Abstract
Aims
This study compared the cause-specific standardized mortality ratios (SMRs) and expected years of life lost (EYLL) among opioid-dependent individuals in the United States and Taiwan.
Methods
Survival data came from two cohorts followed until 2014: The U.S. data were based on a randomized trial of 1,267 opioid-dependent participants enrolled between 2006 and 2009; the Taiwan data were from a study of 983 individuals that began in 2006, when opioid agonist treatment (OAT) was implemented in Taiwan. SMRs were calculated for each national cohort and compared. Kaplan-Meier estimation was performed on the survival data, then lifespans were extrapolated to 70 years (840 months) to estimate life expectancy using a semi-parametric method. EYLLs for both cohorts were estimated by subtracting their life expectancies from the age- and gender-matched referents within the general population of their respective country.
Results
Compared with age- and gender- matched referents, the SMRs were 3.2 for the U.S. sample and 7.8 for the Taiwan sample; the EYLLs were 7.7 and 16.4 years, respectively. Half of decedents died of unnatural causes in both cohorts; overdose deaths predominated in the U.S. and suicide in Taiwan.
Conclusions
Our study identified differences by country in EYLL and causes of deaths. These findings suggest that intervention strategies to reduce mortality risk by overdose (particularly in the U.S.) and suicide (particularly in Taiwan) are urgently needed in these countries.
Keywords: Opioid, Mortality, Overdose, Suicide, Life Expectancy
Introduction
Opioid dependence contributes to a heavy burden of disease globally, including excessive early mortality (Degenhardt et al., 2013). According to a meta-analysis based on 58 studies, the estimated crude mortality rate (CMR) was 21 per 1,000-person years (PY), and a standardized mortality ratio (SMR) of 15 was found among opioid-dependent individuals across the world, with the highest mortality rates in Asia (Degenhardt.et al., 2011). Moreover, variations in years of potential life lost (YPLL) among Western countries were substantial (Darke et al., 2016; Degenhardt et al., 2014; Smyth et al., 2007). Geographic differences in opioid-involved mortality raise questions about causes, but epidemiological studies comparing related phenomena across regions are lacking.
Unnatural causes of death, such as accidental overdose, suicide and homicide, predominate as the reasons for the excess mortality of opioid-dependent individuals (Clausen et al., 2009; Degenhardt et al., 2014; Evans, Li et al., 2015). Previous studies have found regional variations not only in death rates but also in causes of death. For example, overdose mortality accounted for more than half of deaths in one Australian cohort (the ATOS study, Darke et al., 2016) which was followed for 15 years, but for less than 15% of deaths in a national sample in Taiwan followed for one year (Lee et al., 2013). In addition, one systematic review (Darke & Ross, 2002) reported suicide proportions ranging from 3% to 35% among opioid cohorts. However, there has been little research comparing relative causes of death and expected years of life lost (EYLL) for opioid users in distinct nations during similar periods of time.
Opioid agonist treatment (OAT) with either methadone (MET) or buprenorphine (BUP) can reduce mortality, especially during medication-adherent treatment (Evans, Li et al., 2015; Kimber et al., 2010). MET has been available in the United States since the 1960s. In contrast, Taiwan started MET programs in 2006, primarily in response to the HIV/AIDS epidemic among drug users (Chen & Kuo, 2007). The treatment programs in both nations are highly regulated (e.g., they both have restrictive admission criteria and patient compliance requirements). In the United States, methadone programs require a special program license and are often stand-alone programs separated from the mainstream healthcare system. Most methadone programs in Taiwan have been established in the psychiatric department of hospitals, but program regimens are usually restricted to methadone dispensing without psychiatric services, mainly because addiction treatment is not covered by the national universal health insurance (Fan et al., 2013). Additionally, methadone is a Schedule II drug in Taiwan, and there is no take-home allowed in methadone programs. BUP, which was approved by the FDA in 2002 in the United States, can be prescribed by qualified practitioners in the general healthcare settings and does not have the program requirements that methadone has. BUP was not widely available in Taiwan until around 2010, but it is still listed as a Schedule III controlled drug.
Comparing treatment outcomes associated with the distinctive treatment systems and policies in different regions or countries may shed light on strategies needed to improve care and outcomes. Taking advantage of the availability of the opioid cohorts in the United States and Taiwan, this present study aimed to compare the cause-specific SMRs and EYLL among opioid users in the two countries. The similarities or differences between the countries should provide insight as to optimal strategies needed to address the disease burden of opioid use overall, and to each country specifically.
Methods
Data Sources
The U.S. START Study (see Saxon et al., 2013, for details) was a multisite prospective study at eight federally licensed opioid treatment programs across the United States that examined the effects of BUP and MET on indices of liver health in opioid-dependent patients seeking OAT. Eligibility criteria included being age 18 or older and currently opioid dependent. Patients who had medical and psychiatric conditions such as cardiopathy, liver disease, and acute psychosis were excluded from the study. START recruited 1,267 individuals from May 2006 to October 2009.
The Taiwan OAT study (see Chang et al., 2015, for details) was a pilot methadone maintenance treatment (MMT) program started in 2006 by the Taiwan Center for Disease Control (CDC) in four of Taiwan’s 23 administrative regions (3 in northern Taiwan and one in the Jianan Psychiatric Center in the south). The Taiwan CDC also permitted buprenorphine-naloxone (Suboxone ®) to be used in a second pilot study, beginning, as well, in 2006. Among the various hospitals involved in the study, the Jianan Psychiatric Center was the only institution providing both methadone and buprenorphine-naloxone. Inclusion criteria for both pilot studies were: (1) age 20 or older, (2) meeting the DSM-IV (fourth edition of the Diagnostic and Statistical Manual of Mental Disorders) criteria for opioid dependence, and (3) no other OST contraindication, such as severe liver disease or acute psychosis. For the comparisons presented in this paper, we used data from the 983 patients who participated in OAT between March 2006 and July 2008.
Participants
Clinical profiles at baseline for the 1,267 participants in the U.S. START study and the 983 cases in the Taiwan OAT study are provided in Table 1 and have been presented in previous articles (Chang et al., 2015; Hser et al., 2014; Hser et al., 2015). The mean age at baseline was 37.4 for the U.S. START participants and 37.8 for the Taiwan OAT participants. Most U.S. START participants were white (71.5%) and two-thirds were male, whereas almost all Taiwan OAT participants were male (88.3%; all were Asian). The proportion of injection drug use in the past 30 days was 67.8% for the U.S. START participants and 91.0% for the Taiwan OAT participants. The majority of both cohorts were cigarette smokers, with the proportion of smokers being extremely high (99.5%) among the Taiwan OAT participants. Regarding infectious diseases, the proportion of U.S. START patients with hepatitis C (HCV) was significantly lower than that among the Taiwan OAT patients (43.5% vs. 91.4%, p < 0.001), as was the proportion with HIV (1.1% among U.S. START participants vs. 18.1% among Taiwan OAT participants).
Table 1.
Comparisons of demographics and clinical status of the studied cohorts
| Characteristics | U.S./START group
|
Taiwan/OAT group
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total | 1267 | 100.0 | 983 | 100.0 |
| Demographics | ||||
| Sex * | ||||
| Male | 859 | 67.8 | 868 | 88.3 |
| Female | 408 | 32.2 | 115 | 11.7 |
| Age (Mean ± S.D.) | 37.4 ± 11.1 | 37.8 ± 7.7 | ||
| Age strata* | ||||
| 18–34 | 604 | 47.7 | 364 | 37.0 |
| 35–49 | 455 | 35.9 | 542 | 55.1 |
| ≧50 | 208 | 16.4 | 77 | 7.8 |
| Current Cigarettes Smoker | 1126 | 88.9 | 978 | 99.5 |
| Drug use | ||||
| Opioid Injection (past 30 days) * | 870 | 68.7 | 894 | 91.0 |
| Amphetamine (urine positive) * | 114 | 9.0 | 192 | 19.5 |
| Cocaine (urine positive) | 474 | 37.4 | ---- | ---- |
| Cannabinoid (urine positive) | 300 | 23.7 | ---- | ---- |
| Ketamine a | ---- | ---- | 103 | 10.5 |
| Medical co-morbidity | ||||
| HIV antibody positive * | 11 | 1.1 | 176 | 18.1 |
| HCV antibody positive * | 551 | 43.5 | 885 | 91.4 |
| HBV antigen positive * | 5 | 0.4 | 172 | 17.8 |
| Alcohol use b | 340 | 26.9 | 226 | 23.0 |
| Psychiatric medicationc | ||||
| Prescribed medication for mood problems (Lifetime)* | 519 | 60.1 | 35 | 5.4 |
| Prescribed medication for mood problems (past 30 days) * | 240 | 27.8 | 14 | 2.2 |
Self-reported ketamine use during lifetime.
- U.S./START group: Excessive use, or use to intoxication within the past 30 days.
- Taiwan/OAT group: Current alcohol use disorder diagnosed by qualified psychiatrists at assessment.
- U.S./START group: 863
- Taiwan/OAT group: 649
p<0.001
Also presented in Table 1 are measures of receipt of psychiatric medications collected at follow-ups. The majority (60.1%) of the U.S. START participants reported receiving medications for mood problems in their lifetime, and 27.8% had received prescribed medication in the past 30 days. In contrast, fewer than 5.4% of the Taiwan OAT participants ever received psychiatric medication treatment and only 2.2% currently took medication for mood problems.
Mortality and Cause of Death
The date and cause of deaths between the baseline assessment date and 2014 were determined for all the U.S. START participants using the National Death Index (Hser et al., 2015). Deaths among Taiwan OAT participants were identified by record linkage with the Taiwan National Death Certification Registry system, which is regularly managed by the Ministry of Health and Welfare and contains all information reported in death certificates, including name, identification number (ID), date of birth, sex, date of death, and cause of death. In addition, within the Taiwan system, the cause of all deaths from unnatural causes (suicide, overdose, and homicide) was decided upon by a death verdict jointly determined by a prosecutor and a coroner, whose main concern is the possibility of homicide. In a previous study in Taiwan, only 2 out of 117 suicides were judged to have been classified as accidental rather than deliberate (Cheng, 1995). Because the cause of death entry in these national registries is often delayed, there were 6 missing causes of deaths among U.S. START participants at the time we conducted these analyses, and we excluded the 2014 death records from the Taiwan OAT sample due to the potential misclassification of cause of death.
Statistical analysis
We applied the Kaplan-Meier method to estimate survival functions of these two cohorts based on follow-up data from 2006 to 2014. Person-years of follow-up were calculated from the baseline date to the date of death, or were censored on Dec. 31, 2014, and crude mortality rates per 1,000 person-years (PY), with 95% confidence intervals (CIs), were calculated. Standardized mortality ratios (SMRs) were calculated as the observed number of deaths divided by the expected number, with age-, sex-, year-, and cause-specific mortality rates in the U.S. or Taiwan populations used to calculate the expected versus the actual number of deaths (Breslow & Da, 1993). A semi-parametric method for EYLL estimates (Hwang & Wang, 1999) was used to overcome lead time bias between the two cohorts.
Extrapolation of Long-term Survival for the U.S. and Taiwan Samples
For estimating life expectancy (LE) and expected years of life lost (EYLL), we extrapolated a survival function (based on the Kaplan-Meier estimation method) to lifetime by assuming a “constant excess hazard” for opioid-dependent individuals. The method can be summarized as follows: First, we took the hazard functions from the life tables of the National Vital Statistics of the U.S. and Taiwan to create two age- and sex-matched reference populations for the respective samples using the Monte Carlo method (Hwang & Wang, 1999). The survival functions of these two reference populations were estimated objectively, thereby acting as a yardstick for each cohort. Second, we fitted a simple linear regression line to the logit of the ratio of survival functions between the U.S. START (or Taiwan OAT) and U.S. (or Taiwan) referent cohorts to the end of the follow-up. Third, the slope of the estimated straight regression line, together with the survival functions of the reference population beyond the follow-up limit, was used to extrapolate the lifetime survival functions of the two cohorts. In this way, the LE of these two cohorts (with extrapolation up to 840 months) after the baseline assessment was estimated.
Subsequently, the LE and EYLL in the U.S. START and Taiwan OAT cohorts were estimated. The EYLL was defined as the lifetime survival difference between each cohort and its reference population; in other words, the loss in years of LE. This calculation provides a measure of the burden of opioid dependence on an individual via estimation of how much one’s life is likely to be shortened by opioid dependence. It also provided an opportunity for comparing the burden of opioid dependence in different social contexts.
The standard errors of the means were calculated by the bootstrap method for 100 iterations in the U.S. START and Taiwan OAT cohorts. The semi-parametric survival extrapolation method described above has been described in detail in other studies (Chang et al., 2015; Fang et al., 2007; Liu et al., 2013; Andersson et al., 2013). To facilitate the computation, we used an ISQoL (integration of survival and quality of life) software program, which can be freely downloaded from http://www.stat.sinica.edu.tw/jshwang (the present analysis examined only survival).
Validation of the Extrapolation Method
To validate the extrapolation method, the first 4-year survival data were extrapolated up to 8 years to estimate the survival function through the previously described method. Because these two cohorts were actually followed until 2014, we regarded the mean survival duration up to 8-year follow-up, estimated by the Kaplan-Meier method, as the gold standard. The relative bias was computed to compare the difference in values between our extrapolation method and the actual observed data (Kaplan-Meier estimation).
Results
Overall and Cause-specific Mortality
There were 71 deaths (5.6%) in the U.S. START cohort and 107 deaths (10.9%) in the Taiwan OAT cohort by the end of the follow-up. The crude mortality rate among U.S. START participants was lower than in the Taiwan OAT group (8.4 vs. 17.3 per 1,000 person-years), as summarized in Table 2. Compared to the general population of similar age and gender, the standardized mortality ratios (SMRs) were 3.2 and 7.8 for the U.S. START and Taiwan OAT cohorts, respectively.
Table 2.
Estimated standardized mortality ratios (SMRs) for opioid-dependent cohorts in the United States and Taiwan
| Mortality | U.S. START group | Taiwan OAT group | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (person-years, PY) | 8413.75 | 6186.84 | ||||||
| Deaths(n) | 71 | 107 | ||||||
| Expected death (n) | 22.5 | 13.8 | ||||||
| standardized mortality ratio, SMR (95% CI) | 3.2 (2.5–3.9) | 7.8 (6.3–9.3) | ||||||
| Crude mortality rate (CMR; per 1000PY) | 8.4 | 17.3 | ||||||
| Crude overdose mortality rate (CMR; per 1000PY) | 3.4 | 2.1 | ||||||
| Crude suicide mortality rate (CMR; per 1000PY) | 0.1 | 4.4 | ||||||
|
| ||||||||
| Cause-specific | Deaths(n) | SMR |
95% CI
|
Deaths(n) | SMR |
95%CI
|
||
| lower | upper | lower | upper | |||||
|
| ||||||||
| Unnatural | 36 | 8.0 | 5.4 | 10.7 | 52 | 16.6 | 12.1 | 21.1 |
| Overdose | 29 | 48.9 | 31.1 | 66.6 | 13 | 131.4 | 60.0 | 202.8 |
| Suicide | 1 | 0.8 | 0.0 | 6.9 | 27 | 18.1 | 11.3 | 24.9 |
| Disease-related (Somatic) | 27 | 1.7 | 1.1 | 2.3 | 48 | 4.9 | 3.5 | 6.3 |
ICD-9-CM codes: overdose: E850–858, E980; suicide: E950–E959. ICD-10-CM codes: overdose: X40–X44; suicide: X60–X84, Y10–Y34 and Y87.0; Note: 6 missing causes of deaths among START group. 2 and 7 deceased cases were coded as sudden/undefined (Y10–Y34, R00–R99) for START and Taiwan OAT group, respectively. CI: confidence interval.
Among the 71 U.S. START participants who died during the observation period, 50.7% (N = 36) were unnatural deaths, mostly (N=29) due to overdose. Similarly, nearly half (N=52) of the deceased Taiwan OAT sample died due to unnatural causes; 27 of these 52 were due to suicide as opposed to 1 of 36 in the U.S. START study. Overdose mortality (per 1,000 person-years) and SMRs were 3.4 and 48.9 versus 2.1 and 131.4 for the U.S. START study versus Taiwan OAT cohorts. On the other hand, the suicide mortality rate among the Taiwan OAT cohort was 4.4 per 1,000 person-years, representing 18.1-fold age- and gender-standardized mortality increases. The suicide mortality SMR among the U.S. START cohort was 0.8.
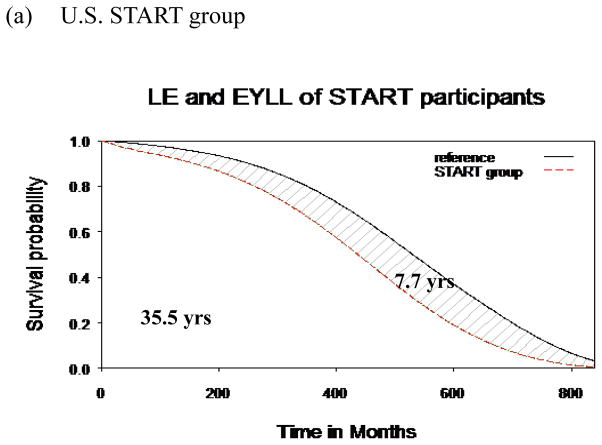
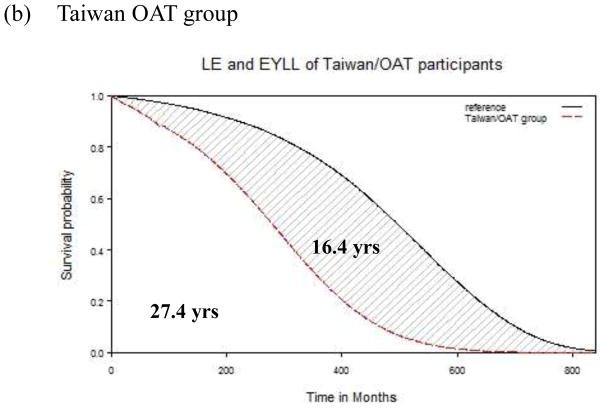
LE and EYLL
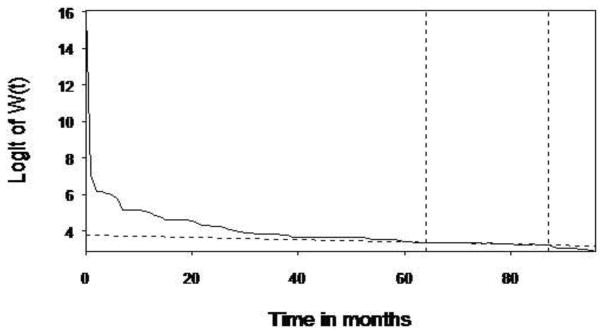
The 8-year follow-up data from both cohorts were used to extrapolate the lifetime survival time up to 840 months. As depicted in Figures 1a and 1b, the LEs were 35.5 and 27.4 years after diagnosis, respectively, for the U.S. START and Taiwan OAT cohorts. Compared with the age- and gender-matched referents, the EYLLs were 7.7 and 16.4 for these U.S. START and Taiwan OAT opioid-dependent participants, respectively. After adjustment for age and gender, the Taiwan OAT sample had an additional loss of 8.7 life-years (=16.4 –7.7) in comparison to the U.S. START sample. Our method of extrapolation is valid if constant excess hazard can be assumed (Breslow & Da. 1993; Fang et al., 2007), and the logit of W(t) of the U.S. START cohort, as an example expressed in Figure 2, showed the fulfillment of the “constant excess hazard” assumption. Similar results were found with the Taiwan OAT cohort (Chang et al., 2015). Thus, we tentatively concluded that our estimation is relatively accurate.
Fig. 1.
Mean survival difference between opioid users and age- and sex- matched reference population after 70 years of extrapolation. (a): the Starting Treatment with Agonist Replacement Therapy (U.S. START) group. (b): Taiwan OAT group.
Fig. 2.
Logit transformation of the survival ratio W(t) of the survival functions of the U.S. START group and that of the age- and gender-matched reference population generated by the Monte Carlo method. The solid line is the linear regression line. The two vertical dotted lines mark the time period when the data were used for extrapolation, while the horizontal dotted line indicates the slope of logit survival ratio. The bottom dotted line is the linear regression line. When the curve of logit survival ratio converges to a stable straight line and its slope is estimable, it means that the assumption of constant excess hazard is fulfilled.
Table 3 presents the results of the validation of the extrapolation method for estimates of survival of each cohort. The first 4-year survival curves of each cohort were extrapolated to the end of 8 years and compared with the Kaplan-Meier estimates based on actual follow-up. The relative biases of extrapolated survivals were all less than 1%, indicating the relative accuracy of this method.
Table 3.
Estimates of mean survival durations for 8 years of follow-up using the extrapolation method based on the first 4 years of follow-up data compared with the Kaplan-Meier estimates based on 8 years of follow-up data.
| Cohort size | 8-year survival based on Kaplan-Meier estimate mean (SE) months | Extrapolation based on the first 4-year follow up mean (SE) months | Relative bias, % | P value | |
|---|---|---|---|---|---|
| U.S./START | 1267 | 91.89(0.23) | 92.07(0.15) | 0.2 | 0.343 |
| Taiwan/OAT | 983 | 88.08(0.6) | 87.66(0.23) | −0.5 | 0.312 |
Abbreviation: SE=standard error.
Discussion
Compared to those reported in the meta-analyses by Degenhardt et al., (2011) the estimates of CMR and SMR in both the U.S. and Taiwan cohorts appear to be low, which could be due to the fact that both of these cohorts comprised treated patients. Yet, the average EYLL of 7.7 for the U.S. START cohort and 16.4 for the Taiwan OAT cohort still suggest serious health burdens in both countries. Despite the CMR of the U.S. START group being lower than that of the Taiwan OAT group, the U.S. START group showed a substantially higher mortality rate from overdose than did the Taiwan OAT group, which is consistent with the generally high overdose death rates across the U.S. in these years (Okie, 2010; Dart et al., 2015; Volkow et al., 2014). Most alarming is that the suicide mortality among the Taiwan OAT group was 20 times greater than that in the U.S. START group.
The overall higher CMR and EYLL in the Taiwan OAT study, relative to those in the U.S. START study could be due to several reasons. Patients in the Taiwan OAT cohort appeared to have higher severity in drug use (91.0 % injection drug use, relative to 68.7% in the U.S. START sample) and higher rates of comorbid HCV and HIV infections. Although not directly observed from the data, given the high rate of suicide deaths in the Taiwan OAT cohort, the combination of severe health conditions and the stigma associated with opioid use and comorbidities could lead to depression and, eventually suicide. In fact, a striking difference in suicide mortality existed between the two groups, with a suicide mortality rate of 1.4% for the U.S. START cohort versus 25.2% for the Taiwan OAT cohort. Further, the SMR for suicide among the Taiwan OAT group was 18.1-fold higher than that of the general population, which is consistent with the previous one-year mortality study from 10,842 opioid users in Taiwan (Lee et al., 2013).
Depression as a risk factor for suicide has particular salience for opioid users (Darke & Ross, 2002; Pan et al., 2014). It is well known that the rates of mental disorder are high among patients with opioid use disorder. But with the concerns about abuse of antidepressants (Holt, 2007), there were cautions about using them for opioid-dependent patients with severe suicidal ideation in Taiwan. However, the high rate of suicide morality and the low rates of psychiatric medication among opioid patients in Taiwan raise the concern of undertreatment. It is unfortunate that despite the fact that most OAT programs in Taiwan are housed within the psychiatric unit of hospitals, these suicide deaths were not prevented. Policymakers in Taiwan need to seriously consider incorporating psychiatric assessments or counseling and other supportive services in order to reduce suicide mortality risks among opioid patients in OAT treatment.
The high overdose mortality found among the U.S. START participants supports the recent decision by the U.S. Department of Health & Human Services to identify opioid use disorder as a national public health crisis (Macrae & Hyde, 2015; National Heroin Task Force, 2015). This public health crisis has attracted renewed interest in and attention to better identifying risk factors and implementing strategies that address the recurring opioid epidemic in the United States. Expanding access to medication-assisted treatment and training for medical professionals on opioid medication prescribing practices are among initiatives recently issued by President Obama to address the prescription opioid abuse and heroin epidemic (The White House, 2016). In Taiwan, access to prescribed opioids is still very low because of strict regulation, which might explain the low overdose mortality.
This study needs to be considered within the context of several limitations. Despite the coincidental timing of the U.S. and Taiwan cohorts with similar inclusion and exclusion criteria, it is difficult to compare all the variables specifically relevant to each of the two different societies. Because similar mortality outcomes were found when we compared findings based on samples from Taiwan with those from the U.S. (Evans, Li et al., 2015; Lee et al., 2013; Evans, Kelleghan et al., 2015) using the SMRs to demonstrate the important mortality issues and EYLL to estimate the health burden in both of our cohorts could be representative of opioid-dependent individuals in their areas. Also, both EYLL estimates of these two cohorts could be conservative because of the selection bias (associated with the clinical trial model in the U.S. START study or with the design of including the first admitted cases to Taiwan OAT treatment) and uncertainty would be inevitable over lifetime extrapolation. Psychiatric diagnoses were not available, and the relationship of access to psychiatric treatment and suicide risks among opioid-dependent individuals needs further investigation. Finally, further causal relationships should not be asserted because of the observational designs involved in the studies that collected data from both cohorts.
Conclusions
Despite different contexts in two vastly different countries, the current estimates of EYLL highlight that opioid dependence and its associated comorbidities and risk factors still contribute severe health burdens across regions. Our comparison of cause-specific SMRs could inform stakeholders as they make health policy modifications relevant to their region. Given the prominent role of overdose in the U.S. START cohort, improving access to medication-assisted treatment (Volkow et al. 2014; Jones et al., 2015) to prevent overdoses or naloxone to treat overdoses (Coffin & Sullivan et al., 2013) will help address the problem. Suicide is preventable; intervention strategies, including regular screening of ideation and depressive symptoms and providing treatment and support among opioid users in OAT treatment, are urgently needed in Taiwan.
Acknowledgments
We extend our sincere appreciation to our participating networks: the Pacific Northwest Node and Evergreen Treatment Services; the Western States Node and CODA Inc. and Bi-Valley Medical Clinic; the New England Node and Connecticut Counseling Centers and Yale and Hartford Dispensary; the Delaware Valley Node and NET Steps; the Pacific Region Node and Matrix Institute; EMMES Corporation (CCC); the Center for Clinical Trials Network (CCTN) and NIDA.
Funding source:
Main study funding was provided by the National Institute on Drug Abuse (NIDA) through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
The Greater New York Node (UG1 DA013035)
Funding was also provided by NIDA through grant number P30DA016383.
Footnotes
Declaration of Interest: Andrew Saxon: receives royalties as an editor for UpToDate. All other authors report no financial or other possible conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson TML, Dickman PW, Eloranta S, Lambe M, Lambert PC. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med. 2013;32:5286–5300. doi: 10.1002/sim.5943. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Da NE. IARC scientific publications 1993. Lyon: International Agency for Research on Cancer; 1987. Statistical methods in cancer research, Vol. II: The design and analysis of cohort studies; p. 82. [PubMed] [Google Scholar]
- Chang KC, Lu TH, Lee KY, Hwang JS, Cheng CM, Wang JD. Estimation of life expectancy and the expected years of life lost among heroin users in the era of opioid substitution treatment (OST) in Taiwan. Drug and Alcohol Dependence. 2015;153:152–158. doi: 10.1016/j.drugalcdep.2015.05.033. [DOI] [PubMed] [Google Scholar]
- Chen YM, Kuo SH. HIV-1 in Taiwan. Lancet. 2007;369:623–625. doi: 10.1016/S0140-6736(07)60291-8. [DOI] [PubMed] [Google Scholar]
- Cheng AT. Mental illness and suicide: a case-control study in East Taiwan. Arch General Psychiatry. 1995;52:594–603. doi: 10.1001/archpsyc.1995.03950190076011. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M. Gossop M Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158:1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- Darke S, Marel C, Mills KL, Ross J, Slade T, Tessson M. Years of potential life lost amongst heroin users in the Australian Treatment Outcome Study cohort, 2001–2015. Drug and alcohol dependence. 2016;162:206–210. doi: 10.1016/j.drugalcdep.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J. Suicide among heroin users: rates, risk factors and methods. Addiction. 2002;97:1383–1394. doi: 10.1046/j.1360-0443.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. New England Journal of Medicine. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Larney S, Randall D, Burns L, Hall W. Causes of death in a cohort treated for opioid dependence between 1985 and 2005. Addiction. 2014;109:90–99. doi: 10.1111/add.12337. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Evans E, Li L, Min J, Huang D, Urada D, Liu L, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction. 2015;110:996–1005. doi: 10.1111/add.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Kelleghan A, Li L, Min J, Huang D, Urada D, et al. Gender differences in mortality among treated opioid dependent patients. Drug Alcohol Depend. 2015;155:228–235. doi: 10.1016/j.drugalcdep.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Tan HK, Chien IC, Chou SY. Prevalence of psychiatric disorders among heroin users who received methadone maintenance therapy in Taiwan. The American Journal on Addictions. 2013;23:249–56. doi: 10.1111/j.1521-0391.2014.12090.x. [DOI] [PubMed] [Google Scholar]
- Fang CT, Chang YY, Hsu HM, Twu SJ, Chen KT, Lin CC, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM. 2007;100:97–105. doi: 10.1093/qjmed/hcl141. [DOI] [PubMed] [Google Scholar]
- Holt M. Agency and dependency within treatment: Drug treatment clients negotiating methadone and antidepressants. Social Science & Medicine. 2007;64:1937–1947. doi: 10.1016/j.socscimed.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll K, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2015 doi: 10.1111/add.13238. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JS, Wang JD. Monte Carlo estimation of extrapolation of quality-adjusted survival for follow-up studies. Stat Med. 1999;18:1627–1640. doi: 10.1002/(sici)1097-0258(19990715)18:13<1627::aid-sim159>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber J, Copeland L, Hickman M, Macleod J, McKenzie J, De Angelis D, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ. 2010;341:c3172. doi: 10.1136/bmj.c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen VC, Tan HK, Chou SY, Wu KH, Chan CH, et al. Suicide and other-cause mortality among heroin users in Taiwan: A prospective study. Addictive behaviors. 2013;38:2619–2623. doi: 10.1016/j.addbeh.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56:309–313. doi: 10.1016/j.ypmed.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Macrae J, Hyde P. HHS launches multi-pronged effort to combat opioid abuse. Published July 27, 2015. Retrieved from http://www.hhs.gov/blog/2015/07/27/hhs-launches-multi-pronged-effort-combat-opioid-abuse.html.
- National Heroin Task Force final report and recommendations. Published December 31, 2015. Retrieved from https://www.justice.gov/file/822231/download.
- Okie S. A flood of opioids, a rising tide of deaths. New England Journal of Medicine. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Pan CH, Jhong JR, Tsai SY, Lin SK, Chen CC, Kuo CJ. Excessive suicide mortality and risk factors for suicide among patients with heroin dependence. Drug Alcohol Depend. 2014;145:224–230. doi: 10.1016/j.drugalcdep.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug and alcohol dependence. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth B, Hoffman V, Fan J, Hser Y. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med. 2007;44:369–374. doi: 10.1016/j.ypmed.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The White House. Fact Sheet: President Obama proposes $1.1 billion in new funding to address the prescription opioid abuse and heroin use epidemic. 2016 doi: 10.3109/15360288.2016.1173760. Retrieved from https://www.whitehouse.gov/the-press-office/2016/02/02/president-obama-proposes-11-billion-new-funding-address-prescription. [DOI] [PubMed]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. New England Journal of Medicine. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]