Abstract
Rationale
Smoking is the leading cause of preventable death in the U.S., but quit attempts result in withdrawal-induced cognitive dysfunction and predicts relapse. Greater understanding of the neural mechanism(s) underlying these cognitive deficits is required to develop targeted treatments to aid quit attempts.
Objectives
We examined nicotine withdrawal-induced inattention in mice lacking the α7 nicotinic acetylcholine receptor (nAChR) using the 5-choice continuous performance test (5C-CPT).
Methods
Mice were trained in the 5C-CPT prior to osmotic minipump implantation containing saline or nicotine. Experiment 1 used 40 mg/kg/day nicotine treatment and tested C57BL/6 mice 4, 28, and 52 h after pump removal. Experiment 2 used 14 and 40 mg/kg/day nicotine treatment in α7 nAChR knockout (KO) and wildtype (WT) littermates tested 4 h after pump removal. Subsets of WT mice were sacrificed before and after pump removal to assess changes in receptor expression associated with nicotine administration and withdrawal.
Results
Nicotine withdrawal impaired attention in the 5C-CPT, driven by response inhibition and target detection deficits. The overall attentional deficit was absent in α7 nAChR KO mice despite response disinhibition in these mice. Synaptosomal glutamate mGluR5 and dopamine D4 receptor expression were reduced during chronic nicotine but increased during withdrawal, potentially contributing to cognitive deficits.
Conclusions
The α7 nAChR may underlie nicotine withdrawal-induced deficits in target detection but is not required for response disinhibition deficits. Alterations to the glutamatergic and dopaminergic pathways may also contribute to withdrawal-induced attentional deficits, providing novel targets to alleviate the cognitive symptoms of withdrawal during quit attempts.
Keywords: Response inhibition, α7 nicotinic acetylcholine receptor, 5-choice continuous performance task, attention, mGluR5, dopamine D4 receptor
Introduction
Tobacco smoking is the leading cause of premature and preventable death, disease, and disability in the United States (United States Department of Health and Human Services 2014). Although the percentage of U.S. adults who smoke cigarettes has declined from 20.9% in 2005, it was still ~16.8%, or ~40 million adults as of 2014 (Jamal et al. 2015). These high rates of tobacco use reflect, to some extent, the difficulty in quitting. Although two-thirds of smokers report a desire to quit, half relapse within the first week after attempting to quit and ~ 90% relapse within a year of quitting (Garvey et al. 1992; Powell et al. 2010; Ashare and Hawk 2012; Jamal et al. 2015).
It is now commonly accepted that the difficulty of smoking cessation is, in part, due to the cognitive deficits associated with nicotine withdrawal (Hall et al. 2015). Patients report difficulty concentrating and confusion with quantified nicotine withdrawal-induced impairments to working memory, attention, response inhibition, reward processing, and reaction time (Hughes 2007; McClernon et al. 2015; van Enkhuizen and Young 2016). In fact, some of these cognitive impairments, including attention deficits and impulse control, before and during withdrawal can predict relapse (Pomerleau et al. 2003; Dolan et al. 2004; Rukstalis et al. 2005; Krishnan-Sarin and Reynolds 2007; Culhane et al. 2008; Powell et al. 2010). Understanding the mechanism(s) underlying withdrawal-induced cognitive deficits may be key toward developing targeted treatments that will remediate these deficits and aid quit attempts (Hall et al. 2015).
Nicotine is the primary psychoactive agent in tobacco smoke. Nicotine is the prototypical ligand of the nicotinic acetylcholine receptors (nAChRs), the most abundant of which are α4β2 and α7 nAChRs (Gotti et al. 2006). Nicotine is a full agonist at both of these nAChRs, although at a higher affinity for α4β2 nAChRs (Gotti et al. 2009). Importantly, nicotine administration can improve cognitive dysfunction during tobacco withdrawal, as can varenicline, a partial agonist of α4β2 and full agonist of α7 nAChRs (Patterson et al. 2009; Ashare and McKee 2012). The efficacy of nicotine and varenicline in improving cognition implicate both the α4β2 and α7 nAChRs in nicotine withdrawal-induced cognitive deficits. Yildirim et al. (2015) found that ABT-089 (a partial α4β2 agonist), but not ABT-107 (an α7 agonist), alleviated the symptoms of withdrawal-induced deficits in contextual fear conditioning in mice. These results importantly suggest that α4β2 antagonism may treat withdrawal-induced cognitive deficits in humans. Unfortunately, these findings are limited to this hippocampal-dependent task. To our knowledge, no studies have examined the necessity of either of these receptors in the development of nicotine withdrawal-induced cognitive deficits. Conducting such studies in humans would prove difficult. Determining the necessity of these receptors in the effects of withdrawal can however be readily studied in transgenic mice (e.g., Stoker et al. 2012a), enabling more targeted examination of putative underlying mechanisms.
Many studies exist determining the necessity of nAChRs in cognitive functioning using transgenic mice. For example, null mutation of the α7 nAChR revealed the importance of this nAChR for reward learning and sustained attention, as measured by the 5-choice serial reaction-time task (5CSRTT), although these mice exhibited normal motivation and punishment-associated learning compared with their wildtype (WT) littermates (Young et al. 2004; Keller et al. 2005; Hoyle et al. 2006; Young et al. 2007; Levin et al. 2009; Young et al. 2011a). Nicotine withdrawal studies in these and β4 nAChR null mice revealed the importance of the α7 and β4 subunits in the somatic signs and anhedonic-like state of nicotine withdrawal (Stoker et al. 2012a). The roles of the α7 and β4 nAChR subunits in withdrawal-induced cognitive dysfunction have yet to be determined. In rat studies, nicotine withdrawal impaired sustained attention (Shoaib and Bizarro 2005; Semenova et al. 2007), an effect that was remediated by varenicline treatment (Jackson et al. 2016). Although measuring sustained attention, the 5CSRTT has no response inhibition component, which is common to human attentional tests (Young et al. 2011b; Lustig et al. 2013) and important during nicotine abstinence (Tsaur et al. 2015). The development of the 5-choice continuous performance test (5C-CPT) for mice (Young et al. 2009), rats (Barnes et al. 2012; Hayward et al. 2016), and humans (Young et al. 2013a) has enabled the assessment of sustained attention and response inhibition by adding a non-target stimulus requiring inhibition, consistent with established human tests (Romberg et al. 2013). Use of the 5C-CPT enabled the double dissociation of response inhibition and premature responses (early responding to no stimuli) driven by reduced dopamine D4 receptor expression and 5-HT2C antagonism, respectively (Young et al. 2011b).
Despite its utility, to date no studies have used the 5C-CPT to investigate the necessity of specific nAChRs on nicotine withdrawal-induced deficits in attention and response inhibition. Furthermore, few studies have investigated the molecular changes that occur in the brain due to nicotine withdrawal. Given that reduced expression of dopamine D4 receptors has been associated with response disinhibition (Young et al. 2011b), we assessed their levels in mice during chronic nicotine administration and withdrawal. In addition, group I metabotropic glutamate receptors (mGluR1 and mGluR5) play an important role in maintaining the reinforcing effects of drugs like nicotine and may undergo adaptations during chronic drug exposure, potentially contributing to the withdrawal-induced changes in behavior (Kenny and Markou 2004). For example, reduced function of mGluR5 receptors may contribute to withdrawal-induced anhedonia and somatic symptoms (Liechti and Markou 2007; Stoker et al. 2012b), since chronic nicotine increased mGluR5 expression during treatment, and levels were restored to baseline 1 day after withdrawal (Pistillo et al. 2016). Hence, we also examined whether nicotine withdrawal-induced changes in mGluR1 and mGluR5 expression might be associated with inattention and assessed receptor levels during chronic nicotine treatment and withdrawal.
In this study, we assessed the cognitive deficits associated with nicotine withdrawal in healthy mice and those lacking α7 nAChRs, hypothesizing, based on rat and human studies (Shoaib and Bizarro 2005; Semenova et al. 2007; Harrison et al. 2009; McClernon et al. 2015), that attention would be disrupted during withdrawal. Furthermore, we hypothesized that mice lacking α7 nAChRs would not exhibit nicotine withdrawal-induced deficits in attention. Finally, we hypothesized that nicotine withdrawal would result in decreased expression of dopamine D4 and glutamate mGluR1 and mGluR5 receptors.
Materials and methods
Mice
Male C57BL/6J mice (n = 27) and male α7 nAChR knockout mice (KO, n = 26) and their WT littermates (n=28) were bred in-house, the latter generated from heterozygous breeding pairs on a C57B/6J background as previously described (Young et al. 2011a; Stoker et al. 2012a). Training began at 3 months old (baseline weight for all mice: 23–35 g, consistent with previous reports, there was no difference in weights between the WT and KO mice). All mice were group-housed (maximum 4 per cage) and maintained in a climate-controlled vivarium with a reversed day/night cycle (lights on at 20:00 h, off at 8:00 h) and tested during the dark phase between 9:00 and 18:00 h. Food (Harlan Teklad, Madison, WI) and water were available ad libitum except during training and testing, when food was restricted to maintain mice at ~85% full body weight (20–30 g). All mice were maintained in an animal facility that meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care. All procedures were approved by the University of California San Diego (UCSD) Animal Care and Use Committee.
5-hole apparatus
Sound-insulated 5-hole operant chambers (25×25×25 cm; Med Associates, Inc., St. Albans, VT) were used as described previously (Young et al. 2015). Each chamber had a house light and fan, with an array of 5 square holes (2.5×2.5×2.5 cm, 2.5 cm above the grid floor) arranged horizontally on a curved wall opposite the liquid delivery magazine (Lafayette Instruments, Lafayette, IN). Each hole had a light-emitting diode (LED) at the back and infrared beams, mounted vertically 3 mm from the opening to detect responses. The food delivery magazine contained a well for liquid reinforcement (strawberry Nesquik® plus non-fat milk, 30 μL), delivered by a peristaltic pump (Lafayette Instruments, Lafayette, IN), with an LED at the top. The magazine also contained an infrared beam 5 mm from the floor, recessed 6 mm to detect head entries. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates, Inc.) using custom programming.
Mouse 5C-CPT
Mice were trained as previously described. Mice were trained initially to retrieve rewards from the magazine, after which they were trained in a fixed ratio 1 (FR1) schedule. During this FR1 schedule, all 5 holes opposite the magazine were illuminated and mice were rewarded for nose poking any of the 5 holes. Sessions lasted 30 min or until 150 trials were completed. FR1 criterion was 70 responses for two consecutive days. Once criterion was reached, mice were only trained 2 days per week while other mice were trained 5 days per week in order to minimize over-training in FR1. After all mice had reached stable performance at criterion, training began on the 5CSRTT, in which only 1 of the 5 nose-poke holes was illuminated. Initially, mice had to make the correct choice (hole poking in the illuminated hole) within 10 s (stimulus duration; SD) or the house light was illuminated for a 4 s time out. The intertrial interval (ITI) was held at a constant 4 s and sessions lasted for 30 min or until 120 trials were completed. Criterion for this stage of training was 30 correct trials with mean correct response latencies less than half the current SD s for two consecutive sessions. Mice then progressed to 8, 4, and 2 s SD trials when meeting the same criterion. Once at 2 s, a variable ITI (3–7 s) was introduced to limit the use of a temporally mediated strategy (Cope et al. 2016) and increase the attentional load of the task. After reaching criterion, mice progressed to the 5C-CPT, in which target trials were the same as in the 5CSRTT (response required in a single lit hole) but non-target trials were added in which all 5 holes were illuminated (requiring the inhibition of responding), with the SD consistent between the two trial-types. If during the non-target trial, the nose poke was inhibited for 2 s, the mouse was rewarded, but a response was punished with a 4 s timeout. Once responding at less than 1.5 s mean correct latency for two consecutive days, mice were moved to a 1.5 SD.
Criterion was set at least 30 correct trials, mean overall correct latency less than 1.5 s, and less than 50% false alarm rate for two consecutive sessions.
After stability in performance was achieved, challenge sessions were used. These sessions were identical to training sessions but lasted for 250 trials with 3–7 s variable ITI and 1.5 s SD. Extended session challenges can be used to assess vigilance decrements (Young et al. 2009). Mice continued to be trained in 30 min or 120 trial sessions between challenge sessions.
Primary outcome variables of the 5C-CPT were hit rate (p[HR], the proportion of correct target responses to missed targets) representing target detection, false alarm rate (p[FA], the proportion of inappropriate responses to the non-target stimulus to correct withdrawals to the non-target stimulus) representing response inhibition, and d′ (a composite parametric measure for the difference between p[HR] and p[FA]) representing vigilance. Secondary outcome variables include the responsivity index (RI) to represent bias of responding, as well as accuracy (the proportion of correct responses to incorrect responses), latencies to correct target responses (mean correct latency, MCL), total trials, the % of omitted trials, and the % of premature responses representing motoric impulsivity/temporal discrimination (Cope et al. 2016). The calculation for each variable is thoroughly described elsewhere (Cope and Young 2016).
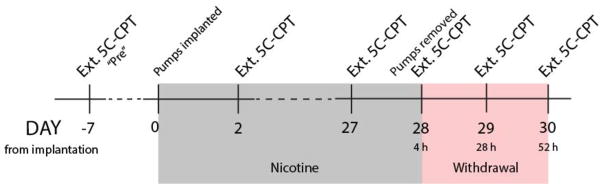
For Experiment 1, mice were tested one week before pump implantation and counterbalanced based on d′, % omissions, p[FA], and MCL for treatment with saline or nicotine (40 mg/kg/day). Mice were tested on the same challenge 2 and 27 days after pump implantation, as well as 4, 28, and 52 h after pump removal (Fig. 1), but trained in the standard task every other day. The 4 h timepoint was chosen based on previous findings of withdrawal-induced anhedonia after 3–6 h of withdrawal from 40 mg/kg/day of chronic nicotine (Stoker et al. 2008; Stoker et al. 2012a). Additional assessments were made 24 and 48 h after the initial testing in keeping with the mice’s normal training/testing schedule.
Figure 1.
Timeline of chronic nicotine testing procedure. Mice were split into two matched treatment groups based on an extended 5C-CPT session (Ext. 5C-CPT) one week (−7 days, “Pre”) before implantation of minipumps (saline or 40 mg/kg/day nicotine). Mice were tested again 2 and 27 days after pump implantation. Minipumps were removed 28 days after implantation, and the mice were tested at 4, 28, and 52 h after pump removal.
For Experiment 2, α7 mutant mice were trained in the 5C-CPT and stability established at a 3–7 s variable ITI and 1.5 s SD over 3 days. These data were averaged and used to counterbalance into treatment groups (saline, nicotine at 14 mg/kg/day, and nicotine at 40 mg/kg/day) based on average d′, p[HR], and MCL. These mice were also challenged in the extended 250-trial session 33 days after pump implantation and 4 h after pump removal. To assess receptor expression during nicotine treatment and withdrawal, 9 WT mice (n=3 per treatment group) were removed 33 days after pump implantation (before pump removal) and 9 additional WT mice (n=3 per treatment group) were removed after the final extended 5C-CPT session (during withdrawal). Performance in the 5C-CPT was assessed only in the mice with a full data-set for all 5C-CPT tests, before, during, and after nicotine.
Drug
(−)Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline solution and pH adjusted to 7±0.5 with sodium hydroxide (Sigma-Aldrich). Nicotine was infused through subcutaneous osmotic minipumps at concentrations of 14 and 40 mg/kg/day (Model 2004, ALZET, Palo Alto, CA). Doses were chosen based on previous reports of effects in mice (Stoker et al. 2008; Portugal and Gould 2009; Hall et al. 2015).
Osmotic mini pump implantation and removal surgery
The ALZET mini-osmotic pump Model 2004 has a reservoir volume of 200 μl and delivers solutions at a pumping rate of 0.25 μl/h (±0.05 μl/h). Pumps were filled and primed in 0.9% saline solution at room temperature for 40–48 h before insertion. Mice were anesthetized with isoflurane (1–3% in oxygen). Before inserting the minipump, the area around the back of the neck was shaved and sterilized with betadine. An incision was made and a pouch large enough for the pump was blunt dissected into the back using scissors. The pre-filled pump was inserted into the pouch with the flow modulator directed posteriorly, away from the wound. The incision was closed with 9 mm wound clips (MikRon Precision, Inc., Gardena, CA) and baytril (5 mg/kg) and flunixamine (2.5 mg/kg) were injected subcutaneously to minimize chances of infection and alleviate pain, respectively. After 28 or 33 days, the nicotine minipumps were surgically removed under isoflurane anesthesia the wound was stapled using aseptic surgery techniques with administration of baytril and flunixamine, as described above.
Cotinine Assessment
Cotinine, a major metabolite in the urine, is a commonly used marker of nicotine exposure (Haufroid and Lison 1998), given the short half-life of nicotine. Therefore, to confirm the presence of nicotine in these mice at levels comparable to human smokers, on day 26 of Experiment 1, urine was collected from mice for assessment of cotinine levels. Samples were shipped to MilleniumHealth Laboratories (San Diego, CA) for analysis.
Synaptosomal isolation and western blot
Synaptosomal isolation and western blots were performed as previously described (Naviaux et al. 2013; Naviaux et al. 2015). Briefly, cerebral samples were collected, homogenized, and synaptosomes isolated by discontinuous Percoll gradient centrifugation. Twelve μg of cerebral synaptosomal protein was loaded. Blots were probed with primary antibodies overnight in a cold room using anti-mGluR1 (#ab27199 from Abcam, Cambridge, MA), mGluR5 (#ab76316 from Abcam), and dopamine D4 receptor (#ADR-004, from Alomone Labs, Jerusalem, Israel) antibodies. After washing, the membranes were blotted with goat anti-rabbit secondary antibody (#31460 from Pierce, Rockford, IL). The proteins of interest were visualized by ECL reagent (#32109) or Pierce SuperSignal™ West Femto Maximum Sensitivity Substrate (#PI-34095) and the immunoblots were exposed to X-Omat Blue films and scanned. The target protein density was normalized by Ponceau S staining and analyzed in GraphPad Prism 6.0 (La Jolla, CA).
Statistics
All primary (d′, p[HR], p[FA]) and secondary outcome measures (RI, accuracy, MCL, total trials, % of omitted trails, and % of premature responses) in the 5C-CPT from Experiment 1 before and during nicotine administration were analyzed using one-way analysis of variance (ANOVA) with treatment group as the between-subjects factor. The same measures from Experiment 1 after withdrawal were analyzed using ANOVA with treatment group as a between-subjects factor and time after withdrawal as a within-subjects factor. Since we hypothesized a priori that the effect of withdrawal would diminish over time, we conducted planned ANOVAs to assess differences in the primary outcome measures specifically at the 4 h time point after withdrawal with treatment group as the between-subjects factor. Primary and secondary 5C-CPT measures from Experiment 2 during and after nicotine administration were analyzed using ANOVA with treatment group and genotype as between-subjects factors. We conducted a priori planned ANOVAs on the primary outcome measures to compare the saline-treated mice and 40 mg/kg/day nicotine-treated group specifically (consistent with Experiment 1) with treatment group and genotype as between-subjects factors. Receptor concentrations were normalized by protein and the concentration of receptor in the saline-treated animals was taken as the 100% reference. Samples were analyzed using two-way ANOVA with treatment group and time point (before or after withdrawal) as between-subjects factors. Tukey post hoc analyses were conducted on all significant main effects and interactions. The alpha level was set to 0.05. All statistics were performed using SPSS (19.0, Chicago, IL) or GraphPad Prism 6.0 (La Jolla, CA).
Results
Experiment 1: the effects of nicotine administration and withdrawal on 5C-CPT performance in C57BL/6J mice
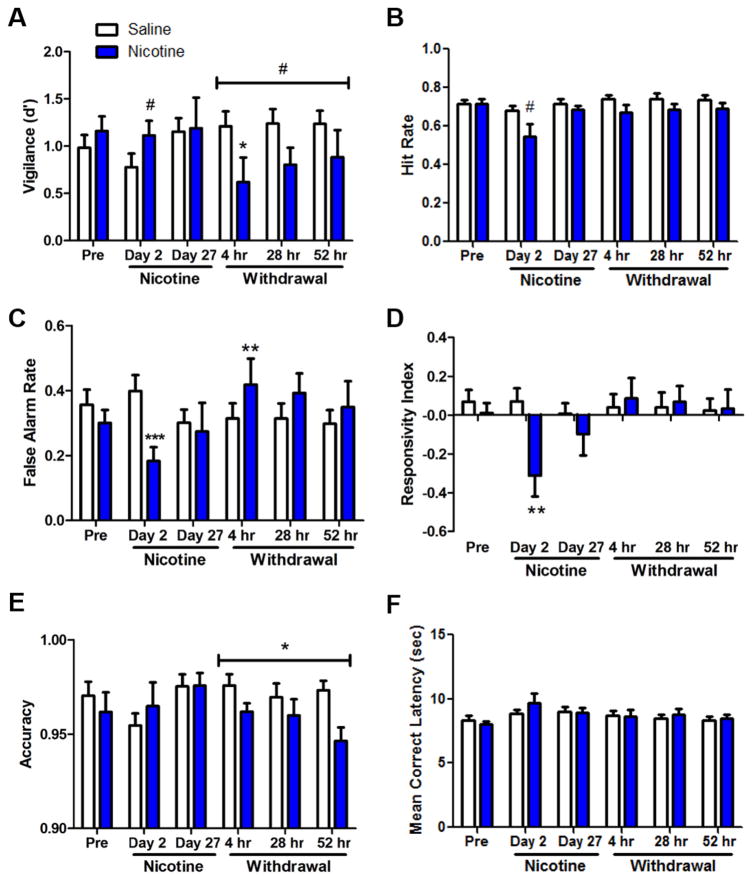
We examined the effects of nicotine administration and withdrawal on 5C-CPT performance in mice, with vigilance represented by d′ (Fig. 2a). Before nicotine administration, there was no significant difference between groups assigned to receive saline or nicotine. Two days after pump implantation, however, there was a trend toward greater d′ for the mice that received nicotine (F(1,25)=3.2, p<0.1). This trend was gone by 27 days after pump implantation. ANOVA across the three time-points following withdrawal (4, 28, and 52 h after pump removal) revealed a trend toward a main effect of nicotine (F(2,50)=3.7, p<0.1) but no main effect of time nor a nicotine × time interaction on d′. An a priori planned comparison revealed that consistent with measurement of anhedonia-like behavior in mice 4 h after pump removal, those withdrawn from nicotine exhibited poorer d′ compared with control mice (F(1,25)=4.2, p<0.05) with a modest to strong effect size (Cohen’s d=0.8).
Figure 2.
Effects of chronic nicotine (40 mg/kg/day vs. saline) administration and withdrawal on performance of C57BL/6J mice in the 5C-CPT. Before nicotine minipump implantation (“Pre”), there were no significant differences between groups on d′ (a), hit rate (b), false alarm rate (c), responsivity index (d), accuracy (e), or mean correct latency (f). Two days after pump implantation, there were trends toward greater d′ and lower hit rate for mice that received nicotine. Nicotine treatment also resulted in significantly lower false alarm rate and more negative responsivity index. No trends or significant effects of nicotine were observed 27 days after nicotine treatment. After pump removal, there were main effects of withdrawal from nicotine treatment on d′ and accuracy, which were both lower for mice that had received nicotine. Data are shown as mean ± SEM, #p<0.1, *p<0.05, *p<0.01, ***p<0.001 when compared with saline.
Further analyses revealed significant effects of nicotine on p[HR] (F(1,25)=4.1, p=0.05, Fig. 2b) and on p[FA] (F(1,25)=11.5, p<0.003, Fig. 2c) 2 days after pump implantation—with both p[HR] and p[FA] lower for mice receiving nicotine—but no significant effects of nicotine on either measure before treatment or 27 days after pump implantation. Although reduced p[HR] and elevated p[FA] were observed during withdrawal, these results were not significant. Specific analysis on p[FA] during only the 4 h withdrawal time point did, however, reveal a significant effect of nicotine withdrawal to increase p[FA] (F(1,25)=3.1, p<0.01) with a modest effect size (Cohen’s d=0.6). At this time point, though it was not statistically significant, the reduction in p[HR] also had a modest effect size (Cohen’s d=0.5).
Two days after pump implantation, there was a main effect of nicotine on RI (F(1,25)=10.2, p<0.001, Fig. 2d), whereby the mice receiving nicotine had a significantly more negative RI than mice receiving saline, indicative of a reduced response rate. The effect of nicotine on RI was not present before nicotine administration, 27 days after pump implantation, or during withdrawal. There was no main effect of nicotine on accuracy before or during nicotine administration but ANOVA revealed a main effect during withdrawal (F(1,25)=7.3, p<0.02, Fig. 2e), with reduced accuracy in animals that received nicotine treatment. Finally, there was no main effect of nicotine on MCL before, during, or after nicotine administration (Fig. 1f). There were neither main effects of time nor nicotine × time interaction on RI, accuracy, or MCL after withdrawal.
Results and statistics for additional secondary measures can be found in Table 1. There were no significant main effects of nicotine or time on total trials. There was a significant effect of nicotine treatment on % omissions 2 days after pump implantation (F(1,25)=4.2, p=0.05)—with increased omissions for nicotine-treated mice—but not 27 days after treatment. After pump removal, the nicotine-treated animals exhibited increased % omissions (F(1,25)=3.0, p=0.1) but this increase was not significant. After pump removal, there was a significant main effect of nicotine withdrawal on premature responses (F(1,25)=9.9, p<0.004), whereby the nicotine-treated mice had a higher percentage of premature responses. This difference was not observed during nicotine treatment, but before nicotine administration the nicotine-treated group already exhibited more premature responses than the saline-treated group (F(1,25)=3.2, p<0.1). Therefore, it is unclear from these data if the effect during the withdrawal period was due to nicotine treatment.
Table 1.
Effects of chronic saline or nicotine (40 mg/kg/day) administration and withdrawal on secondary performance measures for C57BL/6J mice in the 5-chuice continuous performance test.
Effects of chronic nicotine (40 mg/kg/day vs. saline) administration and withdrawal on secondary performance measures for C57BL/6J mice in the 5C-CPT. There were no significant main effects of nicotine treatment or time on total trials. There was a significant effect of nicotine withdrawal 2 days after pump implantation but not 27 days after treatment. After pump removal, there was a trend toward increased omissions for the nicotine-treated animals. Before pump implantation, there was a trend toward increased premature responses for mice assigned to the nicotine-treatment group. This trend was not observed during nicotine treatment but was significant after pump removal.
| Measure | Pre-Treatment | Nicotine Administration | Nicotine Withdrawal | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Day 2 | Day 27 | 4 h | 28 h | 52 h | Nicotine | Time | Nic × Time | ||||||||||||
| Mean ± SEM | Group | Mean ± SEM | Nicotine | Mean ± SEM | Nicotine | ||||||||||||||
| F(1,25) | p | F(1,25) | p | F(1,25) | p | Mean ± SEM | Mean ± SEM | Mean ± SEM | F(1,25) | p | F(2,50) | p | F(2,50) | p | |||||
| Total Trials | Saline | 235.50±4.91 | <2 | ns | 231.00±6.06 | <2 | ns | 238.93±3.34 | <1 | ns | 239.00±3.44 | 234.00±5.28 | 237.86±4.84 | 3.1 | 0.09 | <1 | ns | <1 | ns |
| Nic (40 mg/kg/day) | 243.38±3.77 | 213.23±14.28 | 238.32±3.87 | 223.00±8.70 | 224.77±5.96 | 229.15±5.23 | |||||||||||||
| % Omissions | Sal | 27.88±2.20 | <1 | ns | 31.02±2.54 | 4.2 | 0.05 | 27.75±2.10 | <2 | ns | 10.83±0.99 | 10.98±1.23 | 11.04±1.12 | 3.0 | 0.1 | <1 | ns | <1 | ns |
| Nic (40 mg/kg/day) | 27.41±2.44 | 44.85±6.73 | 30.98±2.05 | 15.81±3.37 | 13.82±1.37 | 13.05±1.23 | |||||||||||||
| % Premature Responses | Sal | 3.09±0.53 | 3.2 | 0.09 | 2.85±0.85 | <1 | ns | 1.66±0.46 | <1 | ns | 1.81±0.34 | 2.14±0.52 | 2.44±0.62 | 9.9 | 0.00 | 2.9 | 0.06 | <2 | ns |
| Nic (40 mg/kg/day) | 6.42±1.93 | 3.40±1.64 | 3.00±1.08 | 4.99±1.48 | 4.69±1.30 | 7.83±1.80 | |||||||||||||
Experiment 2: the effects of chronic nicotine administration and withdrawal on 5C-CPT performance in α7 nAChR KO mice and WT littermates
In this study, the mice were tested in the 5C-CPT before pump implantation and 33 days later. Because the study included the assessment of nicotine withdrawal-induced changes in receptor expression, requiring the loss of a large subset of WT mice, all mice were tested in the 5C-CPT only 4 h after pump removal.
There were no significant differences in rewards collected or days to reach criterion in the stages before 5CSRTT training. When the animals reached asymptotic performance on the 5CSRTT with a constant ITI for 4 consecutive days, KO mice exhibited a lower accuracy than WT mice (F(1,56)=6.3, p<0.02). There was no effect of genotype on the percentage of omitted trials. These findings are consistent with results from Hoyle et al. (2006) using the same task design in α7 nAChR KO mice. A variable ITI was then introduced to limit the use of a temporally mediated strategy and increase the attentional load of the task. When the mice attained stable performance for 4 consecutive days, the KO mice again exhibited a lower accuracy than WT mice (F(1,56)=6.2, p<0.02). The KO mice omitted a greater percentage of trials than the WT mice (F(1,56)=4.3, p<0.05). These results are consistent with the report of Young et al. (2004) on the same task with a variable ITI. As described below, the KO mice continued to exhibit lower accuracy throughout training on the 5C-CPT. No differences to attain criterion in the 5C-CPT stage were observed.
Baseline assessment of performance in mice in the 5C-CPT revealed neither main effects of genotype or nicotine nor assigned nicotine group × genotype interactions on d′, p[HR], p[FA], RI, or MCL (Fs<1, ns). Subsequent analyses of these measures therefore focus on the effects of nicotine 33 days after pump implantation and after 4 h withdrawal.
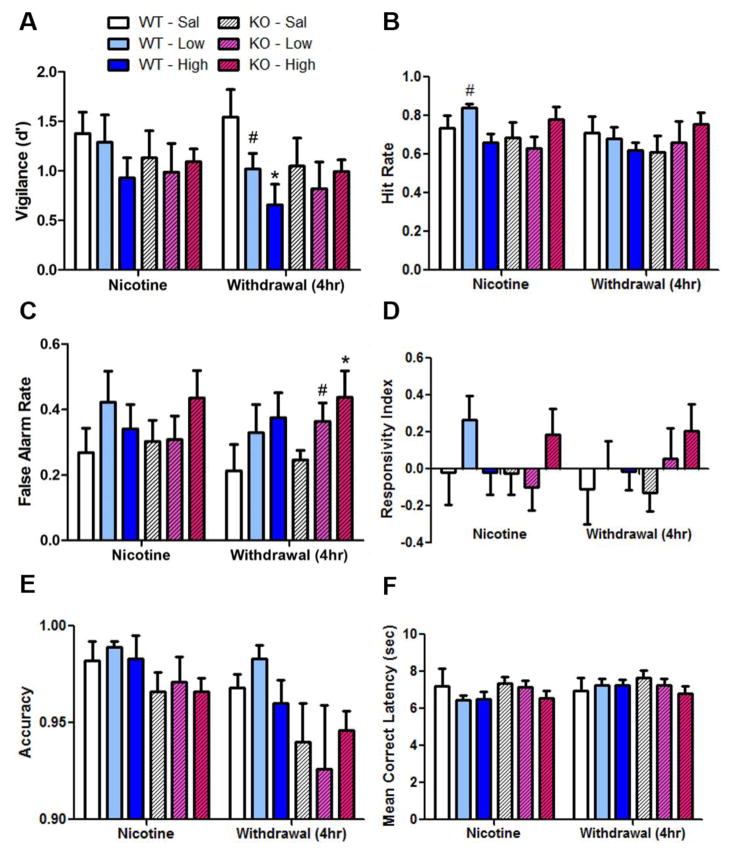
During chronic nicotine treatment on day 33, there were neither main effects of nicotine, genotype, nor nicotine × genotype interactions on d′. In contrast however, 4 h after pump removal the nicotine-treated groups exhibited lower d′ than the saline-treated group (F(2,39)=2.5, p<0.1, Fig. 3a). Given our a priori hypotheses from Experiment 1, we analyzed the effects of the same dose (40 mg/kg/day) on performance in contrast to saline-treated mice. Consistent with Experiment 1, a significant main effect of nicotine 4 h after withdrawal was observed (F(1,26)=4.6, p<0.05), as was a modest nicotine × genotype interaction (F(1,26)=3.6, p<0.1). Post hoc analyses revealed that there was no significant effect of genotype for mice treated with saline alone. The effect of nicotine withdrawal after the 40 mg/kg/day treatment compared with saline-treated mice, however, was significant only in the WT mice (F(1,10)=8.2, p<0.05), but not the KO mice (F<1, ns). The lack of nicotine effect on the KO mice suggests that nicotine withdrawal-induced impaired attention/vigilance requires the α7 nAChR.
Figure 3.
Effects of chronic nicotine (“low” 14 or “high 40” mg/kg/day v. saline) administration and withdrawal on performance of WT and α7 nAChR KO mice in the 5C-CPT. Thirty-three days after nicotine minipump implantation (“Nicotine”), there were no main effects of nicotine treatment or genotype on d′ (a), p[HR] (b), p[FA] (c), RI (d), and MCL (f). There was a significant nicotine × genotype interaction on p[HR] (b), since WT but not KO mice receiving the low dose of nicotine exhibited elevated p[HR]. Four h after pump removal (“Withdrawal”), there were no main effects of nicotine treatment or genotype nor nicotine × genotype interactions on p[HR], RI, or MCL. After pump removal, there was a main effect of withdrawal from nicotine treatment on p[FA] (c), and there was a trend toward an effect of nicotine withdrawal on d′ (a) whereby the effect of nicotine withdrawal to decrease d′ was significant for WT but not KO mice. During and after nicotine treatment, there was a main effect of genotype on accuracy (d) with KO mice exhibiting lower accuracy than WT littermates. Data are shown as mean ± SEM, #p<0.1, *p<0.05 when compared with saline.
Thirty-three days after pump implantation, there were neither main effects of nicotine nor genotype but there was a significant nicotine × genotype interaction on p[HR] (F(2,39)=4.1, p<0.05, Fig. 3b). Post hoc analyses revealed that there was a main effect of nicotine on p[HR] in WT mice (F(2,16)=6.1, p=0.01)—whereby mice receiving the low dose of nicotine exhibited higher p[HR] than mice receiving saline (F(1,10)=4.0, p<0.1) and the high dose of nicotine (F(1,12)=18.0, p<0.001)—but that this effect was absent in KO mice (F<2, ns). Furthermore, the genotypic difference between mice was observed whereby low dose nicotine-treated WT mice exhibited higher p[HR] compared with KO mice (F(1,13)=11.0, p<0.01). This genotype-specific effect suggests that improvement in attention due to chronic nicotine at a low dose relies on α7 nAChRs. Four h after pump removal, there were neither main effects of nicotine or genotype nor nicotine × genotype interactions on p[HR]. An analysis of the high dose revealed no main effects but a slight modest nicotine × genotype interaction (F(1,26)=2.5, p<0.1) since nicotine withdrawal was associated with a statistically insignificant reduction in p[HR] in WT mice but an increase in p[HR] for KO mice.
No significant effects of nicotine, genotype, or their interactions on p[FA] were observed during nicotine treatment. Four h after pump removal however, there was a main effect of nicotine (F(1,39)=3.6, p<0.05, Fig. 3c). Post hoc analyses revealed that both the low dose (F(1,25)=4.2, p=0.05) and high dose of nicotine (F(1,26)=7.3, p<0.02) resulted in significant nicotine withdrawal-induced increases in p[FA]. There was no main effect of gene or nicotine × genotype interaction. Hence, irrespective of genotype, withdrawal from chronic nicotine resulted in response disinhibition.
No significant effects of nicotine, genotype, or their interactions on RI were observed during nicotine treatment or after pump removal (F<1, ns, Fig. 3d). On Day 33 after pump implantation, there was potentially modest a nicotine × genotype interaction (F(2,39)=2.9, p<0.1). No post hoc analyses revealed any significant effects however. No significant effects of nicotine, genotype, or their interactions on MCL were observed during nicotine treatment or after pump removal (Fig. 3f).
There was a significant main effect of genotype on accuracy at baseline (F(1,39)=6.6, p<0.02), during nicotine treatment (F(1,39)=5.0, p<0.05), and 4 h after nicotine withdrawal (F(1,39)=4.9, p<0.05) (Fig. 3e). This main effect of genotype was consistent across these three time periods with KO mice exhibiting lower accuracy than their WT littermates throughout testing. There was no main effect of nicotine or nicotine × genotype interaction on accuracy.
There was no main effect of genotype or nicotine on any of the additional secondary outcome measures (total trials, percentage of omissions, and percentage of premature responses), shown in Table 2. There was a significant nicotine × genotype interaction on the percentage of omitted trials 33 days after pump implantation (F(2,39)=4.1, p<0.05)—whereby the low dose of nicotine resulted in fewer omitted trials compared to saline (F(1,10)=3.3, p<0.1) and the high nicotine dose (F(1,12)=17.6, p=0.001) for the WT mice (overall: F(2,16)=6.1, p<0.02)—but there was no effect of dose on omissions for the KO mice (F<2, ns). This reduction in omitted trials after chronic nicotine administration, like the effect on p[HR], may be reliant on the presence of α7 nAChRs.
Table 2.
Effects of chronic saline (Sal) or nicotine (14 or 40 mg/kg/day) administration and withdrawal on secondary performance measures for wildtype (WT) and α7 nAChR knockout (KO) mice in the 5-choice continuous performance test.
Effects of chronic nicotine (“Nic” at 14 or 40 mg/kg/day vs. saline, “Sal”) administration and withdrawal on secondary performance measures for WT and α7 nAChR KO mice in the 5C-CPT. There was no main effect of genotype or nicotine on total trials, % omissions, or % premature responses. There was a significant nicotine × genotype interaction on % omissions 33 days after pump implantation since the low dose of nicotine resulted in fewer omitted trials compared to saline and the high nicotine dose for the WT mice but not KO mice.
| Pre-Treatment | Nicotine (Day 33) | Withdrawal (4 h) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||
| Mean ± SEM | Genotype | Treatment Group | Genotype × Group | Mean ± SEM | Genotype | Nicotine | Genotype × Nicotine | Mean ± SEM | Genotype | Nicotine | Genotype × Nicotine | |||||||||||
| F(1, 39) | p | F(2,39) | p | F(2,39) | p | F(1, 39) | p | F(2,39) | p | F(2,39) | p | F(1, 39) | p | F(2,39) | p | F(2,39) | p | |||||
| Total Trials | WT - Sal | 113.07±3.44 | <1 | ns | <1 | ns | 2.2 | ns | 243.20±2.01 | <2 | ns | <1 | ns | <2 | ns | 237.80±6.99 | <1 | ns | <1 | ns | <2 | ns |
| WT - Nic (14) | 116.76±1.71 | 245.00±0.00 | 220.00±17.86 | |||||||||||||||||||
| WT - Nic (40) | 116.95±1.69 | 227.00±12.54 | 207.29±19.48 | |||||||||||||||||||
| KO - Sal | 117.70±1.23 | 227.56±11.68 | 219.00±9.29 | |||||||||||||||||||
| KO - Nic (14) | 108.50±5.93 | 224.50±12.64 | 222.13±21.97 | |||||||||||||||||||
| KO - Nic (40) | 113.74±2.43 | 236.56±4.72 | 239.00±5.60 | |||||||||||||||||||
| % Omissions | WT - Sal | 22.03±4.59 | <1 | ns | <1 | ns | <2 | ns | 26.22±6.67 | <1 | ns | <1 | ns | 4.1 | 0.02 | 28.06±8.09 | <1 | ns | <1 | ns | <2 | ns |
| WT - Nic (14) | 15.25±1.38 | 15.66±1.73 | 31.43±6.07 | |||||||||||||||||||
| WT - Nic (40) | 20.41±3.11 | 33.51±4.26 | 37.03±4.01 | |||||||||||||||||||
| KO - Sal | 16.43±1.79 | 30.50±7.38 | 37.84±8.53 | |||||||||||||||||||
| KO - Nic (14) | 21.04±4.69 | 36.44±6.31 | 32.66±10.73 | |||||||||||||||||||
| KO - Nic (40) | 18.04±4.18 | 21.56±6.61 | 23.39±6.01 | |||||||||||||||||||
| % Premature Responses | WT - Sal | 3.79±0.81 | <1 | ns | <1 | ns | <1 | ns | 4.13±1.13 | <1 | ns | <1 | ns | <1 | ns | 3.96±1.71 | 2.0 | ns | <1 | ns | <1 | ns |
| WT - Nic (14) | 8.18±3.66 | 3.50±1.01 | 4.47±1.83 | |||||||||||||||||||
| WT - Nic (40) | 5.37±1.92 | 4.79±2.99 | 6.03±3.35 | |||||||||||||||||||
| KO - Sal | 5.79±2.03 | 4.73±1.85 | 8.04±3.19 | |||||||||||||||||||
| KO - Nic (14) | 6.18±2.02 | 4.50±1.98 | 6.45±2.24 | |||||||||||||||||||
| KO - Nic (40) | 6.25±2.94 | 5.29±1.97 | 12.31±5.66 | |||||||||||||||||||
Cotinine Assessment
Urine cotinine levels were assessed after 26 days of saline or nicotine (40 mg/kg/day) treatment in C57BL/6 mice. Urine cotinine was detected (464.9 ± 73.9 ng/ml) for mice treated with 40 mg/kg/day nicotine but not for those that received saline (0 ± 37.0 ng/ml).
Biochemical analysis
Synaptosomal western blot analyses revealed a main effect of nicotine on mGluR1 receptors during nicotine administration and 4 h after withdrawal (F(1,8)=9.0, p<0.02; Table 3). There was no main effect of time point (F<2, ns) or nicotine × time point interaction (F<1, ns). These findings suggest that nicotine treatment alters mGluR1 and does not normalize within the first 4 h of withdrawal. There were no main effects of nicotine (F<1, ns) or time point (F(1,8)<1, ns) on mGluR5 expression. There was, however, a slight a nicotine × time point interaction (F(1,8)=3.9, p<0.1; Table 3) whereby nicotine-treated mice had reduced mGluR5 expression during nicotine administration but not during withdrawal. Similarly, there were no main effects of nicotine (F<1, ns) or time point (F<2, ns) on dopamine D4 receptor expression (Fs<2, ns), but the nicotine × time point interaction was significant (F(1,8)=7.3, p=0.03; Table 3), again due to reduced D4 receptor expression during nicotine administration but not during withdrawal. Post hoc analyses revealed no significant pair-wise differences in expression of mGluR5 and D4 receptors (split by time point or by treatment group), likely due to low sample size.
Table 3.
Synaptosomal receptor expression (% of saline control with pump). Bolded indicates significant interaction between Saline/Nicotine vs. Pump On/Pump Off.
Effect of chronic nicotine (40 mg/kg/day) treatment on mGluR1, mGluR5, and dopamine D4 receptor expression. After 33 days of chronic nicotine treatment (“Pump On”) and 4 h after pump removal (“Pump Off”), a subset of WT mice were removed and levels of synaptosomal mGluR1, mGluR5 and, D4 receptor expression were analyzed. There was a significant effect of nicotine on mGluR1 expression during nicotine administration and during withdrawal. Significant interactions between synaptosomal expression during nicotine (Pump On) and withdrawal (Pump Off) for mGluR5 (trend) and dopamine D4 receptors were observed, though no significant post hoc differences between levels were observed.
| Receptor | Saline | Nicotine | N per Group | 2-Way ANOVA Interaction F(1,8) | p | ||
|---|---|---|---|---|---|---|---|
| Pump On | Pump Off | Pump On | Pump Off | ||||
| % Control ± SEM | % Control ± SEM | % Control ± SEM | % Control ± SEM | ||||
| mGluR1 | 100 ± 3.7 | 107.±2.3 | 117±6.3 | 127±9.8 | 3 | 0.05 | 0.82 |
| mGluR5 | 100±9.8 | 99±8.0 | 79±6.2 | 110±8.5 | 3 | 3.9 | 0.08 |
| D4R | 100±6.2 | 88.5±12 | 75±2.0 | 101±5.1 | 3 | 7.3 | 0.03 |
Discussion
Withdrawal from nicotine impaired attention/vigilance in mice when measured in a continuous performance test (CPT). Vigilance (d′) can be impaired due to increased misses to targets (inattention) and/or increased responding to non-targets (response disinhibition). The nicotine withdrawal-induced deficit was driven by both. Experiment 1 demonstrated that, consistent with findings in humans, these deficits gradually disappear over time, with deficits in nicotine withdrawal mice observed after 4 but not 52 h after withdrawal. WT mice in Experiment 2 were similarly affected 4 h after nicotine withdrawal. In contrast, mice lacking α7 nAChRs did not exhibit the same nicotine withdrawal-induced deficits due to modest elevations in both target and non-target responding in these mice. Hence, nicotine withdrawal-induced response disinhibition occurred irrespective of the presence of α7 nAChRs, in contrast with withdrawal-induced inattention. Therefore, different mechanisms may underlie withdrawal-induced cognitive deficits in inattention vs. response disinhibition.
Importantly, nicotine withdrawal exerted consistent effects across both studies. Both a reduced hit rate and elevated false alarm rate were observed and although not necessarily significant alone, when combined (a common practice in human CPT testing), significant deficits in performance were observed (Figs. 2a and 3a). This profile of inattention and response disinhibition is consistent with human studies demonstrating delayed reaction times to target stimuli and increased false alarms in CPTs during nicotine abstinence (Harrison et al. 2009; Powell et al. 2010), as well as impaired response inhibition in a stop-signal task, go/no-go tasks, and the Stroop task (Harrison et al. 2009; Kozink et al. 2010; Ashare and Hawk 2012). Also consistent with human testing is the apparent attenuation of withdrawal-induced performance deficits of mice over time. Fig. 1a demonstrates that the greatest deficit during nicotine withdrawal was 4 h after pump removal. Mice undergoing nicotine withdrawal gradually improved in performance over time to the point where there was no difference from their saline-treated controls 52 h later. This gradual improvement was mirrored in the reduction in false alarm rates over time. Hence, we have demonstrated that the use of the CPT in mice during withdrawal produces results that are consistent with human withdrawal/CPT studies and can be useful in the study and development of treatments of withdrawal-induced cognitive deficits.
In addition to nicotine withdrawal effects, Experiment 1 demonstrated that on the second day of nicotine treatment d′ was greater for mice receiving nicotine compared to those receiving saline. Considering d′ alone, the ‘better’ performance of these nicotine-treated mice could be driven not by improved performance on nicotine but by a poorer response of the saline-treated mice to pump implantation. This difference may be due to residual pain from recent surgery or exposure to isoflurane treatment that can differentially affect gene expression and networks (Lowes et al. 2016). The procognitive (Semenova et al. 2007; Wallace and Bertrand 2013) and antinociceptive (Han et al. 2005; Jackson et al. 2009) effects of nicotine in rodents have been well established and may have ameliorated the reduction in performance observed in the saline-treated animals. As described previously, however, vigilance (d′) is a composite measure accounting for responses to targets (p[HR]), as well as inhibition during non-target trials (p[FA]). Although d′ was increased, we found that both p[HR] and p[FA] were reduced in mice that received nicotine. Furthermore, RI was decreased and % omissions increased in these mice, indicative of reduced responding to both target and non-target stimuli. These effects may be due to physical rather than cognitive differences between groups. For example, it is possible that the acidity of the nicotine solution may have contributed to post-operative discomfort that may have affected performance. In any case, all differences in performance between saline- and nicotine-treated groups disappeared by day 27. Hence, after long-term exposure, no improvements in cognition were observed, consistent with a lack of improvement in chronic smokers vs. non-smokers. It is possible that repeated testing under the influence of nicotine may have altered the baseline performance of the mice, compromising the observed effects of withdrawal on performance. Our previous studies, however, observed remarkable stability in baseline performance after multiple tests with the administration of various drugs, including nicotine (Young et al. 2013b), hence the current design. Although we cannot definitively rule out the potential for continued influence of nicotine, it remains unlikely that repeated testing during this administration significantly affected these withdrawal results.
Though not significant across or within every time point during withdrawal, p[HR] was consistently lower and p[FA] consistently higher for mice receiving chronic nicotine treatment compared to mice receiving saline. Specifically at the 4 h time point, withdrawal-induced elevation in p[FA] for mice that received nicotine was statistically significant. The subtle effect of withdrawal on both measures may have been masked due to small sample size. Overall, this experiment illustrates that the 5C-CPT—which includes inhibition from responding to non-target trials—can be used to disassociate sustained attention and disinhibition measures in mice, enabling comparisons to similar measures in humans. Indeed, our finding that impaired attention during nicotine withdrawal in mice is consistent with similar findings in human studies (McClernon et al. 2015; van Enkhuizen and Young 2016).
Further relevance to human testing comes from observations that treatment with 40 mg/kg/day nicotine resulted in urine cotinine levels (~ 450 ng/ml) comparable to the lower range of urine cotinine levels for human smokers, where <10 cigarettes/day = 646 ng/ml, while > 20 cigarettes per day = 1100 ng/ml (Wall et al. 1988; Haufroid and Lison 1998; Parker et al. 2002). Support for using higher doses of nicotine for 28 days comes from studies using lower chronic nicotine levels (14 days at 6.3 mg/kg/day) which produce plasma cotinine levels of 60–180 ng/ml (Portugal et al. 2012), that are low relative to 260–300 ng/ml in human smokers (Benowitz et al. 1983; Shoaib and Stolerman 1999). Hence, the current technique for chronic nicotine treatment with osmotic minipumps resulted in cotinine levels comparable to those seen in human smokers.
We also examined the effects of nicotine withdrawal on 5C-CPT performance in α7 nAChR KO mice and their WT littermates. Consistent with our initial findings, 4 h after pump removal, d′ was lower for nicotine-treated mice than saline-treated mice. More specifically, d′ was significantly lower for mice that received the high dose of nicotine (40 mg/kg/day) compared to mice that received saline providing reproducible evidence for an effect of nicotine withdrawal on cognitive performance. Importantly here however, there was also a modest nicotine treatment × genotype interaction whereby there was a significant effect of nicotine withdrawal on d′ in WT mice but not KO mice. The lack of nicotine treatment effect in the KO mice suggests that α7 nAChRs are required for nicotine withdrawal-induced inattention, which may contribute to the difficulty of quitting smoking in humans. Further analyses revealed that, consistent with Experiment 1 and human studies, the probability of false alarms (p[FA]) is significantly affected by nicotine withdrawal—with elevated p[FA] for mice that received nicotine versus saline. As noted above, d′ incorporates both p[FA] and p[HR]. Given that withdrawal affects p[FA] in both WT and KO mice, the difference in d′ may stem from differential effects of withdrawal on p[HR] in these genotypes. Indeed, there was a modest nicotine × genotype interaction on p[HR] whereby the WT mice that received the high dose of nicotine exhibited reduced p[HR] 4 h after nicotine withdrawal while the KO mice that received nicotine exhibited a slight increase in p[HR]. Although the effects were not statistically significant, even modest effects on p[HR] in combination with changes in p[FA] can profoundly affect overall attentional performance. Lack of significance may be due to the removal of WT mice to assess potential alterations in receptor expression that result from chronic nicotine and its withdrawal, described below. Thus, both inattention and response disinhibition should be considered for remediation to improve cognition during withdrawal.
Our findings support the hypothesis that an α7 nAChR antagonist during nicotine withdrawal may help alleviate the effects of withdrawal on sustained attention. Certainly, α7 nAChR antagonist treatments have improved attention and cognitive performance (Hahn et al. 2011; Burke et al. 2014). Of course, consistent with previous studies, the KO mice exhibited an overall impairment in attention throughout the experiment, as measured by accuracy, as well as slower training (Young et al. 2004; Keller et al. 2005; Hoyle et al. 2006), perhaps driven by reward-related learning deficits (Young et al. 2011a). Therefore, KO mice may have altered baseline behavior that could confound the interpretation of these data. Furthermore, since the null mutation of α7 nAChRs in these mice is constitutive, whether or not the temporary disruption of α7 nAChRs via conditional KO or pharmacological antagonism would also affect accuracy remains to be tested. Nonetheless, the consistency of withdrawal effects in WT mice in both experiments and comparable effects of withdrawal on p[FA] in both WT and KO mice suggest that the differential effects on p[HR] warrant further investigation.
Previous studies have shown that subchronic nicotine treatment improves attention by increasing target responding (Young et al. 2013b). Consistent with these findings, we observed an improvement in p[HR] and reduced omissions due to chronic low—but not high—dose nicotine administration in WT mice. Importantly, we observed that the nicotine-related improvements were observed in WT mice only and not KO mice. This genotype-specific effect further suggests that the cognition-enhancing properties of low doses of nicotine treatment may rely on α7 nAChRs. Combined, these data highlight the fact that although acute nicotine treatment improves attention by increasing target responding, withdrawal from nicotine impairs performance primarily by increasing response disinhibition and reducing accuracy, as observed in the 5CSRTT. Since nicotine withdrawal-induced disinhibition does not seem to rely on α7 nAChRs, another target must be identified for the remediation of this cognitive deficit.
Synaptosomal western blots revealed that mGluR5 and D4 receptor levels were decreased in mice that received chronic nicotine treatment compared to those that received saline. Four h after nicotine pump removal, however, these expression levels were elevated for mice that received nicotine. In contrast, levels of mGluR1 were elevated in mice that received nicotine during administration, as well as during withdrawal. The elevated levels of mGluR5 and D4 receptor after nicotine withdrawal may be overcompensation during the recovery from lack of nicotine. Of course, these are not the only changes associated with withdrawal, and we do not know if the α7 nAChR KO mice would exhibit the same changes in receptor expression. Hence, it is unclear if either of these changes would be suitable targets to treat withdrawal-induced disinhibition. Given that mice with reduced dopamine D4 receptor expression exhibit response disinhibition (Young et al. 2011b), however, agonists at this receptor may remediate response disinhibition that occurs during withdrawal. In fact, D4 receptor agonist treatment improved response inhibition in rats with high p[FA] during 5C-CPT testing (Tomlinson et al. 2015).
Since the success of quitting attempts can be increased by two- or three-fold with the aid of counseling and medications, it is imperative to better understand and develop more accessible treatments to aid smoking cessation (Fiore et al. 2008; Polosa and Benowitz 2011; Ashare and Schmidt 2014). Our studies suggest that a combination of therapeutic interventions—to target the α7 nAChR-dependent changes in target detection (p[HR]) and possibly dopamine D4 receptors in response inhibition (p[FA])—may be necessary to address multiple impairments to attention in people experiencing nicotine withdrawal.
Acknowledgments
We would like to thank Ms. Mahalah Buell and Dr. Lisa Eyler for their assistance in this work. This work was supported by NIH grants R01-MH104344, UH2-MH109168, F31MH109218, and the Clinical Translational Research Institute (CTRI) at UCSD.
Footnotes
Conflicts of Interest
The authors report no conflict of interest.
References
- Ashare RL, Hawk LW. Effects of smoking abstinence on impulsive behavior among smokers high and low in ADHD-like symptoms. Psychopharmacology (Berl) 2012;219:537–47. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, McKee SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Exp Clin Psychopharmacol. 2012;20:63–70. doi: 10.1037/a0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin Drug Discov. 2014;9:579–94. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2012;62:1432–1441. doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Herning RI, et al. Smokers of Low-Yield Cigarettes Do Not Consume Less Nicotine. N Engl J Med. 1983:139–142. doi: 10.1056/NEJM198307213090303. doi:10.1056. [DOI] [PubMed] [Google Scholar]
- Burke DA, Heshmati P, Kholdebarin E, Levin ED. Decreasing nicotinic receptor activity and the spatial learning impairment caused by the NMDA glutamate antagonist dizocilpine in rats. Eur J Pharmacol. 2014;741:132–139. doi: 10.1016/j.ejphar.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Halberstadt AL, van Enkhuizen J, et al. Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: evidence from no-light and pharmacological challenges. Psychopharmacology (Berl) 2016:1–13. doi: 10.1007/s00213-016-4389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Young JW. The Five-Choice Continuous Performance Task (5C-CPT): A Cross-Species Relevant Paradigm for the Assessment of Vigilance and Response Inhibition in Rodents. 2016 doi: 10.1002/cpns.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane MA, Schoenfeld DA, Barr RS, et al. Predictors of early abstinence in smokers with schizophrenia. J Clin Psychiatry. 2008;69:1743–50. doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, et al. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophr Res. 2004;70:263–75. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–76. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey A, Bliss R, Hitchcock J. Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–91. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: Effects on attention and nicotine-induced attentional enhancement. Psychopharmacology (Berl) 2011;217:75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Hall FS, Der-Avakian A, Gould TJ, et al. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev. 2015;58:168–185. doi: 10.1016/j.neubiorev.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-J, Choi S-S, Lee J-Y, et al. Antinociceptive effect of nicotine in various pain models in the mouse. Arch Pharm Res. 2005;28:209–15. doi: 10.1007/BF02977717. [DOI] [PubMed] [Google Scholar]
- Harrison E, Coppola S, McKee S. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Exp Clin Psychopharmacol. 2009;17:91–98. doi: 10.1037/a0015657.Nicotine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufroid V, Lison D. Urinary cotinine as a tobacco-smoke exposure index. A minireview. Int Arch Occup Environ Health. 1998;71:162–168. doi: 10.1007/s004200050266. [DOI] [PubMed] [Google Scholar]
- Hayward A, Tomlinson A, Neill JC. Low attentive and high impulsive rats: A translational animal model of ADHD and disorders of attention and impulse control. Pharmacol Ther. 2016;158:41–51. doi: 10.1016/j.pharmthera.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–23. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jackson A, Silk S, Buhidma Y, Shoaib M. Varenicline, the clinically effective smoking cessation agent, restores probabilistic response reversal performance during withdrawal from nicotine. Addict Biol. 2016 doi: 10.1111/adb.12423. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Walters CL, Miles MF, et al. Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology. 2009;57:347–355. doi: 10.1016/j.neuropharm.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Homa DM, O’Connor E, et al. Current Cigarette Smoking Among Adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64:1233–40. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of alpha7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behav Brain Res. 2005;162:143–52. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: Role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kozink RV, Kollins SH, McClernon FJ. Smoking withdrawal modulates right inferior frontal cortex but not presupplementary motor area activation during inhibitory control. Neuropsychopharmacology. 2010;35:2600–6. doi: 10.1038/npp.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–13. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes DA, Galley HF, Moura APS, Webster NR. Brief isoflurane anaesthesia affects differential gene expression, gene ontology and gene networks in rat brain. Behav Brain Res. 2016;317:453–460. doi: 10.1016/j.bbr.2016.09.045. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, et al. CNTRICS final animal model task selection: Control of attention. Neurosci Biobehav Rev. 2013;37:2099–2110. doi: 10.1016/j.neubiorev.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Addicott MA, Sweitzer MM. Smoking Abstinence and Neurocognition: Implications for Cessation and Relapse. In: Balfour DJK, Munafò MR, editors. The Neurobiology and Genetics of Nicotine and Tobacco, Current topics in behavioral neurosciences. 2015. pp. 193–227. [DOI] [PubMed] [Google Scholar]
- Naviaux JC, Wang L, Li K, et al. Antipurinergic therapy corrects the autism-like features in the Fragile X ( Fmr1 knockout ) mouse model Antipurinergic therapy corrects the autism-like features in the Fragile X ( Fmr1 knockout ) mouse model. Mol Autism. 2015;6:1–19. doi: 10.1186/2040-2392-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naviaux RK, Zolkipli Z, Wang L, et al. Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model. PLoS One. 2013;8:e57380. doi: 10.1371/journal.pone.0057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4:305–309. doi: 10.1080/14622200210142715. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, et al. Varenicline Improves Mood and Cognition during Smoking Abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028.Varenicline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistillo F, Fasoli F, Moretti M, et al. Chronic nicotine and withdrawal affect glutamatergic but not nicotinic receptor expression in the mesocorticolimbic pathway in a region-specific manner. Pharmacol Res. 2016;103:167–176. doi: 10.1016/j.phrs.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011;32:281–9. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Downey KK, Snedecor SM, et al. Smoking patterns and abstinence effects in smokers with no ADHD, childhood ADHD, and adult ADHD symptomatology. Addict Behav. 2003;28:1149–1157. doi: 10.1016/S0306-4603(02)00223-X. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacol Biochem Behav. 2009;92:117–123. doi: 10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, et al. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet. 2012;42:133–150. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, et al. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl) 2010;212:537–49. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Romberg C, Bussey TJ, Saksida LM. Paying more attention to attention: Towards more comprehensive cognitive translation using mouse models of Alzheimer’s disease. Brain Res Bull. 2013;92:49–55. doi: 10.1016/j.brainresbull.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–8. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178:211–22. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self- administration in rats. Psychopharmacology (Berl) 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Role of α7- and β4-Containing Nicotinic Acetylcholine Receptors in the Affective and Somatic Aspects of Nicotine Withdrawal: Studies in Knockout Mice. Behav Genet. 2012a;42:423–436. doi: 10.1007/s10519-011-9511-0.Role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 2012b;221:317–327. doi: 10.1007/s00213-011-2578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Grayson B, Marsh S, et al. Putative therapeutic targets for symptom subtypes of adult ADHD: D4 receptor agonism and COMT inhibition improve attention and response inhibition in a novel translational animal model. Eur Neuropsychopharmacol. 2015;25:454–467. doi: 10.1016/j.euroneuro.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Tsaur S, Strasser AA, Souprountchouk V, et al. Time dependency of craving and response inhibition during nicotine abstinence. Addict Res Theory. 2015;23:205–212. doi: 10.3109/16066359.2014.953940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress A Report of the Surgeon General. A Rep Surg Gen. 2014:1081. [Google Scholar]
- van Enkhuizen J, Young JW. Nicotine withdrawal and attentional deficit studies across species: Conflation with attentional dysfunction in psychiatric patients. In: Scott Hall F, Young JW, Der-Avakian A, editors. Negative Affective States and Cognitive Impairments in Nicotine Dependence. Elsevier; London, United Kingdom: 2016. [Google Scholar]
- Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem Pharmacol. 2013;85:1713–1720. doi: 10.1016/j.bcp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Yildirim E, Connor DA, Gould TJ. ABT-089, but not ABT-107, ameliorates nicotine withdrawal-induced cognitive deficits in C57BL6/J mice. Behav Pharmacol. 2015;26:241–248. doi: 10.1097/FBP.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–55. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, et al. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry. 2013a;3:e324. doi: 10.1038/tp.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kamenski ME, Higa KK, et al. GlyT-1 Inhibition Attenuates Attentional But Not Learning or Motivational Deficits of the Sp4 Hypomorphic Mouse Model Relevant to Psychiatric Disorders. Neuropsychopharmacology. 2015:1–12. doi: 10.1038/npp.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, et al. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013b;240:119–33. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, et al. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes, Brain Behav. 2011a;10:720–733. doi: 10.1111/j.1601-183X.2011.00711.x.Delayed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, et al. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011b;222:183–92. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]