelbaum M, Mandenoff A. Naltrexone suppresses hyperphagia induced in the rat by a highly palatable diet. Pharmacol Biochem Behav. 1981;15:89–91. doi: 10.1016/0091-3057(81)90344-0. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Dopaminergic Regulation of Feeding-Behavior 1. Differential-Effects of Haloperidol Microinfusion into 3 Striatal Subregions. Psychobiology. 1991;19:223–232. [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993;111:207–14. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–86. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–59. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013;37:1985–98. doi: 10.1016/j.neubiorev.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–77. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Spencer RC, Sadeghian K, Mena JD. GABA-Mediated Inactivation of Medial Prefrontal and Agranular Insular Cortex in the Rat: Contrasting Effects on Hunger- and Palatability-Driven Feeding. Neuropsychopharmacology. 2016;41:960–70. doi: 10.1038/npp.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008;53:307–11. doi: 10.1016/j.yhbeh.2007.09.023. author reply 315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–81. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–20. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–64. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103:36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol. 2014;19:652–62. doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M, Duclaux R. Olfactory-gustatory alliesthesia and food intake in humans. J Physiol (Paris) 1973;66:113–35. [PubMed] [Google Scholar]
- Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR, Koch A, Maltby K, Skeggs AL, Napolitano A, Farooqi IS, Bullmore ET, Fletcher PC. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry. 2013;73:887–94. doi: 10.1016/j.biopsych.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JD, Goldfield GS, Finlayson G, Blundell JE, Doucet E. Fasting for 24 hours heightens reward from food and food-related cues. PLoS One. 2014;9:e85970. doi: 10.1371/journal.pone.0085970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SN, Parker LA. Morphine-induced modification of quinine palatability: effects of multiple morphine-quinine trials. Pharmacol Biochem Behav. 1995;51:505–8. doi: 10.1016/0091-3057(95)00042-u. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology. 1983;22:323–8. doi: 10.1016/0028-3908(83)90247-2. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Turkish S. Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol Biochem Behav. 1989;33:17–20. doi: 10.1016/0091-3057(89)90422-x. [DOI] [PubMed] [Google Scholar]
- Craig W. Appetites and Aversions as Constituents of Instincts. Proc Natl Acad Sci U S A. 1917;3:685–8. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590:715–24. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–28. [PubMed] [Google Scholar]
- DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC. Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol. 2012;22:1918–24. doi: 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Jackson T, Chen S, Wang Y, Zhou F, Chen H. Impulse control and restrained eating among young women: Evidence for compensatory cortical activation during a chocolate-specific delayed discounting task. Appetite. 2016 doi: 10.1016/j.appet.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46:745–9. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav. 1992;51:371–9. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- Dukas R. Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond B Biol Sci. 2002;357:1539–47. doi: 10.1098/rstb.2002.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MC, Todd TP, Bouton ME, Green JT. Medial prefrontal cortex involvement in the expression of extinction and ABA renewal of instrumental behavior for a food reinforcer. Neurobiol Learn Mem. 2016;128:33–9. doi: 10.1016/j.nlm.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev. 1990;14:9–22. doi: 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–6. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex. 2007;17:1948–57. doi: 10.1093/cercor/bhl104. [DOI] [PubMed] [Google Scholar]
- Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–25. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–28. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone’s anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav. 1993;46:917–21. doi: 10.1016/0091-3057(93)90222-f. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and nucleus of the solitary tract in mu-opioid induced feeding in the rat. Brain Res. 1998;802:184–8. doi: 10.1016/s0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Grace M, Cleary JP, Billington CJ, Levine AS. Potency of naloxone’s anorectic effect in rats is dependent on diet preference. Am J Physiol. 1996;271:R217–21. doi: 10.1152/ajpregu.1996.271.1.R217. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200:475–86. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–42. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–35. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biol Psychiatry. 2016;80:509–21. doi: 10.1016/j.biopsych.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Akiyama G, Fujii Y, Minowa R, Koshikawa N, Cools AR. Role of AMPA and NMDA receptors in the nucleus accumbens shell in turning behaviour of rats: interaction with dopamine receptors. Neuropharmacology. 2003;44:81–7. doi: 10.1016/s0028-3908(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Mazzucato L, La Camera G, Fontanini A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci. 2013;33:18966–78. doi: 10.1523/JNEUROSCI.2974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI. Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res. 2000;99:29–42. doi: 10.1016/s0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- Kim EM, Quinn JG, Levine AS, O’Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–9. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–21. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain; an interdisciplinary approach. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–56. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H, Macht M, Weyers P, Weijers HG, Janke W. Effects of stressful noise on eating and non-eating behavior in rats. Appetite. 1996;26:193–202. doi: 10.1006/appe.1996.0015. [DOI] [PubMed] [Google Scholar]
- Krebs H, Weyers P, Macht M, Weijers HG, Janke W. Scanning behavior of rats during eating under stressful noise. Physiol Behav. 1997;62:151–4. doi: 10.1016/s0031-9384(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J Neurosci. 2011;31:14861–70. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–53. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3:421–8. doi: 10.1016/0196-9781(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Levine AS, Murray SS, Kneip J, Grace M, Morley JE. Flavor enhances the antidipsogenic effect of naloxone. Physiol Behav. 1982;28:23–5. doi: 10.1016/0031-9384(82)90095-6. [DOI] [PubMed] [Google Scholar]
- Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–52. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–34. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WC. Opiate blockade inhibits saccharin intake and blocks normal preference acquisition. Pharmacol Biochem Behav. 1986;24:833–6. doi: 10.1016/0091-3057(86)90420-x. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221:407–26. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed NH, Przewlocka B, Wedzony K, Przewlocki R. Stimulation of food intake following opioid microinjection into the nucleus accumbens septi in rats. Peptides. 1986;7:711–6. doi: 10.1016/0196-9781(86)90083-5. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, Ehrich EW. A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int J Eat Disord. 2013;46:239–45. doi: 10.1002/eat.22114. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Saggau P. Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol. 2003;90:1936–48. doi: 10.1152/jn.01150.2002. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–60. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33:18540–52. doi: 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32:439–49. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Liang S, Ye Z, Karatayev O, Leibowitz SF. Disturbances in behavior and cortical enkephalin gene expression during the anticipation of ethanol in rats characterized as high drinkers. Alcohol. 2012;46:559–68. doi: 10.1016/j.alcohol.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–24. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–82. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Luscher C. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015;88:553–64. doi: 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Onuki Y, Makino J. Food-carrying behavior increased under risk-approaching signal in rats (Rattus norvegicus) Physiol Behav. 2005;84:141–5. doi: 10.1016/j.physbeh.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Kosheleff A, Maidment NT, Murphy NP. Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology (Berl) 2013;229:105–13. doi: 10.1007/s00213-013-3077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MA, Amarante LM, Liu B, Weikum D, Laubach M. The medial prefrontal cortex is crucial for the maintenance of persistent licking and the expression of incentive contrast. Front Integr Neurosci. 2015;9:23. doi: 10.3389/fnint.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, McCabe MP, Johns HW, Lund DK, Odu F, Sharma R, Thakkar MM, Cornelison DD, Will MJ. Neural activation patterns underlying basolateral amygdala influence on intra-accumbens opioid-driven consummatory versus appetitive high-fat feeding behaviors in the rat. Behav Neurosci. 2015;129:812–21. doi: 10.1037/bne0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 1995;3:269–80. [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37:1529–40. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–41. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–25. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Picetti R, Schlussman SD, Zhou Y, Ray B, Ducat E, Yuferov V, Kreek MJ. Addictions and stress: clues for cocaine pharmacotherapies. Curr Pharm Des. 2013;19:7065–80. doi: 10.2174/13816128113199990610. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Maldonado R, Berrendero F. The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol. 2012;45:424–39. doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2016;221:2937–62. doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25:2498–503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol Psychiatry. 2013;73:360–70. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav. 1996;53:731–4. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rudski JM, Billington CJ, Levine AS. Naloxone’s effects on operant responding depend upon level of deprivation. Pharmacol Biochem Behav. 1994;49:377–83. doi: 10.1016/0091-3057(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–9. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–61. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–5. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, Baldo BA. Endogenous Opioid Signaling in the Medial Prefrontal Cortex is Required for the Expression of Hunger-Induced Impulsive Action. Neuropsychopharmacology. 2015;40:2464–74. doi: 10.1038/npp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–39. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Rosner S. Opioid antagonists for pharmacological treatment of alcohol dependence - a critical review. Curr Drug Abuse Rev. 2008;1:280–91. doi: 10.2174/1874473710801030280. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–94. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s) Brain Res. 1993;604:304–17. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Taki K, Kaneko T, Mizuno N. A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience. 2000;98:221–31. doi: 10.1016/s0306-4522(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A. 2010;107:15235–9. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toates FM. Motivational systems. Cambridge University Press, Cambridge Cambridgeshire; New York: 1986. [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SC, Campbell IC, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR. Naltrexone in alcohol dependence. Lancet. 1995;346:456. doi: 10.1016/s0140-6736(95)91316-5. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Weldon DT, O’Hare E, Cleary J, Billington CJ, Levine AS. Effect of naloxone on intake of cornstarch, sucrose, and polycose diets in restricted and nonrestricted rats. Am J Physiol. 1996;270:R1183–8. doi: 10.1152/ajpregu.1996.270.6.R1183. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–60. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–65. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharmacol Biochem Behav. 1985;23:431–8. doi: 10.1016/0091-3057(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–17. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60:439–46. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–11. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–77. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–44. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun WL, See RE. Orexin Receptor Targets for Anti-Relapse Medication Development in Drug Addiction. Pharmaceuticals (Basel) 2011;4:804–821. doi: 10.3390/ph4060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, Tao WX, Napolitano A, Skeggs AL, Brooke AC, Cheke L, Clayton NS, Sadaf Farooqi I, O’Rahilly S, Waterworth D, Song K, Hosking L, Richards DB, Fletcher PC, Bullmore ET. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry. 2013;18:1287–93. doi: 10.1038/mp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–7. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2:1225–9. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]