Abstract
Objectives
Increased risk of herpes simplex virus 2 (HSV-2) has been proposed as a possible indirect pathway through which hormonal contraceptives (specifically depot medroxyprogesterone acetate [DMPA]) may increase the risk of HIV acquisition among women. We investigated the effects of DMPA on HSV-2 acquisition among female sex workers.
Methods
Longitudinal data were drawn from a prospective cohort of sex workers in Vancouver, Canada. The primary outcome was HSV-2 seroconversion. Extended cox regression analyses were used to model the independent effect of DMPA use on HSV-2 acquisition.
Results
Between January 2010 and February 2014, 149 HSV-2 seronegative women were enrolled, contributing to 228 person-years (py) of follow-up. Of these, 19 (13.3%) reported DMPA use. There were 39 HSV-2 seroconversions (12 among DMPA users and 27 among non-users) over the study period (median follow-up of 18.6 months (IQR 8.4–29.9)), resulting in an overall incidence rate of 17.1 cases per 100 py (95% CI 12.4 to 23.6). Incidence rates were higher among DMPA users (57.4 cases per 100 py, 95% CI 31.4 to 105.0) compared with non-users (13.1 cases per 100 py, 95% CI 8.9 to 19.1). After adjusting for key confounders, use of DMPA remained an independent predictor of HSV-2 acquisition (adjusted HR 4.43, 95% CI 1.90 to 10.35).
Conclusions
The high observed incidence rates of HSV-2, together with a strong association between DMPA exposure and HSV-2 acquisition, raise serious concerns about the provision of optimal reproductive and sexual healthcare to sex workers in this setting. Given the known links between HSV-2 and HIV, our findings underscore the need for further research to better understand the potential association between DMPA and increased risk of HSV-2 and other STIs to help inform the development of safer reproductive choices for women worldwide.
Keywords: HSV-2, HIV, medroxyprogesterone acetate, sex workers, contraceptive agents, reproductive health
INTRODUCTION
Unintended pregnancy remains a significant global public health challenge, with worldwide rates of around 41% [1]. Unintended pregnancy, in turn, has negative impacts on women’s and newborn’s health, as well as imposes a substantial social and economic burden for women and their families, as well as to the larger society [1 2]. Access to effective, safe and acceptable contraceptives that are tailored to suit individual women’s needs is crucial to women’s reproductive choice and control. In particular, long-acting, reversible contraceptive (LARC) methods offer a highly effective female-controlled option that does not require daily compliance [2].
A growing body of data suggests that depot medroxyprogesterone acetate (DMPA, Depo-Provera®), one of the most widely used hormonal LARC, may increase the risk of acquiring and transmitting HIV and other sexual transmitted infections (STIs) among women [1 3–5]. This is particularly concerning given the widespread use of DMPA, especially in regions with both high HIV and unintended pregnancy burden [1].
The underlying biological mechanisms through which DMPA use may contribute to increased risk of HIV acquisition are not well understood. Proposed mechanisms include thinning or disruption of the cervicovaginal epithelium, interferences with innate and adaptive soluble and cellular local and systemic immune response, and alteration in the vaginal microbiome [6]. DMPA may also indirectly raise the risk of HIV by facilitating herpes simplex virus type 2 (HSV-2) genital infection, a well-known risk factor for HIV acquisition as well as substantial morbidity on its own [6 7].
Despite high rates of unmet reproductive health needs and heightened vulnerability to STIs among women sex workers, research on the effects of DMPA on HSV-2 and HIV acquisition among this population is limited, particularly outside Sub-Saharan Africa [8 9]. We therefore conducted this study to investigate the effect of DMPA use on HSV-2 acquisition within a longitudinal community cohort of street and off-street sex workers in Vancouver, Canada.
METHODS
The reporting of this study complies with the STROBE guidelines for observational studies [10].
Study Design and population
Data for this analysis was drawn from An Evaluation of Sex Workers’ Health Access (AESHA), an ongoing prospective cohort of more than 740 women sex workers in Metro Vancouver that initiated enrolment in 2010. Eligibility criteria include, age ≥14 years, self-identification as women (including transgender women), and having exchanged money for sex in the previous 30 days. Participants are recruited through a combination of outreach to outdoor/ public, indoor sex work venues, and off-street self-advertizing spaces across Metro Vancouver, using time-location sampling [11]. Sampling and recruitment procedures have been described in detail elsewhere [12].
The current analysis was restricted to female sex workers who were seronegative for HSV-2 at baseline and who completed at least one follow-up visit between January 1, 2010 and February 28, 2014.
Procedures
After providing written informed consent, at baseline and semiannually thereafter, participants complete an interviewer-administered questionnaire. The questionnaire collects sociodemographic information, sex work patterns, sexual health (including contraceptives and condom use), healthcare services access and utilisation, and physical, social-structural exposures of the working and living environments. In addition, at each visit, a project nurse offers participants HIV and Hepatitis C (HCV) and other STIs testing, alongside pretest and post-test counselling. STI testing includes HSV type-specific serology (TSS), non-treponemal (rapid plasma reagin (RPR)) and treponemal tests (Treponema pallidum haemagglutinin assay (TPHA)) for all samples with positive RPR, and nucleic acid amplification test (NAAT) testing for Neisseria gonorrhoeae and Chlamydia trachomatis in urine specimens. HSV-2 diagnosis is done using a two-step approach. First, serum samples are screened with a non-specific EIA HSV IgG. If this test is reactive, the serum is tested for anti-HSV-2 using the TSS Focus HerpeSelect-2 IgG EIA (Focus Diagnostics, Cypress, California, USA) [13]. Sensitivity and specificity of TSS Focus HerpeSelect-2 IgG EIA are 96–100% and 96–97%, respectively [14]. The project nurse also provides basic treatment for STIs onsite, as well as referrals to health services when needed. This includes treatment for primary or recurrent episodes of genital herpes with either acyclovir or valacyclovir, in accordance with provincial STI treatment guidelines [15]. Participants receive an honorarium of $C40 at each visit for their time and expertise.
Measures
The primary outcome was time to HSV-2 seroconversion, defined as the time interval from study recruitment to the estimated date of HSV-2 acquisition during follow-up. Date of HSV-2 seroconversion was estimated as the midpoint between the last HSV-2 negative test and the first HSV-2 positive test. Participants who remained HSV-2 seronegative throughout the study period were censored at the time of their last HSV-2 serologic assay before February 28, 2014.
The primary explanatory variable of interest was a time-varying measure of self-reported DMPA use for pregnancy prevention in the 6-month period preceding the study interview (yes vs. no). Participants were defined as DMPA users at each follow-up visit if they had been prescribed and received DMPA at any time in each 6-month period. We also considered several secondary factors that in previous analyses have been shown to potentially confound the relationship between hormonal contraception and HIV/STIs risk [4 8 16 17]. Time-fixed variables of interest at baseline included the following socio-demographic characteristics: age (per year older), Indigenous ancestry (yes vs. no), and highest educational attainment (high school or postsecondary education vs. less than high school completion). Time-varying variables included individual-level factors such as HIV status and incident STIs (syphilis, N. gonorrhoeae and C. trachomatis) based on biological testing (positive vs. negative); interpersonal-level factors such as average number of clients per week, number of male non-commercial partners, and inconsistent use of condoms by both clients and male non-commercial partners (yes vs. no); and characteristics of the work environment such as primary place of servicing clients (formal sex work establishment/in-call venue; informal indoor/out-call venues; or outdoor/public space). Among participants without a history of syphilis at baseline (i.e., RPR and TPHA negative), incident syphilis was defined as a positive TPHA and RPR titres ≥1:8, while for those with a history of syphilis (i.e., TPHA positive), it was defined as a fourfold increase in RPR titres between visits. All time-varying variables were time-updated at each semi-annual follow-up visit and refer to the 6-month period prior to the interview. Missing data was overall low, with all key variables having <1% of missing data.
Statistical Analysis
Initially, we examined baseline characteristics of the study sample stratified by DMPA exposure at any time during the study period. Categorical variables were analyzed using the Pearson’s chi-squared test (or Fisher’s exact test in the presence of small cell counts) and continuous variables were analyzed using the Wilcoxon rank sum test.
Next, we calculated incidence density rates of HSV-2 infection using the person-time method. We also estimated the cumulative incidence, stratified by DMPA exposure at some point during the study period, using the Kaplan Meier method, and compared their survival curves using the log-rank test.
To assess the independent effect of DMPA use on HSV-2 infection risk, we used the extended Cox regression analyses. First, a bivariable Cox regression model was run to estimate the unadjusted hazard ratios of DMPA exposure and secondary variables on the risk of HSV-2 acquisition. Then, a multivariable confounding model was constructed using a variable selection process described by Maldonado and Greenland [18] that we have used extensively in previous research. As a first step, we fitted a full model that included the primary explanatory variable of interest, and potential confounders that were chosen a priori (i.e., age) or associated in the unadjusted analyses at p<0.20. We noted the value of the coefficient associated with DMPA exposure in the full model, and then, in a stepwise manner, removed the secondary explanatory variable corresponding to the smallest relative change on the effect of DMPA use on HSV-2 risk. This iterative process was continued until the minimum relative change exceeded 5%. The remaining variables were considered as confounders in the multivariable analysis.
In a sensitivity analysis, based on a priori conceptual evidence of potential confounding effects [4 8 16 17], all variables were forced into the final model. All statistical analyses were performed using the SAS software version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Between January 2010 and February 2014, 744 women sex workers were enrolled in AESHA cohort. Among the 231 women sero-negative for HSV-2 at baseline, 143 had at least one follow-up visit and were thus included in this analysis, contributing to 228 person-years (py) of follow-up. Baseline characteristics of study participants, stratified by use of DMPA at some point during the study period are presented in Table 1.
Table 1. Baseline characteristics, stratified by DMPA use at some point during the study period, of HSV-2 seronegative female sex workers in Vancouver, Canada (N=143).
| Characteristic | Total, n (%) (N = 143) | DMPA Use | p - value | |
|---|---|---|---|---|
|
| ||||
| Yes, n (%) (N = 19) | No, n (%) (N = 124) | |||
| Individual | ||||
| Age (med, IQR) | 34 (27–41) | 27 (23–33) | 35.5 (28–42) | 0.004 |
| Aboriginal ancestry | 40 (28.0) | 11 (57.9) | 29 (23.4) | 0.002 |
| High school completion | 93 (65.0) | 8 (42.1) | 85 (68.6) | 0.024 |
| HIV seropositive* | 7 (4.9) | 4 (21.1) | 3 (2.4) | 0.006 |
| STI* | 5 (3.5) | 3 (15.8) | 2 (1.6) | 0.017 |
| Interpersonal | ||||
| Average number of clients per week (med, IQR)* | 9 (5–15) | 9 (5–18) | 9 (5–15) | 0.852 |
| Inconsistent condom use by clients* | 25 (17.5) | 8 (42.1) | 17 (13.7) | 0.006 |
| Number of male intimate/non-commercial partners (med, IQR)* | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.149 |
| Inconsistent condom use by non-commercial partners* | 62 (43.4) | 10 (52.6) | 52 (41.9) | 0.214 |
| Environmental | ||||
| Primary place of servicing clients* | ||||
| Formal sex work/in-call establishment | 70 (48.9) | 5 (26.3) | 65 (52.4) | REF |
| Informal indoor venue | 29 (20.3) | 4 (21.1) | 25 (20.2) | 0.303 |
| Outdoor/public space | 44 (30.8) | 10 (52.6) | 34 (27.4) | 0.022 |
Time-updated variable using last 6 months as a reference point.
DMPA: depot medroxyprogesterone acetate; IQR: Interquartile range; STI: sexual transmitted infection
Overall, 19 women (13.3%) reported use of DMPA for pregnancy prevention at some point during the study period. As shown in Table 1, there were significant differences between DMPA users and non-users. Specifically, DMPA users were younger, had lower educational levels, and were more likely to be of Indigenous ancestry. DMPA users were also more likely to be living with HIV, have a concurrent STI, and report inconsistent use of condoms by clients.
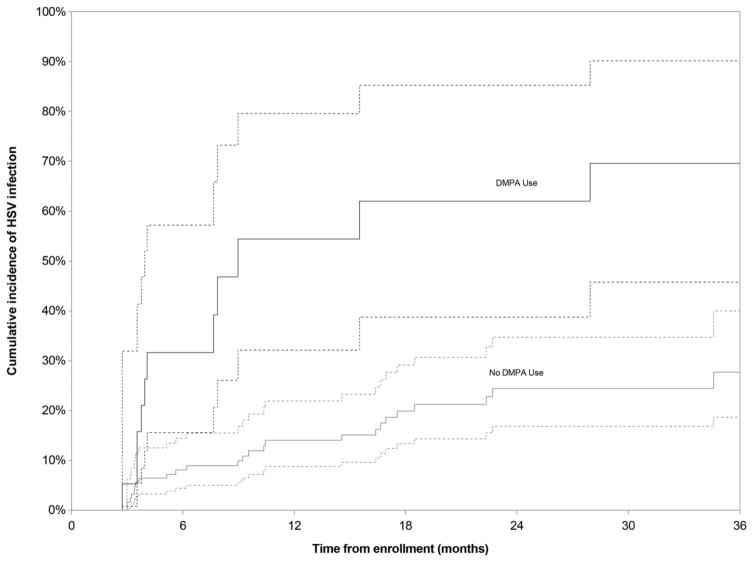
There were 39 HSV-2 seroconversions (12 among DMPA users and 27 among non-users) over the study period (median follow up of 18.6 months [IQR 8.4–29.9]), resulting in an overall incidence rate of 17.1 cases per 100 py (95% confidence interval [CI] 12.4–23.6). Incidence rates were significantly higher among DMPA users (57.4 cases per 100 py, 95% CI 31.4–105.0) than among non-users (13.1 cases per 100 py, 95% CI: 8.9–19.1). As indicated in Figure 1, the cumulative incidence of HSV-2 infection after 24 months of follow-up was 62.0% (95% CI 38.7%–85.2%) among DMPA users compared to 24.4% (95% CI 16.8%–34.7%) among non-users (log-rank p<0.001). When the sample was stratified by consistent use of DMPA throughout the study period, cumulative incidence at 24 months among consistent DMPA users (n=8) was 81.3% (95% CI 46.5%–98.9%), considerably higher than among inconsistent or non-users (25.8%, 95% CI 18.4%–35.7%, p<0.001).
Figure 1. Cumulative incidence of HSV-2 infection, stratified by DMPA use at some point during the study period, among female sex workers in Vancouver, Canada, 2010–2014.
Dotted lines represent the 95% confidence interval
In addition to DMPA exposure, secondary variables that were positively associated with HSV-2 acquisition in unadjusted Cox analyses included: indigenous ancestry, HIV infection, and primarily servicing clients outdoors or in informal indoor settings (compared to formal sex work establishments) (Table 2).
Table 2. Bivariable Cox analyses of potential confounders associated with HSV-2 acquisition among female sex workers in Vancouver, Canada (N=143).
| Characteristic | Unadjusted | |
|---|---|---|
|
| ||
| Hazard Ratio (95% CI) | p - value | |
| Age† | ||
| (per year older) | 0.99 (0.96 – 1.02) | 0.545 |
| Aboriginal ancestry† | ||
| no | Reference | |
| yes | 2.10 (1.13 – 3.92) | 0.020 |
| High school completion† | ||
| no | Reference | |
| yes | 0.65 (0.35 – 1.21) | 0.175 |
| HIV seropositive*† | ||
| no | Reference | |
| yes | 3.65 (1.06 – 12.56) | 0.040 |
| STI* | ||
| no | Reference | |
| yes | 2.11 (0.63 – 7.00) | 0.224 |
| Average number of clients per week*† | ||
| (per one client increase) | 1.03 (1.00 – 1.06) | 0.060 |
| Inconsistent condom use by clients*† | ||
| no | Reference | |
| yes | 1.99 (0.96 – 4.15) | 0.065 |
| Number of male intimate/non-commercial partners | ||
| (per one partner increase) | 0.99 (0.97 – 1.01) | 0.413 |
| Inconsistent condom use by non-commercial partners* | ||
| no | Reference | |
| yes | 0.80 (0.41 – 1.57) | 0.516 |
| Primary place of servicing clients*† | ||
| Formal sex work establishment | Reference | |
| Informal indoor venue | 3.33 (1.27 – 8.75) | 0.015 |
| Outdoor/public space | 5.41 (2.16 – 13.53) | <0.001 |
Time-updated variable using last 6 months as a reference point.
Considered as potential confounders in the multivariable model selection process.
CI: confidence interval; STI: sexual transmitted infection
Table 3 shows the unadjusted and adjusted hazard ratios between DMPA use and HSV-2 infection. In adjusted analyses, DMPA exposure remained independently associated with increased risk of HSV-2 acquisition (adjusted hazard ratio [AHR] 4.43, 95% CI 1.90–10.35). Results from the sensitivity analysis (i.e., using a fixed multivariable model with all variables) were not significantly different (AHR 3.51 (95% CI: 1.31–9.37). Other covariates that were positively associated with increased risk of HSV-2 acquisition in the fixed full multivariable model included inconsistent condom use by clients (AHR 2.24 (95% CI: 1.01–4.95) and servicing clients outdoors (compared to formal sex work establishments, AHR 4.47 (95% CI: 1.35–14.75).
Table 3. Bivariable and multivariable Cox analyses for the association between DMPA use and HSV-2 acquisition among female sex workers in Vancouver, Canada (N=143).
| Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI)† | |
|---|---|---|
|
|
||
| DMPA use* | 6.04 (2.79 – 13.06) | 4.43 (1.90 – 10.35) |
Participant used DMPA for pregnancy prevention in the last 6 months.
Adjusted for primary place of servicing clients, time-updated to refer to the past 6 months. Age, Aboriginal ancestry, educational attainment, HIV status, number of clients per week and inconsistent condom use by clients were included in the full model, but removed in the backwards selection approach.
DMPA: depot medroxyprogesterone acetate; CI: confidence interval
To further assess the robustness of the findings, in sub-analyses, we examined the effect of DMPA exposure on HSV-2 acquisition among participants who were HIV negative at baseline. Adjusted Cox analyses among the sample of 136 HIV negative participants, resulted in similar estimates than in the full sample (AHR 3.97, 95% CI 1.64–9.60, data not shown).
DISCUSSION
This study documented high overall incidence rates of HSV-2 among a cohort of women sex workers in Vancouver. These rates are comparable to estimates among women sex workers in Africa, which in turn are among the highest HSV-2 incidence rates reported [19 20]. Of particular concern is that after controlling for confounders, DMPA exposure remained independently associated with approximately 4-times increased risk of HSV-2 acquisition. Importantly, these findings held when using a fixed full multivariable model, as well as when restricting the analyses to HIV negative participants at baseline.
To our knowledge, only two other studies have previously assessed the potential association between DMPA use and HSV-2. Both were conducted among African women, and showed conflicting results. Chohan et al. found no association between DMPA use and HSV-2 infection among sex workers in Kenya [9]. Conversely, and similar to the findings of the present analysis, Grabowski et al. [21] found that, among Ugandan women in stable heterosexual partnerships, women using DMPA consistently had double the risk of acquiring HSV-2.
There is a paucity of research examining the underlying mechanisms through which DMPA could increase the susceptibility to HSV-2. It has been suggested that the hypoestrogenic environment created by DMPA exposure may result in thinning of the vaginal epithelium and changes in soluble mucosal defense, which in turn may facilitate HSV-2 infection [6]. Indeed, studies in mouse models have demonstrated that DMPA increases the risk of HSV-2 infection up to 100-fold and hampers immune responses to HSV-2 [22 23]. Although these findings provide biological plausibility for a potential link between DMPA use and HSV-2 acquisition, they should be interpreted with caution since, due to inter-species differences in local immune responses and vaginal microbiome and relatively higher DMPA doses used in animal studies, they might not completely reflect actual events in women’s genital tract [6]. Further prospective studies among women at-risk for HSV-2 infection are needed to ascertain the human health relevance of animal model results.
Our findings add to the ongoing debate on the potential impact of DMPA on HIV risk [3 4 6]. A well-established body of literature supports a negative synergy between HSV-2 and HIV. HSV-2 infection has been associated with an approximately three-fold increased risk of HIV acquisition [7], with higher risk among individuals with recent HSV-2 infection [8 24]. In addition, among people living with HIV, increases in plasma and genital HIV viral load have been observed during clinical and subclinical reactivation of herpetic genital lesions [25 26], suggesting that co-infection with HSV-2 might play an important role in HIV transmission and progression [27]. Thus, if DMPA use is conclusively demonstrated to increase the risk of HSV-2 acquisition, this might have important implications for family planning at an individual level (e.g., informed choice regarding contraceptives) as well as for public health interventions (e.g., tailored public health messages about contraceptive options for women at-risk of/living with HIV and their sexual partners). The development of sensitive and evidenced-based interventions in this area would benefit greatly from additional research on the social pathways through which biological risks pertaining to HSV-2 and other outcomes (e.g., HIV) are realized – and potentially exacerbated.
An additional and extremely relevant finding of this analysis is that, in agreement with early caution raised by public health scholars in Canada, the UK and Australia [28 29], use of DMPA was disproportionately higher among the most vulnerable women in this sample, including younger women, women of Indigenous ancestry, those with lower educational levels, and women living with HIV. These women are already at increased risk of poor health outcomes due to socio-economic and political inequities; and the use of DMPA for pregnancy prevention might further expose them to other health risks, including potential risks of STIs and bone mineral density loss [30]. Altogether, these findings raise strong concerns regarding prescribing practices and proper informed consent in marginalized populations. Qualitative research is urgently needed to better understand and promote sex workers’ exercise of their rights within the asymmetrical power dynamics between healthcare providers and clients.
The current analysis has a number of limitations that should be noted. First, our study relied on self-reported data, including sexual behaviours and use of DMPA, which might be subject to recall, social desirability and misclassification biases. Particularly, given the community-based nature of our cohort we did not have access to participants’ medical records to confirm DMPA use, including frequency and date of administration. However, all interviews were conducted in private and safe environments by experienced interviewers with strong community rapport, facilitating accurate responses. Further, we are not aware of any reason why there would be systematic differences in reporting sensitive data between sex workers who reported or not use of DMPA. Second, our small sample size, relatively short follow-up period and small number of incident HSV-2 infections may have limited our statistical power, as reflected by the wide CIs for some of the estimates. Therefore, although DMPA remained independently and strongly associated with HSV-2 acquisition even after adjustment for all potential known confounders, these findings need to be confirmed in a larger study. Third, exposure to DMPA was not randomised, and therefore we cannot exclude selection biases and residual confounding. While a randomised controlled trial would account for these potential biases and provide more definitive answers, such a trial might prove challenging, including feasibility issues due to the long duration of follow-up required for results to be available. Fourth, our findings are based on a cohort of street and off-street women sex workers in Vancouver, Canada; and as such they might not be generalisable to women in the general population or in other geographical areas. Fifth, our dichotomous measure of condom use (consistent/inconsistent) may not fully capture the range of possible frequencies of condom use. However, contrary to its high effectiveness for HIV prevention, condom effectiveness to prevent HSV-2 acquisition is more controversial with some meta-analyses suggesting an effectiveness of only 30% [31]. Sixth, there is variable delay between HSV-2 primary infection and seroconversion. As a result, we could have inadvertently included women who despite being seronegative for HSV-2 at baseline were already infected. Similarly, it is possible that at the end of the study period, some women who were still seronegative for HSV-2 had actually recently acquired HSV-2. Finally, given the lack of clinical information (eg, presence of genital ulcers) we cannot exclude that HSV-2 seroconversions reflected orolabial infections. Nevertheless, given that in adults HSV-2 is nearly always sexually transmitted [19], and the hypothesised pathways trough which DMPA may increase the risk of HSV-2, it is likely that the majority of seroconversions observed correspond to genital infections.
In summary, this study found high incidence rates of HSV-2 in a cohort of street and off-street sex workers in Vancouver, Canada. We also found a strong association between DMPA exposure and HSV-2 acquisition, as well as disproportionately higher use of DMPA among the most marginalised women in our study. Taken together, these issues raise concerns about the provision of optimal reproductive and sexual healthcare to women sex workers in Vancouver. These results point to an urgent need to engage women sex workers in the development of tailored health services that comprehensively address their particular reproductive and sexual health needs, including improving access to a wider range of contraceptive methods. In addition, healthcare providers should inform and advise women of the potential risks associated with DMPA use. Finally, given the known links between HSV-2 and HIV, our findings further underscore the need for studies with larger sample sizes, longer follow-up periods, and specifically designed to test the potential associations between different contraceptives and risk of HSV-2 and other STIs. These will be essential to help inform the development of safer reproductive choices, including LARCs for women worldwide.
KEY MESSAGES.
This study documented extremely high incidence rates of HSV-2 among sex workers in Vancouver, Canada.
Use of DMPA was associated with increased risk of HSV-2 acquisition.
Women sex workers should be engaged in the development of health services that comprehensively address their particular reproductive and sexual health needs.
Further research is needed to better understand associations between different contraceptives and risk of HSV-2 and other STI to help inform the development of safer reproductive choices.
Acknowledgments
The authors thank all those who contributed their time and expertise to this project, including participants, partner agencies and AESHA research team and Community Advisory Board. The authors particularly thank Melissa Braschel for her statistical advice and support.
Funding
This research was supported by operating grants from the US National Institutes of Health (R01DA028648) and Canadian Institutes of Health Research (CIHR, HHP-98835), and MacAIDS. KS is partially supported by a Canada Research Chair in Global Sexual Health and HIV/AIDS and Michael Smith Foundation for Health Research (MSFHR). JSGM is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307). MES is supported by a MSFHR postdoctoral fellowship award. PD is supported by CIHR and MSFHR postdoctoral fellowship awards.
Footnotes
Contributors
KS, MES and PD designed the study. PN did the statistical analysis. MES wrote the initial draft. All authors contributed to the interpretation of the findings, the critical revision of the manuscript for intellectual content, and approved submission of the final manuscript.
Ethics approval Providence Health Care/University of British Columbia Research Ethics Board (H09-0280).
Competing interests
JSGM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
References
- 1.Haddad LB, Polis CB, Sheth AN, et al. Contraceptive methods and risk of HIV acquisition or female-to-male transmission. Current HIV/AIDS reports. 2014;11(4):447–58. doi: 10.1007/s11904-014-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal PD, Voedisch A, Gemzell-Danielsson K. Strategies to prevent unintended pregnancy: increasing use of long-acting reversible contraception. Hum Reprod Update. 2011;17(1):121–37. doi: 10.1093/humupd/dmq026. [DOI] [PubMed] [Google Scholar]
- 3.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15(2):181–9. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best practice & research Clinical obstetrics & gynaecology. 2009;23(2):263–84. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Murphy K, Irvin SC, Herold BC. Research gaps in defining the biological link between HIV risk and hormonal contraception. American journal of reproductive immunology. 2014;72(2):228–35. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. Aids. 2007;21(13):1771–7. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 9.Chohan V, Baeten JM, Benki S, et al. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sexually transmitted infections. 2009;85(7):489–92. doi: 10.1136/sti.2009.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stueve A, O’Donnell LN, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: results with young Latino men who have sex with men. American journal of public health. 2001;91(6):922–6. doi: 10.2105/ajph.91.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon K, Bright V, Allinott S, et al. Community-based HIV prevention research among substance-using women in survival sex work: the Maka Project Partnership. Harm reduction journal. 2007;4:20. doi: 10.1186/1477-7517-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahariadis G, Severini A. Evaluation of a novel serology algorithm to detect herpes simplex virus 1 or 2 antibodies. Sexually transmitted diseases. 2010;37(11):696–9. doi: 10.1097/OLQ.0b013e3181e2cdab. [DOI] [PubMed] [Google Scholar]
- 14.LeGoff J, Pere H, Belec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83. doi: 10.1186/1743-422X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.British Columbia Sexual Tramsmitted Infections Treatment Guideline. Vancouver, BC: BC Centre for Disease Control; 2014. [Google Scholar]
- 16.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185(2):380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 17.Polis CB, Westreich D, Balkus JE, Heffron R Participants of the HCHIVOAM. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. Aids. 2013;27(Suppl 1):S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 19.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS one. 2015;10(1):e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajagopal S, Magaret A, Mugo N, Wald A. Incidence of herpes simplex virus type 2 infections in Africa: a systematic review. Open Forum Infect Dis. 2014;1(2):ofu043. doi: 10.1093/ofid/ofu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabowski MK, Gray RH, Makumbi F, et al. Injectable Hormonal Contraception Use and Women’s Risk for HSV-2 Acquisition. Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, Washington. 2015. Asbtract #28. [Google Scholar]
- 22.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77(8):4558–65. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77(18):9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Wald A, Hubbard A, et al. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. Aids. 2007;21(12):1515–23. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- 25.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. The Journal of infectious diseases. 2002;186(12):1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 26.Todd J, Riedner G, Maboko L, et al. Effect of genital herpes on cervicovaginal HIV shedding in women co-infected with HIV AND HSV-2 in Tanzania. PloS one. 2013;8(3):e59037. doi: 10.1371/journal.pone.0059037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375(9717):824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shea L. Reflections on Depo Provera: Contributions to Improving Drug Regulation in Canada. Toronto, ON: Women and Health Protection; 2007. [Google Scholar]
- 29.Morgan J. Depo-provera and the regulation of indigenous women’s reproduction. Simon Fraser University; 2007. [Google Scholar]
- 30.US Food and Drug Administration. Black box warning added concerning long-term use of Depo-Provera Contraceptive Injection. Secondary Black box warning added concerning long-term use of Depo-Provera Contraceptive Injection. 2004 http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm.
- 31.Martin ET, Krantz E, Gottlieb SL, et al. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med. 2009;169(13):1233–40. doi: 10.1001/archinternmed.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]