Abstract
Background
Forced expiratory volume in 1 second (FEV1) is an established marker of cystic fibrosis (CF) disease progression that is used to capture clinical course and evaluate therapeutic efficacy. The research community has established FEV1 surveillance data through a variety of observational data sources such as patient registries, and there is a growing pipeline of new CF therapies demonstrated to be efficacious in clinical trials by establishing improvements in FEV1.
Results
In this review, we summarize from a statistical perspective the clinical relevance of FEV1 based on its association with morbidity and mortality in CF, its role in epidemiologic studies of disease progression and comparative effectiveness, and its utility in clinical trials. In addition, we identify opportunities to advance epidemiologic research and the clinical development pipeline through further statistical considerations.
Conclusions
Our understanding of CF disease course, therapeutics, and clinical care has evolved immensely in the past decades, in large part due to the thoughtful application of rigorous research methods and meaningful clinical endpoints such as FEV1. A continued commitment to conduct research that minimizes the potential for bias, maximizes the limited patient population, and harmonizes approaches to FEV1 analysis while maintaining clinical relevance, will facilitate further opportunities to advance CF care.
Keywords: disease progression, FEV1 endpoints, longitudinal, lung function, spirometry
1. Introduction
Cystic fibrosis (CF) is a heterogeneous disease characterized by a multitude of clinical and biologic indications; however, the hallmark clinical course of CF is progressive loss of lung function with eventual respiratory failure. It can be argued within the context of CF that lung function is perhaps the most prominent measure of disease severity, progression, and therapeutic efficacy. While pulmonary function tests can yield measurements of lung capacity, forced expiratory flow, vital capacity and residual volume, the primary spirometric result of interest in CF is forced expiratory volume in 1 second (FEV1), an index of airway obstruction that has played a critical role in both clinical care and research.
Over the past 50 years, FEV1 decline has been consistently associated with morbidity and mortality among individuals with CF (1) (2), as well as greater risk of pulmonary exacerbation, hospitalizations and colonization with Pseudomonas aeruginosa (3, 4). FEV1 has also been an important primary endpoint in pivotal clinical trials assessing the efficacy of new therapies (5) (6). Historically, researchers have used varied approaches to analyze FEV1, which has limited comparisons across studies and universal interpretation of findings. Given the importance of FEV1 in CF research and care and the analytic challenges that emerge, this review highlights the use of FEV1 in epidemiologic studies and clinical trials, describes the heterogeneity among analytic methods related to FEV1, and provides recommendations for continued application of methods to optimally utilize this endpoint. The recommendations suggested within this review may be useful to CF researchers, epidemiologists and statisticians, as well as clinicians who wish to apply research findings to patient care.
2. FEV1 in CF Epidemiologic Studies: Analytic Approaches for Establishing Associations with Morbidity and Mortality
Patient registries and large scale, epidemiological cohort studies are instrumental to understanding the natural history of disease, identifying risk factors for clinical outcomes, and generating hypotheses for prospective interventional studies. Much of the epidemiologic evidence of FEV1-specific associations has been derived from CF patient registries, which actively collect clinical data on lung function and other markers of disease (7) (8) (9) (10) (11). The CF research community is fortunate to have access to a wealth of longitudinal data for these purposes, which together with the recent advances in analytical methodologies provide a tremendous opportunity to further our understanding of the mechanisms behind disease progression.
Key Points: For advanced statistical methods to be effectively utilized by the clinical research community they ought to.
Be accessible and enable researchers to implement and replicate the methodology.
Offer significant advancement over traditional, simpler methodology.
Produce clinically relevant estimates of effect that can be interpreted by clinicians.
2.1 FEV1 as a Predictor of Survival
The importance of FEV1 in CF disease progression has its origin in survival analyses, which have led to prognostic tools for mortality(12) (13) (2). These models feature survival as the outcome, employing Cox regression to estimate the hazard ratio or comparative risk of dying over the follow up period between cohorts with higher and lower FEV1. Alternative approaches have utilized logistic regression to estimate the odds of dying over a short time period(14). Predominant approaches are shown in the first column of Table 1. The choice of methodology depends on the question as well as the available data. If death occurs within a short time frame of the exposure, or time to death is not of interest, then logistic regression analyses may be appropriate. However, in studies with longer follow-up, varying follow-up times, and even loss to follow-up, the Cox proportional hazard model is preferred. While logistic regression may be an easier model framework for clinical researchers to implement and interpret, its use for modeling death probabilities could lead to bias and inaccurate survival predictions in the presence of incomplete follow-up data.
Table 1.
Heterogeneity in the analytic approaches in CF epidemiologic research of FEV1.
| Role of FEV1 | ||
|---|---|---|
|
| ||
| Predictor/Marker | Longitudinal Outcome | |
|
|
|
|
| Survival | Rate of Decline | Modeling Covariance* |
|
|
|
|
Cox Regression(2)
|
Marginal Models
|
Unstructured covariance
|
Abbreviations: generalized estimating equations (GEE); hazard ratio (HR); odds ratio (OR). *Extent of correlation between temporal FEV1 measurements should be examined a priori, in order to determine an appropriate covariance model.
Strictly speaking, most of the published studies using FEV1 to predict survival are derived from population studies, and cannot necessarily be extrapolated to the individual patient. While some studies have evaluated overall goodness of fit of their models, a limited number of studies have validated the predictive models against the probability of dying for individual patients (14, 15). Ideally, for prediction models to be utilized for individual patient predictions, appropriate methods that assess prediction accuracy, including sensitivity, specificity, and positive and negative predictive values, are necessary. There are several analytic approaches that can be used to validate a prediction model, including (1) splitting the cohort into training and testing set(s), such as in k-fold cross-validation; (2) bootstrapping, which involves simulation and training/testing a number of datasets; (3) receiver-operator characteristics (e.g. area under the curve). Lastly, it may be helpful to validate a prediction model using an independent cohort, but differences in healthcare systems or underlying populations should be noted. For example, application of the 5 year survivorship model of CF by Liou and colleagues(2) to an Italian cohort by Buzzetti and colleagues(16) demonstrated lack of accuracy in predicting survival; this was likely due to significant differences between the model building and validation cohorts.
2.2 Modeling Longitudinal Change in FEV1 including Rate of Decline
FEV1 progression over time is often estimated using either marginal models or mixed effects (conditional) models summarized in the second column of Table 1. Determining which of these two approaches to use for FEV1 analysis depends on whether it is of interest to make inferences about the CF population as a whole (e.g. “on average”) or about individuals with CF. For example, Dasenbrook and colleagues determined the impact of MRSA acquisition on lung function decline using a marginal model (17). Their study objective was to understand how the average rate of FEV1 decline in the CF population would differ between groups with and without persistent methicillin-resistant Staphylococcus aureus (MRSA) infection. In another study, the aim was to estimate the effect of persistent MRSA infection on accelerating an individual patient’s FEV1 decline; the authors employed a mixed effects model to enable this estimation (18). This approach provided subject-specific deviations from the cohort’s overall average FEV1 progression.
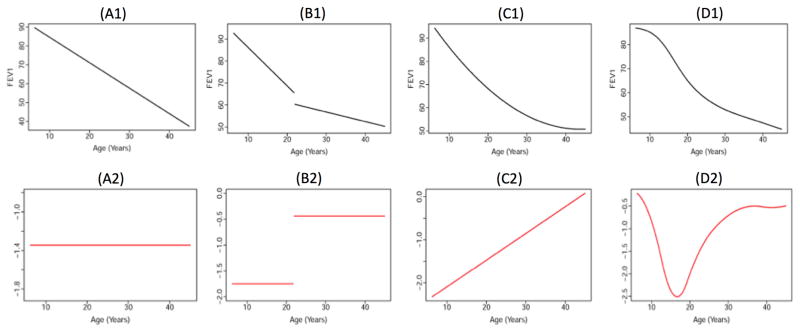
Finding predictors and/or treatments that are associated with slower rate of FEV1 decline remains an important clinical objective within the context of epidemiologic and comparative effectiveness, but it is unclear how to operationalize this measure as a rate of change (19). The predominant analytic approach has been to estimate longitudinal changes in FEV1 using slopes (Figure A1), which assumes a constant rate of decline for the population and to incorporate random effects for subject-specific variation in the aforementioned mixed effects models (20) (21) (22). Measuring FEV1 change as a slope provides readily interpretable information on the rate of decline, assuming that FEV1 changes at the same rate (Figure A2). However, recent epidemiologic studies suggest that FEV1 progression is not constant (i.e. linear) over age. For example, to capture nonlinear progression with slopes, researchers have stratified analyses by age(17) or employed change-point models (23) (24) (25) (26) (Figure B1), which enable modeling of different rates of decline depending upon the age strata (Figure B2). Another example is to include a quadratic term to the model(27), which provides a more smooth, curvilinear shape for overall progression (Figure C1); the resulting rate of change varies over time in a linear fashion. The assumption is that FEV1 decline becomes less severe as a person with CF ages (Figure C2); this is indicative of the “ceiling” and “floor” effects that have been noted with FEV1 decline, as younger individuals with higher lung function tend to have steeper decline than older individuals with lower lung function who exhibit the “floor” effect(21). An alternative is to model FEV1 progression with splines(15) (28) (29). Similar to change point models, this approach provides nonlinear fitting within more precise intervals of age (Figure D1) and can identify intervals of severe decline (Figure D2). When working with longitudinal FEV1, exploratory analyses should include 1) plots of observed patient trajectories both over follow up and patient age; 2) graphical assessment of the degree of nonlinearity present in the data. Correctly fitting the null model for FEV1 progression (i.e. without including explanatory variables) is a prelude to identifying risk factors for more progressive decline.
FIGURE 1. Shapes of FEV1 progression assumed in CF epidemiologic studies.
The top panel (A1–D1) shows age-related FEV1 (expressed as % of predicted on the y-axis) over age (in years on the x-axis). The bottom panel (A2–D2) contains the corresponding rates of change, or derivatives, for the FEV1 curves from the top panel (expressed as annual rate of change in FEV1% predicted). Each black line or curve represents the age-related FEV1 trend that has been proposed in epidemiologic studies of long-term CF disease progression, including: linear progression (A1) that corresponds to a constant rate of decline (A2); piecewise or stratified models by age (B1) that correspond to constant rate of decline within each stratum (B2); curvelinear progression modeled with a quadratic term (C1), yielding rate of decline that becomes less severe with age (C2); a semiparametric model for more curvature at specific intervals of age (D1), producing rate of decline that can vary with age in a nonlinear manner (D2). Additional description is provided in Section 2.2.
When modeling longitudinal data, special considerations need to be made for sources of variability. In most longitudinal studies, FEV1 variability is comprised of three sources: between patients, within an individual patient, and measurement error. Appropriate covariance models (for the correlation of FEV1 measurements collected over time), specified in the third column of Table 1, can account for each of these three sources of variation, thereby improving individual predictive accuracy of the FEV1 trajectory. This is important because correlation between a CF patient’s initial and subsequent FEV1 observations may be sustained over as much as 15 years(22). Further, increased FEV1 variability has been associated with worse decline in pulmonary function (30). Not accounting for these sources of variation can lead to higher Type I error rates (false positives) when, for instance, testing for differences in rate of decline between different patient groups, diminished precision of model estimates, and incorrect conclusions about the degree of influence that certain predictors have on FEV1 decline.
Key Points: Longitudinal Analysis of FEV1 Data.
Marginal models (such as through generalized estimating equations [GEE] that account for repeated measures) provide average rates of change in FEV1.
Mixed models are more complex than marginal models, but enable estimation of both the average rates of change and individual patient trajectories.
When it is of clinical interest, methods are available to estimate FEV1 variability and correlation between measurements within an individual patient.
Exploratory analyses can be used to determine the assumption of linearity of the slope over time in epidemiologic studies. Either marginal models or mixed models can accommodate non-linearity.
2.3 Key Sources of Bias in the Analysis of FEV1 in Epidemiologic Studies
Perhaps the bias most pertinent to longitudinal FEV1 analysis is bias due to missing data, particularly with dropout or delayed entry. For example, an extreme form of dropout bias can occur when patients have died over the follow up period of interest. Most longitudinal modeling approaches produce estimates of longitudinal FEV1 that ignore this sentinel event which may lead to optimistic projections of FEV1 over time as those with presumably the worst lung function are dropping out of the analyses. Left truncation due to delayed entry, which has had relatively less attention until recently, can occur because a patient’s FEV1 data is not observed at the earliest time of valid collection (typically 6 years of age) or due to CF diagnosis at a later age (31). Universal US newborn screening and prolific modern genotyping will reduce issues related to left truncation bias in CF registries going forward; it will not however address issues related to unavailable pulmonary function testing among very young children. Currently, this bias is often addressed by including birth cohort or age at CF diagnosis as a model covariate or by performing stratified analyses based on these variables. Principled missing data assumptions and methods should be selected to address these biases, and have been covered in the statistics literature (32).
Jointly modeling longitudinal FEV1 and survival can minimize bias arising from delayed entry, non-random dropout and loss to follow up. For example, in a study of center-specific FEV1 over time and survival for a cohort of delF508 homozygous CF patients, longitudinal FEV1 and survival were modeled simultaneously (33). This type of approach is referred to as a joint model in the statistics literature and continues to gain traction, with CF registry data used as an exemplar for methods development (34). Left-truncation may also be incorporated in joint models of FEV1 and survival (35). These studies show improved predictive accuracy for both survival and FEV1, particularly when there is a high probability of censoring. Although the models require more careful interpretation and sophisticated software, introductory examples are provided in the chronic disease literature (36).
Comparative effectiveness registry-based studies in which FEV1 is the outcome of interest may be subject to confounding by indication, which is a type of treatment-selection bias that occurs in observational studies and often results from sicker patients being more likely to receive the treatment of interest. In particular, confounding by indication affects clinical outcomes based on a physician’s decision to treat a CF patient, for example, in reaction to symptoms or changes in FEV1. These decisions often affect both the exposure and outcome of interest. This type of bias creates statistical challenges in the development of models to examine comparative effectiveness, and the issues have been highlighted over the years (37) (38),(39). A pervasive example is when using a retrospective cohort to compare clinical outcomes either between patients receiving a particular therapy versus not or pre- to post therapy, as has been done for commonly used CF therapies such as inhaled tobramycin(25), rhDNase(24), and inhaled corticosteroid therapy(23). Propensity-score matching(40) and instrumental variables(41) approaches have been used in epidemiologic studies of treatment effectiveness and FEV1 decline to improve upon methods to adjust for confounding that are limited to relatively few matching variables.
Key Points: Addressing Bias in Epidemiologic Studies.
Selection bias should be addressed via sensitivity analyses to evaluate the impact of cohort selection on findings.
Confounding by indication cannot be avoided but has been addressed in prior CF studies using propensity scores or instrumental variables.
Missing data should be thoroughly evaluated and described for all epidemiologic studies. Appropriate sensitivity analyses should be conducted using missing data methodology to evaluate the impact of missing data on key results.
2.4 Choice of Reference Equations for use of FEV1 as Percentage of that Predicted for a Comparator Population
Lung size and function are related to sex, body size and age, such that lung function increases rapidly during childhood and slowly declines in adulthood. Lung function outcomes are typically compared to a reference population using reference equations, which account for height, age, sex and ethnicity. Applicable in some regard to both epidemiologic studies and clinical trials is the choice of reference equations for FEV1, which may impact interpretation of results. Over short periods of time, and within an individual, the raw unit for FEV1, liters, is appropriate for analysis; however, comparisons over extended time or across patients of varying age, height, sex and ethnicity may need to be standardized in the absence of a control group. There are numerous reference equations for FEV1; most recently, the CF community adopted equations developed by Wang(42) and Hankinson(43) to express FEV1 in terms of percent predicted. Equations by Wang and colleagues(42) are typically used for males between ages of 6–17 years and females between ages 6–15 years; Hankinson equations(43) are used for subsequent years thereby creating a discontinuity in FEV1 predicted as a patient ages from one standard to the other. The advent of newer multi-ethnic all-age reference equations by the Global Lung Function Initiative (GLI) provides a more streamlined conversion of FEV1 in liters to sex-age-height and ethnic specific percent-predicted values (44). GLI reference equations are now being used in multiple national patient registry reports (45) (46) (47) (48). Furthermore, updated epidemiologic studies of FEV1 progression, particularly for patients during adolescence and early adulthood, are needed due to differences in percent-predicted values obtained using GLI reference equations, compared to aforementioned methods (29). From a statistical perspective, the variability of lung function measurements varies with age and percent predicted may not be the optimal way to track changes in FEV1 in an individual over time. The GLI also allow calculation of z-scores, which may provide an alternative way to track changes over time. CF-specific standardizing equations have been proposed(49); however, evolving therapies that improve the lung function of the CF population can render such equations obsolete within a few years.
Key Points: Selection of Reference Equations.
In many studies and in particular for longitudinal epidemiologic studies of pediatric patients, it is useful for FEV1 values to be interpreted as a standardized outcome (% predicted or z-scores) using a standard reference equation.
The GLI reference equations are endorsed by the major respiratory societies, and have been adopted by many CF registries. The use of GLI equations for epidemiological and clinical studies will help facilitate comparison between studies.
3. FEV1 in CF Clinical Trials: A Critical Clinical Endpoint with Numerous Analytic Options
While there has been a dramatic increase in median survival over the last two decades, respiratory failure remains the leading cause of death among individuals with CF (45) (46) (47) (48). The strong relationship between FEV1 and the pathophysiology of this chronic respiratory disease, combined with the ability to be objectively and reliably measured relative to other endpoints, has made FEV1 a key endpoint to measure both efficacy and safety in CF clinical trials.
3.1 Regulatory Considerations
While FEV1 is a necessary safety endpoint in nearly all CF clinical trials, it is important to note that the Food and Drug Administration (FDA) and European Medical Agency (EMA) will determine the sufficiency of FEV1 to establish efficacy based on each investigational product and its mechanism of action. FDA-approved therapies for CF based on randomized, placebo-controlled trials, including rhDNase(50), inhaled tobramycin(51), aztreonam for inhalation solution(52, 53), ivacaftor(54), and lumacaftor-ivacaftor(55) all demonstrated significant acute and sustained improvements in lung function. However, ultimate approval for these therapies also relied on significant reductions in pulmonary exacerbations or need for rescue antibiotics. In the case of one of the aztreonam pivotal trials, lung function was a secondary endpoint to a validated patient reported outcome due to FDA requirement.(52, 53) Therapies that are not expected to elicit acute improvements in FEV1 may have a difficult path to regulatory approval. Anti-inflammatories in CF are an example where chronic use may slow the rate of FEV1 decline (56); however, conducting a pivotal trial in this setting becomes daunting with requirements for large sample size and long durations.(57)
3.2 Heterogeneity in the Analysis of FEV1 in CF Clinical Trials
Despite the widespread acceptance of FEV1 as a key clinical efficacy measure (as a primary clinical trial endpoint or otherwise), there is significant variability in the analytic execution of this endpoint which compromises the ability to compare treatment effects across studies. This heterogeneity can be summarized by two key analytic choices: (1) units of FEV1: liters vs. percentage of predicted (and the many prediction equations mentioned in the previous section), and (2) measure of change: absolute vs. relative. Table 2 demonstrates the heterogeneity in FEV1 results reported across key pivotal trials for select chronic therapies that have been adopted into CF clinical care.
Table 2.
Heterogeneity in the reporting of FEV1 endpoints in pivotal clinical trials for select chronic CF therapies.
| Change in FEV1 | FEV1 Units | |
|---|---|---|
| Liters | % Predicted | |
| Relative Change | rhDNase(50), Hypertonic saline(62), Aztreonam(52) | Inhaled tobramycin(51), Azithromycin(63) (64), Aztreonam(52), Ivacaftor(54), Tobramycin inhalation powder ((65)), Mannitol ((66)), Ataluren ((67)) Lumacaftor-Ivacaftor(55) |
| Absolute Change | Azithromycin(63) (64), Hypertonic saline(62), Mannitol(66) | Azithromycin (64), Ivacaftor (54), Ivacaftor-Lumacaftor(55) |
Analytic differences in the use of FEV1 have varied across classes of therapies and regulatory divisions, and there has been no formal guidance on which approach is optimal. The choice between use of liters and percent predicted is often based on clinical interpretation; in particular, since FEV1 % predicted is used more frequently clinically to monitor disease progression for individuals with CF, the clinical community is able to better interpret a change in FEV1 % predicted as opposed to a change in liters. Use of % predicted in a clinical trial does however require more rigor, specifically in terms of specification of how the reference equations will be applied longitudinally and minimizing the impact of height measurement error. Additionally, the use of FEV1% predicted in trial eligibility criteria relies on the standardized use of reference equations across participating centers or centralized calculation through an electronic data capture system or telephone based randomization system.
When change in FEV1 % predicted is a clinical trial endpoint, proper application of the reference equations is imperative. For instance, the aforementioned discontinuity between Wang(42) and Hankinson(43) reference equations could artificially induce changes in lung function due to aging across the equations –not treatment effect. Trial investigators and statisticians can mitigate this by fixing an equation for each individual (their starting age dictates the standardization equation that will be used throughout the trial). However, this does not help for a very long study where a teen ages out of the Wang equations entirely, nor is it conducive to interpretation or reproducibility(58). A single set of reference equations such as the GLI will mitigate this(44), but still rely on the accuracy of height measurements at a given time point. Thus, FEV1 on the liters scale becomes attractive from an analytic perspective, as it will not be impacted by analytic choices in the application of the reference equations nor imprecise measurements of height. In pediatric studies however, both somatic and lung growth must be considered when interpreting change in FEV1 in liters, as improvements due to growth could be misinterpreted as improvements due to treatment in the absence of a placebo group. This is one concern regarding the use for FEV1 in liters in randomized, placebo controlled trials -that it is unknown whether a beneficial treatment effect on lung function is due to direct improvements in the airways or attributable to improvements in growth. On the other hand, improvements in lung function could be argued to be important regardless of mechanism. The choice between use of liters or % predicted as a primary trial endpoint ultimately depends on the characteristics of the study population, the duration of the study, as well as the resources available to accurately compute and implement the chosen reference equations. However, changes in both liters and % predicted should be reported for clinical trials to illustrate the totality of evidence –regardless of the unit chosen for the primary endpoint.
Beyond the decision to analyze FEV1 in terms of liters or as a percentage of predicted is the choice to measure absolute versus relative change, a decision which is also most often driven by clinical interpretation. When trials include participants with significant differences in disease severity, it could be argued for interpretation purposes that the impact of a 5% absolute improvement in FEV1 % predicted is much greater in a participant with a baseline FEV1 of 40% as compared to a participant with a baseline FEV1 of 90%, as this 5% absolute improvement would correspond to 12.5% and 5.6% relative improvements in these participants, respectively. On the other hand, maintaining the measurement of improved change on the absolute scale ensures the trial population overall is benefiting regardless of disease severity. To this end, a 5% absolute increase in FEV1 % predicted is a harder target to reach as compared to a 5% relative improvement. While simulation studies can be performed to examine the impact of these analytic decisions on power and sample size, the parameters to do these simulations are highly dependent on the patient population and expected effect of the specific therapy of interest. Ultimately, examination of each of these measures from earlier phase 1 and 2 data should be used to inform and justify the endpoints used for the pivotal phase 3 study.
Key Point: Ensure Consistency in Clinical Trial Reporting.
Requirements provided by clinicaltrials.gov will encourage sponsors to adhere to standardized reporting of study results.
It is imperative for the community to encourage reporting of FEV1 absolute and relative changes and associated variability in both liters and % predicted to ensure comparisons across CF therapies.
Choice of FEV1 endpoint (liters vs. % predicted) and change estimate (relative or absolute) for pivotal CF trials should be clinically motivated by the class of therapy and patient population of interest, with careful consideration of the relative analytic performance of these measures in early phase studies.
There will be challenges for the use of FEV1 in future clinical trials in an era of disease modifying therapies. As individuals with CF have increased access to therapies that will halt disease progression, the likelihood of a therapeutic agent demonstrating rapid acute improvement in lung function will be diminished. In some patient populations this may be overcome by increasing the sample size of the interventional study to detect smaller effect sizes, but in patients with milder disease where FEV1 is normal there is a need for more sensitive outcome measures, including new lung function tests that can detect early progression of lung disease. This is certainly already the case among the CF pediatric population, many of whom are too young to perform spirometry, or in whom FEV1 is normal well into adulthood. Continued efforts towards the validation of lung function measurements in infants and preschool children, combined with the association of these new measures with long term morbidity, will be critical for advancing the development pipeline of therapies for CF.
4. Conclusions
Studies of CF progression, whether observational or interventional in nature, require careful consideration of the FEV1 outcome. In the orphan disease setting, where there is a limited number of patients available to participate in prospective studies, utilization of retrospective registry data is crucial and resource sparing for addressing many research questions. The ability to combine and compare data from completed CF studies, in addition to promoting transparency of methods, is therefore a necessity. The CF community must continue to work towards consistent data collection and analytic approaches to enable future ancillary studies not only with respect to FEV1, but across all key clinical outcomes. The opportunities to advance methodology are numerous, but must be cogently described and applied to maintain clinical relevance. Researchers must balance the clinical relevance in interpretation of results with the benefits of the sophisticated statistical analyses. Multi-disciplinary efforts between researchers, clinicians, and statisticians will be critical to ensure we are optimally positioned to capture a remarkable and hopeful slowing of disease progression among our CF population as new therapies and advances in clinical care are adopted.
Acknowledgments
We thank the handling editor and reviewers for their thoughtful comments, which have substantially improved the quality and content of the work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest
Author RS received support for this work from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) under award number K25 HL125954. Author SLH received support for this work from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award number P30 DK089507. NMH received support for this work from the NIH UL1TR000423.
References
- 1.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. American Journal of Epidemiology. 1996;143(10):1007–17. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 2.Liou TG, Adler FR, FitzSimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. American Journal of Epidemiology. 2001;153(4):345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatric Pulmonology. 2001;32(4):277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. American Journal of Respiratory and Critical Care Medicine. 2010;182(5):627–32. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing Outcome Measures for the New Era of Drug Development in Cystic Fibrosis. Proceedings of the American Thoracic Society. 2007;4(4):370–7. doi: 10.1513/pats.200703-040BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clinical Investigation (Lond) 2012;2(2):163–75. doi: 10.4155/cli.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatric Pulmonology. 1999;28(4):231–41. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Stern M, Wiedemann B, Wenzlaff P German Cystic Fibrosis Quality Assessment G. From registry to quality management: the German Cystic Fibrosis Quality Assessment project 1995 2006. European Respiratory Journal. 2008;31(1):29–35. doi: 10.1183/09031936.00056507. [DOI] [PubMed] [Google Scholar]
- 9.Buzzetti R, Salvatore D, Baldo E, Forneris MP, Lucidi V, Manunza D, et al. An overview of international literature from cystic fibrosis registries: 1. Mortality and survival studies in cystic fibrosis. Journal of Cystic Fibrosis. 2009;8(4):229–37. doi: 10.1016/j.jcf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore D, Buzzetti R, Baldo E, Forneris MP, Lucidi V, Manunza D, et al. An overview of international literature from cystic fibrosis registries 2. Neonatal screening and nutrition/growth. Journal of Cystic Fibrosis. 2010;9(2):75–83. doi: 10.1016/j.jcf.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Annals of the American Thoracic Society. 2016;13(7) doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 12.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. New England Journal of Medicine. 1992;326(18):1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 13.Hayllar KM, Williams S, Wise AE, Pouria S, Lombard M, Hodson ME, et al. A prognostic model for the prediction of survival in cystic fibrosis. Thorax. 1997;52(4):313–7. doi: 10.1136/thx.52.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. American Journal of Respiratory and Critical Care Medicine. 2002;166(12 Pt 1):1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 15.Aaron SD, Stephenson AL, Cameron DW, Whitmore GA. A statistical model to predict one-year risk of death in patients with cystic fibrosis. Journal of Clinical Epidemiology. 2014 doi: 10.1016/j.jclinepi.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Buzzetti R, Alicandro G, Minicucci L, Notarnicola S, Furnari ML, Giordano G, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. Journal of Cystis Fibrosis. 2012;11(1):24–9. doi: 10.1016/j.jcf.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2008;178(8):814–21. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 18.Sawicki GS, Rasouliyan L, Pasta DJ, Regelmann WE, Wagener JS, Waltz DA, Ren CL. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatric Pulmonology. 2008;43(11):1117–23. doi: 10.1002/ppul.20914. [DOI] [PubMed] [Google Scholar]
- 19.Szczesniak RD, McPhail GL, Li D, Amin RS, Clancy JP. Predicting future lung function decline in cystic fibrosis patients: Statistical methods and clinical connections. Pediatric Pulmonology. 2015 doi: 10.1002/ppul.23357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. Journal of Pediatrics. 1997;131(6):809–14. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 21.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. The Journal of Pediatrics. 2007;151(2):134–9. e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Robinson D, Whitehead M, Diderichsen F, Olesen HV, Pressler T, Smyth RL, et al. Understanding the natural progression in% FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax. 2012;67(10):860–6. doi: 10.1136/thoraxjnl-2011-200953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren CL, Pasta DJ, Rasouliyan L, Wagener JS, Konstan MW, Morgan WJ, et al. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. The Journal of Pediatrics. 2008;153(6):746–51. e2. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Jacobs JR, Yegin A, et al. Clinical use of dornase alpha is associated with a slower rate of FEV1 decline in cystic fibrosis. Pediatric Pulmonology. 2011;46(6):545–53. doi: 10.1002/ppul.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Morgan WJ. Clinical use of tobramycin inhalation solution (TOBI®) shows sustained improvement in FEV1 in cystic fibrosis. Pediatric Pulmonology. 2013 doi: 10.1002/ppul.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss A, Juarez-Colunga E, Nathoo F, Wagner B, Sagel S. A comparison of change point models with application to longitudinal lung function measurements in children with cystic fibrosis. Statistics in Medicine. 2016 doi: 10.1002/sim.6845. [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2007;176(11):1084–9. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szczesniak RD, McPhail GL, Duan LL, Macaluso M, Amin RS, Clancy JP. A semiparametric approach to estimate rapid lung function decline in cystic fibrosis. Annals of Epidemiology. 2013;23(12):771–7. doi: 10.1016/j.annepidem.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Stanojevic S, Bilton D, McDonald A, Stocks J, Aurora P, Prasad A, et al. Global Lung Function Initiative equations improve interpretation of FEV1 decline among patients with cystic fibrosis. European Respiratory Journal. 2015 doi: 10.1183/09031936.00187314. ERJ-01873–2014. [DOI] [PubMed] [Google Scholar]
- 30.Morgan WJ, VanDevanter DR, Pasta DJ, Foreman AJ, Wagener JS, Konstan MW, et al. Forced Expiratory Volume in 1 Second Variability Helps Identify Patients with Cystic Fibrosis at Risk of Greater Loss of Lung Function. The Journal of Pediatrics. 2015 doi: 10.1016/j.jpeds.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 31.VanDevanter DR, Pasta DJ, Konstan MW. Improvements in Lung Function and Height among Cohorts of 6-Year-Olds with Cystic Fibrosis from 1994 to 2012. The Journal of Pediatrics. 2014;165(6):1091–7. e2. doi: 10.1016/j.jpeds.2014.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang KM, Little TD. Principled Missing Data Treatments. Prevention Science. 2016 doi: 10.1007/s11121-016-0644-5. [DOI] [PubMed] [Google Scholar]
- 33.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Statistics in Medicine. 2002;21(9):1271–87. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 34.Barrett J, Diggle P, Henderson R, Taylor-Robinson D. Joint modelling of repeated measurements and time-to-event outcomes: flexible model specification and exact likelihood inference. Journal of the Royal Statistical Society, Series B: Statistical Methodology. 2015;77(1):131–48. doi: 10.1111/rssb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccorelli AV, Schluchter MD. Jointly modeling the relationship between longitudinal and survival data subject to left truncation with applications to cystic fibrosis. Statistics in Medicine. 2012;31(29):3931–45. doi: 10.1002/sim.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asar O, Ritchie J, Kalra PA, Diggle PJ. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. International Journal of Epidemiology. 2015;44(1):334–44. doi: 10.1093/ije/dyu262. [DOI] [PubMed] [Google Scholar]
- 37.Rothman KJ, Wentworth CE. Mortality of cystic fibrosis patients treated with tobramycin solution for inhalation. Epidemiology. 2003;14(1):55–9. doi: 10.1097/00001648-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Schechter MS. Patient registry analyses: seize the data, but caveat lector. The Journal of Pediatrics. 2008;153(6):733–5. doi: 10.1016/j.jpeds.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Sawicki GS, Goss CH. Tackling the increasing complexity of CF care. Pediatric Pulmonology. 2015;50(S40):S74–S9. doi: 10.1002/ppul.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schechter MS, Quittner AL, Konstan MW, Millar SJ, Pasta DJ, McMullen A. Long-term effects of pregnancy and motherhood on disease outcomes of women with cystic fibrosis. Annals of the American Thoracic Society. 2013;10(3):213–9. doi: 10.1513/AnnalsATS.201211-108OC. [DOI] [PubMed] [Google Scholar]
- 41.VanDyke RD, McPhail GL, Huang B, Fenchel MC, Amin RS, Carle AC, et al. Inhaled tobramycin effectively reduces FEV1 decline in cystic fibrosis. An instrumental variables analysis. Annals of the American Thoracic Society. 2013;10(3):205–12. doi: 10.1513/AnnalsATS.201209-082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatric Pulmonology. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 43.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. American Journal of Respiratory and Critical Care Medicine. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 44.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. European Respiratory Journal. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cystic Fibrosis Foundation. Patient Registry Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2014. [Google Scholar]
- 46.UK Cystic Fibrosis Registry. 2015 Annual Data Report. 2016. [Google Scholar]
- 47.CF Canada. The Canadian Cystic Fibrosis Registry: 2013 Annual Report. 2015. [Google Scholar]
- 48.CF Australia. Cystic Fibrosis in Australia 2013: 16th Annual Report. 2015. [Google Scholar]
- 49.Kulich M, Rosenfeld M, Campbell J, Kronmal R, Gibson RL, Goss CH, et al. Disease-specific reference equations for lung function in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2005;172(7):885–91. doi: 10.1164/rccm.200410-1335OC. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine. 1994;331(10):637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 51.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. New England Journal of Medicine. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 52.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2008;178(9):921–8. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Retsch-Bogart GZ, Quittner AL, Gibson RL, Oermann CM, McCoy KS, Montgomery AB, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest Journal. 2009;135(5):1223–32. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New England Journal of Medicine. 2011;365(18):1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. New England Journal of Medicine. 2015 doi: 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- 56.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. New England Journal of Medicine. 1995;332(13):848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 57.Davis PB, Byard PJ, Konstan MW. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatric Research. 1997;41(2):161–5. doi: 10.1203/00006450-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Kirkby J, Aurora P, Spencer H, Rees S, Sonnappa S, Stocks J. Stitching and switching: the impact of discontinuous lung function reference equations. European Respiratory Journal. 2012;39(5):1256–7. doi: 10.1183/09031936.00173011. [DOI] [PubMed] [Google Scholar]
- 59.Konstan MW, Wagener JS, VanDevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV 1 in adults with cystic fibrosis. Journal of Cystic Fibrosis. 2012;11(5):405–11. doi: 10.1016/j.jcf.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic pseudomonas aeruginosa infections, and dornase alfa use. The Journal of Pediatrics. 2008;153(6):752–7. doi: 10.1016/j.jpeds.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Liou TG, Elkin EP, Pasta DJ, Jacobs JR, Konstan MW, Morgan WJ, et al. Year-to-year changes in lung function in individuals with cystic fibrosis. Journal of Cystic Fibrosis. 2010;9(4):250–6. doi: 10.1016/j.jcf.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. New England Journal of Medicine. 2006;354(3):229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 63.Saiman L, Marshall B, Mayer-Hamblett N, Burns J, Quittner A, Cibene D, et al. a multicenter, randomized, placebo controlled, double-blind trial of azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Journal of the American Medical Association. 2003;290:1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 64.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. Journal of the American Medical Association. 2010;303(17):1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 65.Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. Journal of Cystic Fibrosis. 2011;10(1):54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilton D, Bellon G, Charlton B, Cooper P, De Boeck K, Flume PA, et al. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. Journal of Cystic Fibrosis. 2013;12(4):367–76. doi: 10.1016/j.jcf.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Kerem E, Konstan MW, De Boeck K, Accurso FJ, Sermet-Gaudelus I, Wilschanski M, et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respiratory Medicine. 2014;2(7):539–47. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]