Abstract
Methuosis is a form of non-apoptotic cell death involving massive vacuolization of macropinosome-derived endocytic compartments, followed by a decline in metabolic activity and loss of membrane integrity. To explore the induction of methuosis as a potential therapeutic strategy for killing cancer cells, we have developed small molecules (indole-based chalcones) that trigger this form of cell death in glioblastoma and other cancer cell lines. Here we report that in addition to causing fusion and expansion of macropinosome compartments, the lead compound, 3-(5-methoxy-2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one (MOMIPP), disrupts vesicular trafficking at the lysosomal nexus, manifested by impaired degradation of EGF and LDL receptors, defective processing of procathepsins, and accumulation of autophagosomes. In contrast, secretion of the ectodomain derived from a prototypical type-I membrane glycoprotein, β-amyloid precursor protein, is increased rather than diminished. A closely related MOMIPP analog, which causes substantial vacuolization without reducing cell viability, also impedes cathepsin processing and autophagic flux, but has more modest effects on receptor degradation. A third analog, which causes neither vacuolization nor loss of viability, has no effect on endolysosomal trafficking. The results suggest that differential cytotoxicity of structurally similar indole-based chalcones is related, at least in part, to the severity of their effects on endolysosomal trafficking pathways.
Keywords: chalcones, cell death, macropinocytosis, endosomes, lysosomes, methuosis
Introduction
Previous reports from our laboratory have described a form of non-apoptotic cell death termed methuosis, which is characterized morphologically by massive accumulation of cytoplasmic vacuoles derived from macropinosomes, without the usual hallmarks of apoptosis (Maltese and Overmeyer 2014; Maltese and Overmeyer 2015). Cell death coincides with a decline in metabolic activity and loss of membrane integrity, which cannot be prevented by the broad-spectrum caspase inhibitor, z-VAD-fmk. Methuosis was initially described in glioblastoma (GBM) cells ectopically expressing activated Ras or Rac1 (Overmeyer et al. 2008). Subsequent studies have identified methuosis-like cell death phenotypes in other contexts, including lung adenocarcinomas with activating mutations in both K-Ras and EGFR (Unni et al. 2015), prostate cancer cells exposed to an oligonucleotide aptamer, AS1411 (Reyes-Reyes et al. 2015), thyroid carcinoma cells in which miR-199a-3p is ectopically expressed (Minna et al. 2014), and GBM cells treated with small molecules (Overmeyer et al. 2011; Kitambi et al. 2014). To begin to explore the therapeutic potential of inducing methuosis in GBM and other cancers, we have developed a series of synthetic indole-based chalcones (indolyl- pyridinyl-propenones) that can elicit this form of cell death at low micromolar concentrations (Overmeyer et al. 2011; Robinson et al. 2012). The first in our series of methuosis-promoting compounds was termed MIPP; an abbreviation for 3-(2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one) (Overmeyer et al. 2011). MIPP triggers the formation of waves of macropinosomes in GBM cells within 2 h. Unlike normal macropinosomes, the nascent vesicles coalesce to form large vacuoles that accumulate until they fill most of the cytoplasmic space. Over the next 24–48 h, the influx of new macropinosomes diminishes and the vacuolated cells detach from the substratum and begin to rupture. The vacuoles caused by MIPP acquire markers of late endosomes, such as Rab7 and LAMP1, but they appear to remain distinct from lysosomes and autophagosomes (Overmeyer et al. 2011). In contrast to the methuosis phenotype induced by activated Ras (Bhanot et al. 2010; Overmeyer et al. 2008) or AS1411 (Reyes-Reyes et al. 2015), the accumulation of vacuoles caused by MIPP is not dependent on activation of the Rac1 GTPase (Overmeyer et al. 2011; Maltese and Overmeyer 2015). Beyond these rudimentary observations, little is known about the specific sequence of subcellular events or the molecular pathways that ultimately lead to metabolic crisis and loss of cell viability associated with methuosis.
Structure-activity relationship (SAR) studies of a directed library of MIPP-related compounds identified a more potent 5-methoxy derivative, termed MOMIPP, which shows methuosis-inducing activity against both temozolomide-resistant and parental U251 GBM cells (Robinson et al. 2012). These initial SAR studies also supported the idea that vacuolization of macropinosomes is an important component of the cell death program, since minor structural changes that eliminated the ability of these compounds to induce vacuolization also eliminated their ability to kill GBM cells (Robinson et al. 2012). However, more recent studies with a larger series of compounds revealed that certain modifications of the MOMIPP scaffold, such as replacement of the methyl group on the 2-position of the indole ring with a propyl group, can substantially attenuate cytotoxicity without eliminating the induction of cellular vacuolization (Trabbic et al. 2014; Trabbic et al. 2015). These observations prompted us to test the hypothesis that closely related indole-based chalcones might have differential effects on intracellular trafficking pathways, despite the common morphological hallmark of vacuole accumulation. Herein we define the effects of the methuosis inducer, MOMIPP, and its non-lethal vacuole-inducing 2-propyl analog, MOPIPP, on endocytic trafficking pathways in GBM cells. The results provide several new insights into the mechanism of action of the indole-based chalcones. First, the vacuoles induced by these compounds arise from pre-existing endosomes in addition to nascent macropinosomes. Second, defects in vesicular trafficking extend beyond macropinocytosis to include disruption of late endosome and autophagosome fusion with lysosomes. Third, despite similarities in the extent of cellular vacuolization, there are substantial differences in the severity of the block in endolysosomal trafficking and degradation of cell surface receptors induced by closely related indole chalcones. These variations may underlie the differential cytotoxicity of these compounds.
Materials and methods
Cell culture
U251 human glioblastoma cells were obtained from the DCT Tumor Repository (National Cancer Institute) and were maintained in Dulbecco s modified Eagle medium (DMEM), supplemented with 10% (v/v) fetal bovine serum (FBS) (JR Scientific) at 37°C with 5% CO2 /95% air.
Compounds
The indole-based chalcones, MOMIPP, MOPIPP and meta-MOMIPP, were synthesized as described previously (Trabbic et al. 2014). They were dissolved in DMSO to generate 10 mM stock solutions and then diluted in culture medium to a final concentration of 10 μM for all experiments, unless otherwise indicated.
Live cell imaging with fluorescent tracers
Dextran Alexa Fluor-568 (10,000 MW, anionic, fixable) was purchased from Life Technologies. For live-cell imaging studies, 150,000 cells were plated in 35 mm dishes. One day later, the cells were incubated for 24 h with fluorescent dextran (0.5 mg/ml), together with each of the indicated indole-based chalcones, in phenol red-free DMEM containing 10% FBS. Magic Red™ RR was purchased from ImmunoChemistry Technologies. One day after plating, cells were treated with compounds for 24 h and then incubated with Magic Red for 1 h according to directions supplied by the manufacturer. The expression vector, pEGFP-Rab7 (WT), was provided by Cecilia Bucci, University of Salento. U251 cells were nucleofected as previously described (Overmeyer et al. 2011). The day after nucleofection, the cells expressing EGFP-Rab7 were treated with 10 μM MOMIPP, MOPIPP, meta-MOMIPP or an equivalent volume of DMSO and monitored 24 h later by fluorescence microscopy. Acridine Orange (AO) was used to visualize acidic intracellular compartments. After treatment with compounds for 24 h, cells were incubated with 2.5 μg/ml AO in phenol red-free DMEM for 45 min at 37°C. Following incubation with the indicated probes, all cultures were washed twice with phenol red-free DMEM and live cells were examined by fluorescence and phase-contrast microscopy using an Olympus IX70 inverted microscope equipped with a heated stage, a DP80 digital camera and cellSens imaging software. Filter combinations utilized for green or red fluorescence were as follows: green (excitation, 470/440 nm; emission, 525/550 nm), red (excitation, 560/540 nm; emission, 620/660 nm).
Cell viability
Cell viability was assessed by measuring cellular ATP using the CellTiter Glo® luminescence assay according to the manufacturer’s protocol (Promega Corp.). Cells were seeded in white-walled opaque 96-well plates (2000 cells/well), with four replicate wells for each culture condition. After addition of compounds at the indicated concentrations, cell viability was assayed at a 48 h end-point. Luminescence was read using a Berthold Tech Centro XS3 LB 960 luminometer and data were acquired with the MikroWin software.
Immunofluorescence
Mouse monoclonal antibody against LAMP1 (H4A3-s) was obtained from the Developmental Studies Hybridoma Bank. Rabbit polyclonal antibody against LC3 (Cat. No. AP1802a, Lot SH050530i) was obtained from Abgent. Mouse monoclonal antibody against p62 (SQSTM1) (D3, Cat. No. 28359, Lot F112) was from Santa Cruz Biotechnology. For immunofluorescence microscopy, cells were seeded at 150,000 cells per dish in 35 mm dishes with glass coverslips. After 24 h the cells were incubated for the indicated time in medium containing test compounds, then washed with PBS and fixed with ice-cold methanol. Target proteins were localized by immunofluorescence microscopy as described previously (Johnson et al. 2006). Secondary antibodies used for detection of bound primary antibodies were: goat anti-mouse IgG conjugated to Alexa Fluor 568 (Cat. No. A11031, Lot 482209A) or goat anti-rabbit IgG conjugated to Alexa Fluor 488 (Cat. No. A11034, Lot 4662488), both obtained from Life Technologies.
For visualization of EGFR internalization, U251 cells were seeded at 100,000 cells per dish in 35 mm dishes with glass coverslips and cultured in DMEM containing 10% FBS for 1 day. The cultures were switched to serum-free medium and maintained for 10 h to allow the EGFR to accumulate on the cell surface. At that point the medium was supplemented with test compounds (10 μM) or an equivalent volume of DMSO, and incubation was continued for 12 h. To initiate EGFR internalization, cells were pre-treated for 30 min with serum-free medium containing 10 μg/ml cycloheximide and then 100 ng/ml recombinant EGF (Sigma Chemical Co) was added to the medium. Coverslips were processed for immunofluorescence microscopy just prior to addition of EGF (time-0) and at 1 h after addition of the growth factor. Briefly, cells were washed with PBS and fixed with ice-cold methanol. Target proteins were localized using a rabbit monoclonal antibody against EGFR (4267, Cell Signaling Technology) and the mouse monoclonal antibody against LAMP1 noted above. Secondary antibodies used for detection of bound primary antibodies were: goat anti-mouse IgG conjugated to Alexa Fluor 568 or goat anti-rabbit IgG conjugated to Alexa Fluor 488, both obtained from Life Technologies.
Western blot analysis
U251 cells were seeded at 1 x 106 cells per 10 cm dish and maintained for 24 h in DMEM containing 10% FBS. Fresh medium containing the indicated compounds at 10 μM final concentration, or an equivalent volume of DMSO, was then added and cells were harvested after 24 h. In some experiments examining the expression of LC3, the following lysosomal protease inhibitors were included in the medium during the incubation with the compounds: 10 μg/ml pepstatin A and 10 μg/ml E-64D (Peptide Institute Inc.). Cells were lysed in SDS sample buffer (Laemmli 1970) and the protein concentration was determined by colorimetric assay using Bio-Rad reagent (Bio-Rad). Equal amounts of protein (generally 80–100 μg) were subjected to SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot procedures described previously (Maltese et al. 2001). Rabbit anti-p44/42 MAPK (Erk1/2) (Cat. No. 9102S, Lot 23), rabbit anti-phospho-p44/42 MAPK (Erk1/2) (T202/Y204) (Cat. No. 9101S, Lot 27), rabbit anti p38 MAPK (Cat. No. 9212S, Lot 23) and rabbit anti-phospho-p38 MAPK (T180/Y182) (Cat. No. 9215S, Lot 7) were obtained from Cell Signaling Technology. Rabbit anti-Cathepsin D (Cat. No. K50161R, Lot 1F1669) (Biodesign International), goat anti-Cathepsin L (Cat. No. 6498, Lot K2812) (Santa Cruz Biotechnology), mouse anti-β-Amyloid Precursor Protein (LN27, Cat. No. 130200, Lot 1469060A) (Invitrogen/Life Technologies), and mouse monoclonal antibodies against α-tubulin (Cat. No. T5168, Lot 016K4886) and LDH (Cat. No. L-7016, Lot 044K4807) (Sigma Chemical Co.) were purchased from the indicated suppliers. HRP-coupled goat anti-mouse (Cat. No. 554002, Lot 4078712) and goat anti-rabbit (Cat. No. 554021, Lot 3199578) secondary antibodies were from BD Biosciences. Chemiluminescent signals were quantified using an Alpha Innotech FluorChem HD2 imaging system with Alpha View software.
Secretion of the β-amyloid precursor protein ectodomain
To assay the secreted form of amyloid precursor protein (s-APP), U251 cells were plated at 500,000 cells per 60 mm dish and grown for a day with DMEM containing 10% FBS. The medium was then replaced with serum-free DMEM containing test compound at 10 μM final concentration. As a positive control to distinguish the effects disrupting trafficking through the Golgi apparatus, cells were treated with 1 μg/ml Brefeldin A (Cayman Chemical Co.). At a 16-h end-point, medium containing secreted proteins was collected and centrifuged at 1,400 x g for 5 min at 4° C. The supernatant was then concentrated to 500 μl by centrifugation at 4,000 x g in an Amicon Ultra-4 filtration unit (Millipore). The concentrated medium containing secreted proteins was mixed with 2x SDS sample buffer, and one-fifth of the total was subjected to SDS-PAGE and immunoblot analysis using the LN27 anti-βAPP monoclonal antibody noted above.
EGF Receptor internalization and degradation
U251 cells were plated at 1 x 106 cells per 10 cm dish and cultured in DMEM containing 10% FBS for 1 day. The cultures then were switched to serum-free medium and maintained for 10 h to allow the EGFR to accumulate on the cell surface. At that point the medium was supplemented with test compound (10 μM) or an equivalent volume of DMSO, and incubation was continued for 12 h. To measure EGFR turnover, cells were pre-treated for 30 min with serum-free medium containing 10 μg/ml cycloheximide and then 100 ng/ml recombinant EGF was added to the medium. Parallel cultures were harvested just prior to addition of EGF (time-0) and at 30 min, 1 h, 2 h and 4 h after addition of the growth factor. Cells were washed twice with cold PBS and solubilized directly in SDS-sample buffer. Equal amounts of protein (80 μg) from each culture were subjected to SDS-PAGE and immunoblot analysis using a mouse antibody against EGFR (BD Biosciences), or a rabbit anti-phospho-EGFR (Y1068) (Cell Signaling Technology) as described above.
LDL Receptor internalization and degradation
U251 cells were plated at 1 x 106 cells per 10 cm dish and cultured in DMEM containing 10% FBS for 1 day. The cells were switched to serum-free medium for 10 h. Compounds were then added and incubation was continued for 16 h. Cells were then pre-treated with 10 μg/ml cycloheximide for 30 min and receptor internalization and degradation were stimulated by addition of FBS (20% v/v). Cells were harvested at 4 h and 8 h after addition of serum. Time-0 samples were harvested just prior to addition of FBS. Cells were washed twice with HBSS and solubilized in SDS sample buffer. Equal amounts of protein (100 μg) were subjected to SDS-PAGE and immunoblot analysis using an antibody against the human LDL receptor (Abcam, EP1553Y; ab52818)
Results
Morphological characterization of vacuole compartments induced by indolyl- pyridinyl- propenones in glioblastoma cells
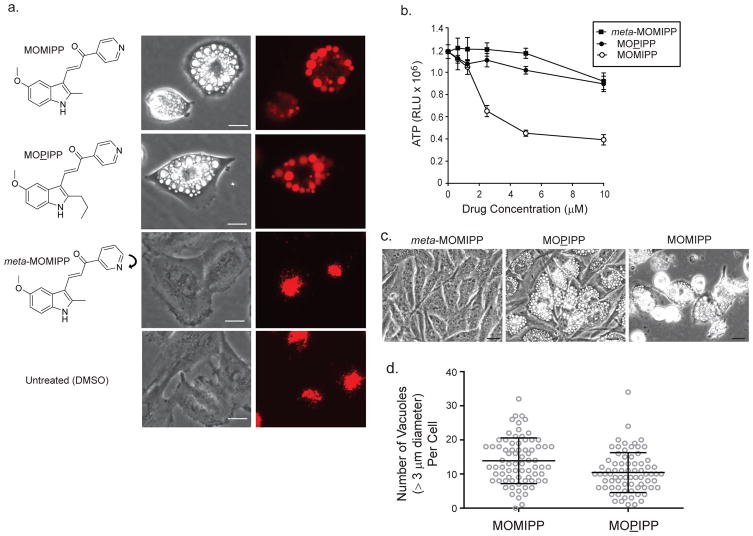
Our prior studies using time-lapse microscopy showed that treatment of GBM cells with the prototype compound, MIPP, caused nascent macropinosomes to merge with each other, resulting in the formation of large phase-lucent vacuoles. Consistent with their origination from macropinosomes, many of the vacuoles incorporated extracellular fluid-phase tracers and did not stain with markers for endoplasmic reticulum or mitochondria (Overmeyer et al. 2011). These early studies did not rule out the possibility that some of the accumulated vacuoles might also arise from swelling or coalescence of pre-existing endosomes or lysosomes. To address this possibility, we treated U251 GBM cells with our newer compound, MOMIPP, for 24 h, with the continuous presence of fluorescent dextran in the medium (Fig. 1a). As expected, many of the MOMIPP-induced phase-lucent vacuoles were strongly labeled with the fluid-phase tracer. However, we consistently noted a sub-population of phase-lucent vacuoles that did not incorporate dextran. Similar observations were recorded when cells were incubated with MOPIPP, a closely related structural analog of MOMIPP that induces cellular vacuolization but does not cause cell death (Fig. 1b &c). Thus, in addition to stimulating vacuolization of newly generated macropinosomes and endosomes, both MOMIPP and MOPIPP appear to induce vacuolization of intracellular vesicular compartments that exist prior to the simultaneous addition of the drug and the fluid-phase tracer. In control cells treated with vehicle (DMSO) or the inactive analog, meta-MOMIPP, dextran labeling was confined to clusters of much smaller juxtanuclear vesicles (Fig. 1a). Meta-MOMIPP induces neither vacuolization nor cell death, due to alteration of the indole nitrogen from the para to meta configuration (Trabbic et al. 2014) (Fig. 1b & c). By phase contrast microcopy, the vacuoles induced by the lethal MOMIPP and the non-lethal MOPIPP appeared generally similar in size and quantity per cell. To obtain a more quantitative assessment, we counted the number of phase-lucent vacuoles meeting or exceeding an arbitrary threshold of 3μm diameter in images of 75 cells treated with each compound for 24 h. This analysis did not reveal a significant difference in the average number of vacuoles per cell (Fig 1d). It was not possible to accurately count the large number of vacuoles below the 3 μm threshold, so it remains possible that differences exist at that level.
Fig. 1.
Different biological activities of closely related indole-based chalcones in U251 glioblastoma cells. a) Cells were co-incubated with Dextran Alexa Fluor-568 and the indicated compounds (10 μM). After 24 h, phase-contrast and fluorescent images of the live cells were acquired. The same field of cells is depicted in the matching phase-contrast and fluorescent images. b) Cells were treated with compounds at the indicated concentrations for 48 h. Cell viability was assessed using the CellTiter Glo® ATP assay. Values are means (± SD) from four replicates. c) Phase-contrast images show the morphology of cells treated for 48 h with the indicated compounds at 10 μM. Scale bars in all of the images represent 20 μm. d) Cells were treated for 24 h with 10 μM MOMIPP or MOPIPP. For each group, digital images of 75 individual cells were manually scored for the number of phase-lucent vacuoles/cell. The threshold for counting vacuoles was arbitrarily set at a diameter of ≥ 3 μm. The means (± SD) for the two groups were not significantly different (p ≥ 0.05) as determined by Student s t-test.
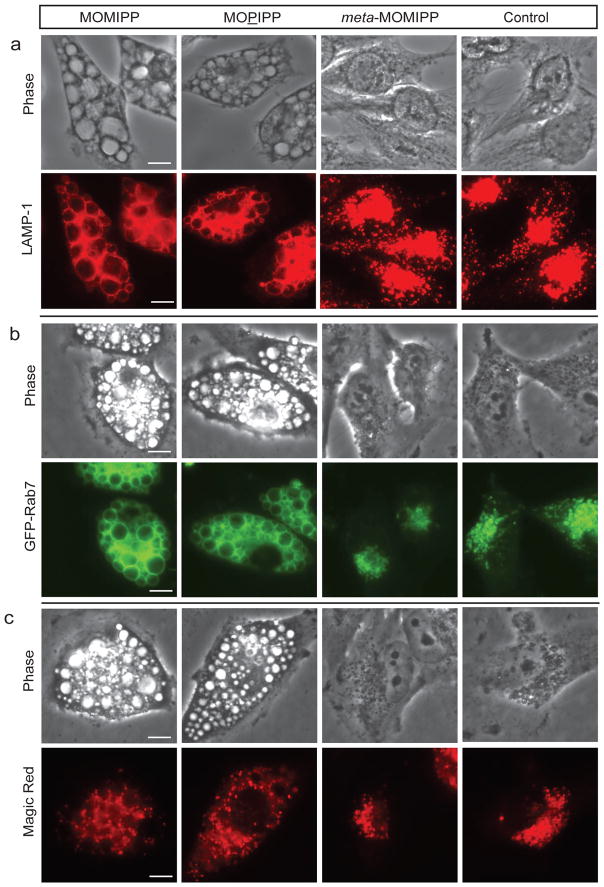
Essentially all of the larger vacuoles induced by MOMIPP and MOPIPP exhibited characteristics of late endosomes, including the presence of LAMP1 and GFP-Rab7 in their limiting membranes (Fig. 2 a & b). The vacuoles were distinct from mature lysosomes detected with the cathepsin-B substrate, Magic Red™, which appeared as smaller punctate structures in areas between the vacuoles (Fig. 2c).
Fig. 2.
Localization of endolysosomal markers in U251 cells treated with different indole-based chalcones. a) Cells were treated for 24 h with the indicated compounds (10 μM) or an equivalent volume of DMSO (control) and then fixed and processed for immunofluorescence microscopy to localize LAMP1. b) U251 cells expressing EGFP-Rab7 were treated with compounds at 10 μM and live-cell fluorescence images were obtained after 24 h. c) Cells were treated with compounds for 24 h and then incubated in medium with Magic Red™ RR for 1 h prior to live-cell imaging. The scale bars for all panels are 20 μm.
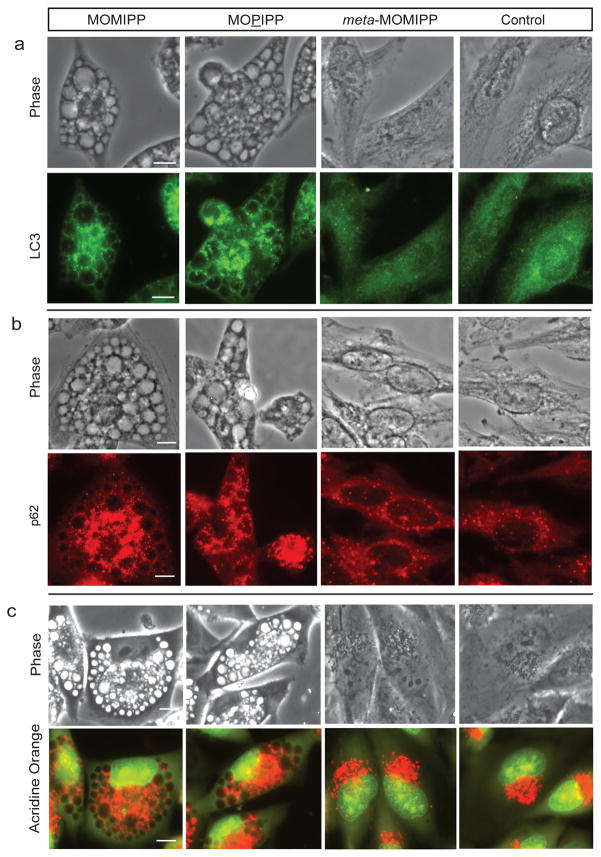
Autophagosomes are double-membrane vesicles that develop from cup-shaped isolation membranes (phagophores), which surround regions of cytoplasm and organelles destined for degradation (Dunn 1994; Klionsky et al. 2014). The contents of autophagosomes are degraded when these structures merge with lysosomes to become autolysosomes (Gordon and Selgen 1988; Dunn 1990; Lawrence and Brown 1992). Microtubule-associated protein 1A/1B-light chain 3 (LC3) is the most widely used molecular marker for autophagosomes (Mizushima 2004). LC3 exists in a cytosolic form (LC3I) and a form that is conjugated to phosphatidylethanolamine on the inner and outer autophagosome membranes (LC3II) (Kabeya et al. 2000). Immunostaining with an antibody against LC3 revealed weak diffuse staining in control and meta-MOMIPP-treated cells, contrasting with intense punctate fluorescence in cells treated with MOMIPP or MOPIPP (Fig. 3a). Interestingly, although the strongest LC3 fluorescence was seen in smaller punctate structures dispersed throughout the cells, we noted a relatively weak signal associated with membranes surrounding the vacuoles. This finding is consistent with a previous report indicating that LC3II can be recruited to macropinosomes in addition to autophagosomes (Florey et al. 2011). Supporting the distinction between autophagosomes and macropinosomes, the autophagosome cargo protein, p62 (SQSTM1), was detected in the smaller punctate structures, but not in the larger macropinosome-derived vacuoles (Fig. 3b). To determine if the contents of the large vacuoles in the cells treated with MOMIPP or MOPIPP are acidic, live cells were stained with acridine orange (AO), a weak base that fluoresces red when it is protonated and sequestered in acidic compartments like lysosomes and autolysosomes. The results indicate that the majority of the vacuoles do not sequester AO (Fig. 3c). This cannot be attributed to global neutralization of endolysosomal compartments or disruption of lysosomal integrity, since the vacuolated cells exhibited robust AO staining of smaller punctate structures surrounding the vacuoles, similar to the pattern observed with the cathepsin substrate, Magic Red.
Fig. 3.
Localization of autophagosome markers in U251 cells treated with different indole-based chalcones. Cells were treated for 24 h with the indicated compounds (10 μM) or an equivalent volume of DMSO (control) and then fixed and processed for immunofluorescence microscopy to localize LC3 (panel a) or p62 (panel b). Images for control and treated cells were captured at the same exposure setting. c) Cells treated with the indicated compounds for 24 h were incubated with 2.5 μg/ml acridine orange (AO) for 45 min and live cells were examined by fluorescence microscopy. AO fluoresces red in acidic compartments. The scale bars are 20 μm.
MOMIPP and MOPIPP inhibit fusion of autophagosomes with lysosomes
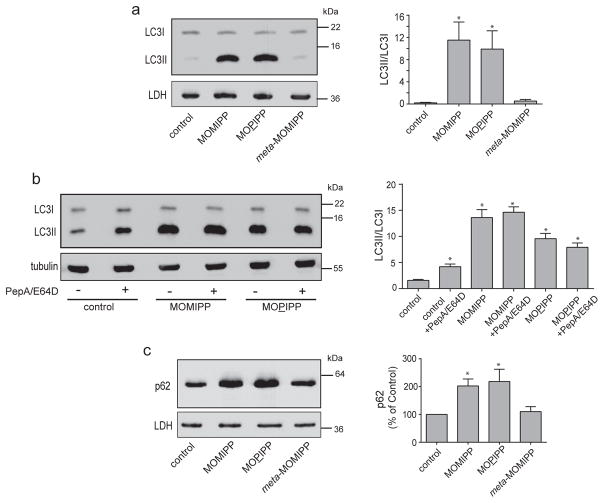
In light of the results in Figure 3, where the increase in punctate LC3 fluorescence suggested an increase in the number of autophagosomes in cells treated with MOMIPP or MOPIPP, we performed western blot analysis for LC3 (Fig. 4a). The results reveal a major increase in the relative amount of LC3II versus LC3I in cells treated with the vacuole-inducing compounds, compared to cells treated with vehicle or the inactive analog, meta-MOMIPP. Treatment of cells with lysosomal protease inhibitors typically results in an increase in the amount of LC3II, since lysosomal degradation of LC3II associated with autophagosomes is prevented (Taneda et al. 2005; Mizushima and Yoshimori 2007). As shown in Figure 4b, the increase in LC3II observed in cells treated with MOMIPP was much greater than the increase in LC3II caused by treatment of control cells with lysosomal protease inhibitors. A similar effect was observed with MOPIPP. When lysosomal protease inhibitors were combined with MOMIPP or MOPIPP, there was no additional increase in the amount of LC3II (Fig. 4b). These observations could be consistent with two possible scenarios: In the first, the vacuole-inducing compounds cause accumulation of LC3II mainly by interfering with the fusion of autophagosomes with lysosomes. In the second, the increase in LC3II reflects an increased steady-state level of autophagic activity, with no substantial impairment of autophagosome trafficking. To further explore these possibilities, we performed western blot analysis to measure the levels of p62 (SQSTM1) in cells treated with MOMIPP, MOPIPP, or meta-MOMIPP (Fig. 4c). p62 binds to ubiquitinated proteins and mediates their incorporation into autophagosomes (Bjorkoy et al. 2005; Pankiv et al. 2007). Since the p62 “cargo” is degraded when autophagosomes merge with lysosomes, the steady-state level of p62 is expected to decline if autophagic flux is increased (Kuma et al. 2004). We observed the opposite effect in cells treated with MOMIPP or MOPIPP (Fig. 4C); i.e., a significant accumulation of p62, suggestive of decreased autophagic flux.
Fig. 4.
MOMIPP and MOPIPP cause accumulation of autophagosome markers. A, Cells were treated for 24 h with the indicated compounds (10 μM) or an equivalent volume of DMSO (control) and harvested for western blot analysis using an antibody against LC3. Lactate dehydrogenase (LDH) served as a loading control. b) Cells were pre-treated with (+) or without (-) a combination of lysosomal protease inhibitors (pepstatin A and E-64D) for 1 h and then the indicated compounds were added. Incubation with the compounds was continued for 24 h, with or without protease inhibitors, and the cells were harvested for western blot analysis for LC3, using α-tubulin as a loading control. c) Cells were treated for 24 h with the indicated compounds (10 μM) or an equivalent volume of DMSO (control) and harvested for western blot analysis using an antibody against p62. Representative western blots are shown on the left, while the bar graphs on the right depict the mean ± SEM derived from quantification of the immunoblot signals from three independent experiments. Asterisks denote values that were significantly elevated compared to the control (Student’s t-test, p ≤ 0.05).
Lysosomal degradation of epidermal growth factor receptor is impaired in vacuolated cells
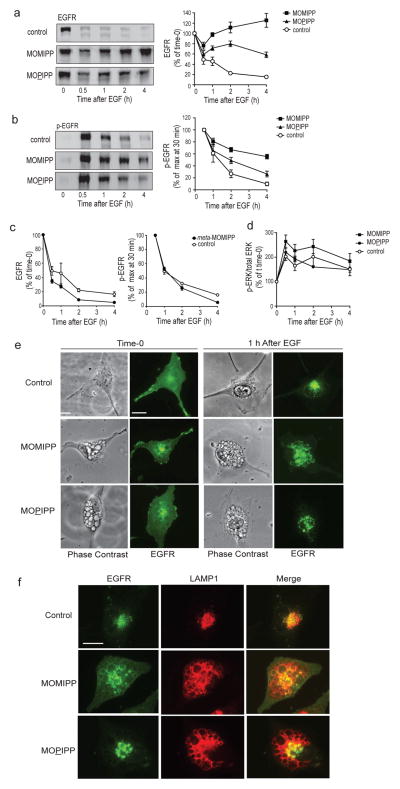
Macropinocytosis is a clathrin-independent endocytic process (Kerr and Teasdale 2009; Swanson and Watts 1995). Thus far it has not been determined whether extreme vacuolization of macropinosomes and late endosomes induced by compounds like MOMIPP might also affect clathrin-dependent endocytic pathways. To address this question, we examined the degradation of the epidermal growth factor receptor (EGFR). Turnover of ligand-activated EGFR depends on trafficking of the internalized receptor complex to multivesicular endosomes (MVEs), where the ESCRT machinery sorts the ubiquitinated receptor to intraluminal vesicles that are ultimately degraded when late endosomes merge with lysosomes (Madshus and Stang 2009; Tomas et al. 2014). Ligand-dependent turnover of the EGFR can be assessed by initially maintaining U251 GBM cells in serum-free medium without EGF, which attenuates receptor internalization and allows EGFR to accumulate on the cell surface. Then, with simultaneous addition of EGF to activate the receptor and cycloheximide to block new protein synthesis, the lysosome-dependent degradation of the internalized EGFR can be tracked by immunoblot analysis (Johnson et al. 2006). Addition of EGF to cells pretreated with vehicle (DMSO), initiated a substantial decline in the amount of full-length EGFR within a period of 4 h (Fig. 5a). In contrast, the clearance of full-length EGFR was strongly inhibited when EGF was added to cells that had been pretreated with MOMIPP for 12 h (Fig. 5a). The non-cytotoxic vacuole-inducing analog, MOPIPP, also impaired the EGF-induced degradation of EGFR, but the effect was not as severe as with MOMIPP (Fig. 5a). The basis for the initial dip in the total EGFR immunoblot signal after 30 min, followed by a small increase in cells treated with MOMIPP or MOPIPP, is not clear. One possibility is that the robust phosphorylation of the receptor, which occurs at 30 min and then dissipates (see Fig. 5b), may have a small effect on the affinity of the receptor for the EGFR antibody. In addition, we have noted that the cycloheximide concentration used in this study reduces incorporation of 3H-leucine into total protein by approximately 80% (not shown), suggesting that a low level of residual EGFR synthesis might gradually add to the pool of non-degraded EGFR.
Fig. 5.
MOMIPP and MOPIPP inhibit degradation of EGFR. a & b) Cells were pre-incubated in serum-free medium containing the indicated compounds (10 μM) or an equivalent volume of DMSO (control) for 12 h. EGF was then added (in the presence of cycloheximide) and parallel cultures were harvested at the specified time intervals after addition of the growth factor. Each cell lysate was subjected to western blot analysis using antibodies for total EGFR (panel a) or tyrosine-phosphorylated EGFR (Y1068) (panel b). Representative western blots are shown on the left, while the bar graphs on the right depict results derived from quantification of the immunoblot signals from six independent experiments (mean ± SEM). c) The same experiment was performed using meta-MOMIPP instead of the active compound (n = 3). d) Cells were pre-treated with the indicated compounds and then stimulated with EGF as noted for a-c. At intervals following addition of EGF, cells were lysed and subjected to western blot analysis for total ERK1/2 or phosphorylated ERK1/2 (T202/Y204). The graph depicts the ratios of p-ERK/total-ERK, expressed as percent of the value determined at time-0, just prior to addition of EGF (mean ± SEM from three independent experiments). e) Immunofluorescence images show the localization of EGFR at time-0 and at 1 h after addition of EGF in cells pre-treated with the indicated compounds as described in Materials and Methods. The same cells are shown in the adjacent phase contrast images. f) Cells pre-treated with the indicated compounds were visualized by immunofluorescence microscopy using antibodies directed against EGFR (green) or LAMP1 (red). All cells are shown at the same magnification, with the scale bar in the first panel representing 20 μm.
The decreased turnover of the EGFR in cells treated with MOMIPP or MOPIPP was not due entirely to inaccessibility of the receptor to ligand at the cell surface, since the addition of EGF triggered robust receptor tyrosine phosphorylation (Y1068) within 30 min, similar to what occurs in the control cells (Fig. 5b). Consistent with the reduced degradation of total EGFR, the phosphorylated form of the receptor (p-EGFR) was more persistent in cells treated with MOMIPP, compared with the control (Fig. 5b). An intermediate effect on p-EGFR turnover was observed with MOPIPP. Two factors contribute to turnover of p-EGFR; lysosomal degradation, and interaction of p-EGFR on the endosomal surface with protein tyrosine phosphatase 1B at points of contact with the ER (Eden et al. 2010; Tomas et al. 2014). Thus, the eventual diminution of receptor phosphorylation in cells treated with MOMIPP, despite a block in receptor degradation, could reflect the direct action of phosphatases. To confirm that the effects of MOMIPP and MOPIPP on EGFR degradation are related to the extreme vacuolization of endocytic compartments, similar studies were conducted with the closely related analog, meta-MOMIPP, which does not induce vacuolization. The latter compound did not inhibit turnover of total EGFR or p-EGFR (Fig. 5c). To explore the potential consequences of the changes in EGFR degradation and phosphorylation for downstream signaling, we compared the effects of EGF stimulation on the phosphorylation state of ERK in control cells versus cells treated with either MOMIPP or MOPIPP. As expected, ERK phosphorylation was increased immediately following addition of EGF under all conditions (Fig. 5d). However, over time, the marked differences in EGFR turnover in cells treated with MOMIPP or MOPIPP (Fig. 5a) did not seem to cause significant differences in ERK phosphorylation. Similar results were obtained when AKT phosphorylation was assessed (not shown). These findings may be attributable to the multiplicity of other factors that can drive the activation of ERK and AKT in GBM, including signals emanating from other receptor tyrosine kinases (e.g., PDGFR) and integrin complexes or inactivating mutations in PTEN and NF1 (Furnari et al. 2007; Verhaak et al. 2010).
In conjunction with the altered turnover of the EGFR, immunofluorescence microscopy revealed a striking difference in the subcellular localization of the receptor in control cells versus MOMIPP-treated cells. Prior to addition of EGF, the EGFR in control cells was localized predominantly on the cell surface and the juxtanuclear region (Fig. 5e). Within 1 h after adding the growth factor, the fluorescence transitioned to a cluster of punctate LAMP1-positive structures, consistent with the expected trafficking of the receptor to late endosomes and lysosomes (Fig. 5e&f). In cells pre-treated with MOMIPP, EGFR was found on the cell surface at time-0, but some fluorescence was also detected in vacuolar structures (Fig. 5e&f). Upon stimulation of the MOMIPP-treated cells with EGF, the receptor was internalized, but it accumulated in a subpopulation of large LAMP1-positive vacuoles, rather than in the smaller punctate structures seen in the control (Fig. 5e&f). Interestingly, cells treated with MOPIPP generally showed less accumulation of EGFR in large vacuoles after addition with EGF (Fig. 5e&f). Thus, the severity of impairment of EGFR degradation seemed to correlate with the extent to which the receptor was sequestered in the vacuoles.
Lysosomal degradation of low density lipoprotein receptor is inhibited in vacuolated cells
To distinguish whether the effects of MOMIPP on EGFR degradation are related to a specific effect on the EGFR pathway or instead reflect a generalized perturbation of endocytic trafficking that could affect other receptors, we expanded these studies to include the low-density lipoprotein receptor (LDLR). The LDLR plays a key role in cellular cholesterol homeostasis by mediating the uptake of extracellular LDL, a major sterol carrier (Goldstein and Brown 2015). Upon binding of LDL, the receptor enters the endocytic pathway where LDL is released in acidic late endosomes. The LDL particle is subsequently degraded in lysosomes, releasing cholesterol, while the receptor is recycled back to the cell surface. Normal turnover of the LDLR occurs in lysosomes. Several regulatory proteins have been shown to modulate LDLR degradation. One example is proprotein convertase subtilisin/kexin type 9 (PCSK9) which, upon association with LDLR, prevents its dissociation from LDL and promotes lysosomal degradation instead of recycling (Poirier et al. 2009). Another regulatory protein termed IDOL is an E3 ubiquitin ligase that stimulates degradation of LDLR via clathrin-independent endocytosis and trafficking to lysosomes through MVE intermediates (Scotti et al. 2013). The extent to which these two regulatory mechanism may overlap in specific tissues and cell types remains to be established, but it is worth noting that both PCSK9 and IDOL are expressed in glioblastoma cells (Guo et al. 2011; Piao et al. 2015).
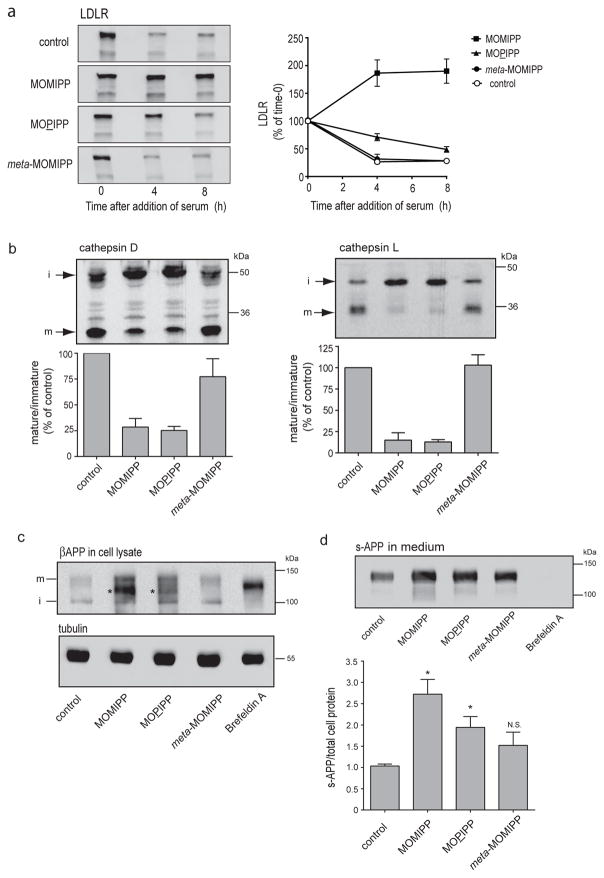
The results depicted in Fig. 6a demonstrate that when serum is added back to serum-starved U251 cells (in the presence of cycloheximide to block new protein synthesis), the amount of endogenous LDLR falls by approximately 75% within 4 h, consistent with ligand-stimulated internalization and endolysosomal degradation. The inactive indole-chalcone, meta-MOMIPP, had no effect on LDLR turnover. In contrast, MOMIPP completely blocked LDLR degradation. As noted earlier, incorporation of 3H-leucine into protein is reduced but not entirely eliminated with the concentration of cycloheximide used in these studies. Therefore, modest time-dependent increase of LDLR in the MOMIPP-treated cells may be related to a low rate of residual protein synthesis. As in the studies of EGFR, the non-lethal vacuole-inducing analog, MOPIPP, had a distinctly smaller effect on LDLR turnover compared with MOMIPP (Fig. 6a).
Fig. 6.
a) MOMIPP and MOPIPP inhibit degradation of LDLR. Cells were pre-incubated in serum-free medium containing the indicated compounds (10 μM) or an equivalent volume of DMSO (control) for 12 h. Serum was then added (in the presence of cycloheximide) and parallel cultures were harvested at the specified time intervals. Each cell lysate was subjected to western blot analysis using antibodies for total LDLR. Representative western blots are shown in the upper panel. The graph below depicts the results derived from quantification of the immunoblot signals from three independent experiments (mean ± SEM). b) Posttranslational maturation of procathepsins D and L is inhibited in cells treated with MOMIPP or MOPIPP. Cells were treated for 24 h with the indicated compounds (10 μM) or an equivalent volume of DMSO (control) and harvested for western blot analysis with antibodies against cathepsin D or cathepsin L. Representative blots from three separate experiments show the immature procathepsin (i) and the mature proteolytically cleaved cathepsin (m). The bar graphs depict the results from three independent experiments (mean ± SEM), with the ratio of mature to immature protein in the drug-treated cultures normalized to the ratio detected in the control. c) Effects of MOMIPP and MOPIPP on posttranslational processing of βAPP. U251 cells were plated in complete medium and grown for 24 h. They were then switched to serum-free medium containing the indicated compounds (10 μM) or DMSO (control) for 16 h. Separate cultures were incubated for the same period of time with Brefeldin A (1 μg/ml). Equal amounts of protein from the cell lysates were subjected to western blot analysis using an antibody against βAPP. d) To detect s-APP released from the cells, one-fifth of the volume of concentrated medium collected in the preceding experiment was subjected to western blot analysis with the antibody against βAPP. Each value for s-APP in the medium was normalized to the total cell protein in the same culture. The bar graph depicts the results (mean ± SEM) from three independent experiments. Asterisks denote values that were significantly elevated compared to the control (Student s t-test, p ≤ 0.05).
Vacuolization affects the trafficking and post-translational processing of cathepsins
The preceding studies with EGFR and LDLR suggest that the vacuole-inducing indole-based chalcones cause a general block in endolysosomal trafficking. However, it is conceivable that inhibition of receptor degradation also could be related to defective receptor ubiquitination or ESCRT function, thus preventing receptor sorting into intraluminal vesicles of the MVEs. To further explore the possibility of a global block in trafficking between late endosomes and lysosomes, we focused on the aspartic protease, cathepsin D, and the cysteine protease, cathepsin L. Both proteins are synthesized as inactive proenzymes that are glycosylated in the Golgi apparatus and then transported to endosomes en route to the lysosomes. N-glycosylated procathepsin D (51–53 kDa) associates with the cation-independent mannose 6-phosphate receptor in the trans-Golgi network (TGN) and is delivered to the endosomal compartment, where it is activated by removal of the pro-peptide to generate an intermediate that migrates at 46–48 kD on SDS gels. The latter is cleaved to the active mature lysosomal form, which contains two non-covalently linked chains of 31 kDa and 14 kDa (Rijnboutt et al. 1992; Delbruck et al. 1994). Similarly, procathepsin L (42 kDa) is converted via a 34 kDa intermediate into a mature lysosomal form that consists of two polypeptides (26 kDa and 7 kDa) linked by a disulfide bond (Smith et al. 1989; Katunuma 2010). When we carried out western blot analysis of cathepsin D and cathepsin L (Fig. 6b), we observed that both MOMIPP and MOPIPP caused major reductions in the relative amounts of the lower molecular weight lysosomal forms of the enzymes compared to cells treated with DMSO or meta-MOMIPP. In conjunction with the decline in the mature lysosomal forms of the cathepsins, there was a corresponding accumulation of the higher molecular weight precursor forms. These findings are consistent with a block in trafficking of the cathepsins to lysosomes.
MOMIPP and MOPIPP do not inhibit secretion of the β-amyloid precursor protein (βAPP) ectodomain
The results of the preceding studies suggest that the vacuolization of macropinosomes and endosomes induced by MOMIPP and MOPIPP causes general disruption of trafficking pathways that terminate at lysosomes. To determine whether exocytic trafficking pathways might also be altered, we evaluated the effects of these compounds on the post-translational processing of βAPP, a type-1 membrane glycoprotein with three major isoforms. βAPP695 is expressed mainly in neurons, whereas βAPP751 and βAPP770 are expressed ubiquitously (Sisodia 1992; Zhang et al. 2011). Nascent βAPP undergoes N-glycosylation in the ER, followed by O-glycosylation in the Golgi apparatus (Haas et al. 2012). The latter maturation step causes a substantial shift in electrophoretic mobility of the protein on SDS gels (Weidemann et al. 1989). Upon its transport from the TGN to the cell surface, βAPP is cleaved within its transmembrane domain by a metalloprotease, α-secretase, releasing a soluble ectodomain (s-APP) (Sisodia 1992; Kuhn et al. 2010). βAPP can undergo alternative cleavage by an aspartyl protease, β-secretase (BACE), releasing a slightly shorter ectodomain and leaving behind a C-terminal tail containing an intact amyloid β-peptide (Aβ) domain (Sinha et al. 1999; Vassar et al. 1999). The latter can be released via proteolytic cleavage by γ-secretase (De Strooper et al. 2012). BACE and γ-secretase appear to reside in endolysosomal compartments (Haas et al. 2012). Our previous studies have demonstrated that the post-translational processing of βAPP and the secretion of the s-APP ectodomain can serve as useful readouts for assessing the integrity of exocytic trafficking pathways in cultured cells (Dugan et al. 1995; McConlogue et al. 1996).
As shown by the western blots in Fig. 6c, control U251 cells, or cells exposed to meta-MOMIPP, contain a readily detectable immature form of βAPP at approximately 105 kDa and a less prominent mature (O-glycosylated) form at approximately 135 kDa. In the cells treated with the vacuole-inducing compounds, similar amounts of the immature and mature forms are observed, but accumulation of a prominent intermediate form of βAPP can be seen (asterisks), especially in the cells treated with MOMIPP (Fig. 6c). The mobility of this intermediate is similar to, but not identical to, the accumulated form of intracellular βAPP detected in cells treated with Brefeldin A, an agent that disrupts trafficking between the ER and Golgi apparatus and prevents O-glycosylation without blocking N-glycosylation (Lippincott-Schwartz et al. 1989; Ulmer and Palade 1991). Despite the accumulation of the intracellular βAPP intermediate, we found that the amount of s-APP released into the medium (normalized to total cellular protein) was not decreased in cells treated with MOMIPP or MOPIPP (Fig. 6d). On the contrary, the amount of secreted s-APP was significantly increased in cells treated with MOMIPP, and to a lesser extent, with MOPIPP. This suggests that exocytic trafficking and secretase cleavage of the O-glycosylated form of APP remains intact, or may even be enhanced, despite the extensive vacuolization of endosomal compartments induced by these compounds.
Discussion
Compounds that trigger non-apoptotic cell death are of interest as potential therapeutics that might offer advantages in treating cancers like GBM, which develop resistance to conventional apoptosis-inducing drugs (Robinson et al. 2012; Kornienko et al. 2013; Kitambi et al. 2014; Maltese and Overmeyer 2014). Previous studies have established that specific indole-based chalcones (e.g., MIPP and MOMIPP) can cause cell death in GBM and other types of tumor cells via a novel non-apoptotic mechanism dubbed methuosis (Maltese and Overmeyer 2014). Although the specific molecular targets of these compounds have not yet been identified, they elicit a distinctive phenotype characterized by massive accumulation of cytoplasmic vacuoles derived from macropinosomes. At the present time the specific mechanisms that lead to loss of cell viability have not been completely defined.
The results presented here demonstrate that our lead methuosis-inducing compound, MOMIPP, has effects that extend beyond the perturbation of macropinocytosis. Our current working model summarizing the effects of MOMIPP on intracellular vesicular trafficking is depicted in Fig. 7. Specifically, we have shown that the compound disrupts ligand-induced turnover of distinct cell surface receptors (EGFR and LDLR) that depend on endocytic trafficking to lysosomes for degradation. MOMIPP also impairs posttranslational trafficking and maturation of lysosomal proteases (cathepsins D and L), suggesting that it causes a global disruption of endolysosomal trafficking at the junction where late endosomes converge on lysosomes.
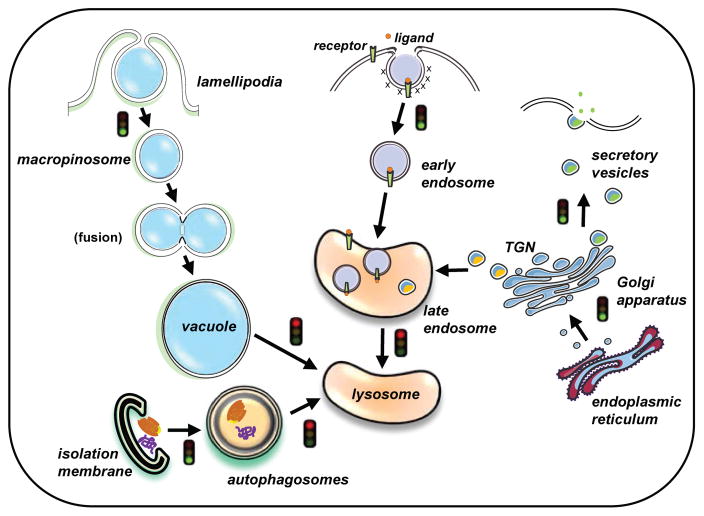
Fig. 7.
A working model describing the current understanding of MOMIPP’s effects on vesicular trafficking. MOMIPP initially stimulates the biogenesis and homotypic fusion of macropinosomes. The resulting fluid-filled vacuoles fail to merge with lysosomes and thus accumulate in the cytoplasm (Maltese and Overmeyer, 2015). The present study indicates that some vacuoles also are derived from expansion of pre-existing late endosomes. The trafficking of cell surface receptors (e.g., EGFR, LDLR) to the lysosomes is blocked, presumably at the junction where late endosomes merge with lysosomes. Likewise, protease precursors routed from the trans-Golgi network (TGN) to the lysosomes via the late endosome compartment are unable to reach their destination for the final step of processing/maturation. The fusion of autophagosomes with late endosomes and/or lysosomes also appears to be inhibited in cells treated with MOMIPP, resulting in accumulation of autophagosomes. A concomitant increase in autophagosome biogenesis may contribute to the increased pool of autophagosomes. In contrast to the disruption of endolysosomal trafficking, the ER → Golgi → plasma membrane secretory pathway appears to remain intact in cells treated with MOMIPP. The non-lethal vacuole-inducing compound, MOPIPP, has similar effects on trafficking of macropinosomes, autophagosomes, and lysosomal proteases, but its effects on endocytic trafficking of receptors appear to be considerably less severe than those of MOMIPP, possibly explaining its lack of cytotoxicity.
In accord with this idea, autophagic flux also appears to be blocked in cells exposed to MOMIPP, based on 1) the large increase in LC3II observed by immunofluorescence and western blot analysis and 2) the accumulation of the autophagosome cargo protein, p62. The concept that perturbation of endolysosomal trafficking could lead to a disruption of autophagic flux is entirely consistent with historical evidence that maturation of nascent autophagosomes to autolysosomes proceeds via fusion with both early and late endosomes (Eskelinen 2005; Razi et al. 2009). Indeed, recent studies have demonstrated molecular interactions between components of the basal autophagy machinery (ATG12-ATG3) and the endosome-associated protein Alix, an important regulator of late endosome function and distribution (Murrow et al. 2015).
While the latter findings are consistent with inhibition of autophagic flux by MOMIPP, it remains unclear why the level of LC3II in cells treated with the compound is substantially higher than it is in control cells where basal autophagic flux is blocked with lysosomal protease inhibitors (Fig. 4b). One possibility is that MOMIPP has a dual effect, increasing autophagosome biogenesis at the same time that fusion of autophagosomes with endosomes/lysosomes is impaired. This could be envisioned if failure of autophagosome → lysosomal trafficking impedes degradation of misfolded protein aggregates or damaged organelles, creating a feed-forward loop where autophagosome biogenesis is elevated as a compensatory mechanism. An alternative explanation for the high accumulation of LC3II in cells treated with MOMIPP, compared to cells treated with protease inhibitors alone, is that some LC3II is sequestered in the MOMIPP-induced vacuoles derived from macropinosomes. This could result in an additive increase when combined with the pool of LC3II that accumulates in autophagosomes.
In contrast to the striking inhibitory effects of MOMIPP on lysosome-directed trafficking of cell surface receptors, cathepsins, and autophagosome cargo (p62), the compound did not impair secretion of the ectodomain (s-APP) derived from the endogenous membrane glycoprotein, βAPP. In fact, secretion of this protein was actually increased (Fig. 6d). At first glance, the increased secretion of s-APP in MOMIPP-treated cells seems inconsistent with the marked accumulation of what appears to be an immature βAPP intermediate (Fig. 6c). We speculate that this incongruity may be related to the nature of the βAPP intermediate. In cells treated with the Golgi-disrupting agent, Brefeldin A, the accumulation of an incompletely processed βAPP intermediate is accompanied by complete loss of the mature 135 kDa form, consistent with known ability of Brefeldin A to block secretory protein trafficking. On the other hand, in the cells treated with MOMIPP, the pool of mature 135 kDa βAPP is not diminished, suggesting that the accumulating intermediate may arise from a mechanism other than disruption of secretory trafficking between the Golgi apparatus and the cell surface. Prior studies have revealed that at steady-state only a small fraction of intracellular βAPP is transported from the TGN to the cell surface for processing by α-secretase (Haas et al. 2012), and that a significant portion of intracellular βAPP may be diverted from the Golgi apparatus to MVEs (Edgar et al. 2015), autophagosomes (Yu et al. 2005), and lysosomes (Tam et al. 2014). In those compartments the protein can undergo cleavage by BACE and γ-secretase (Huse et al. 2000; Pasternak et al. 2004; Yu et al. 2004) or be subjected to degradation by lysosomal proteases (Caporaso et al. 1992; Dash and Moore 1993). With this in mind, it is conceivable that the accumulation of the βAPP intermediate in cells treated with MOMIPP may be related to the inability of these cells to degrade endosomal and/or autophagosomal pools of βAPP diverted from the Golgi apparatus. In any case, the abundant amount of s-APP secreted from cells treated with MOMIPP at least suggests that a sufficient amount of mature O-glycosylated βAPP is generated in the Golgi apparatus to drive α-secretase cleavage at the cell surface. Since the antibody that we used to detect s-APP does not discriminate between the ectodomain polypeptides released by α-secretase and BACE, it remains possible that the pool of s-APP released from MOMIPP-treated cells is augmented by ectodomains derived from BACE cleavage of immature βAPP in endosomal or autophagosomal compartments. The latter could be released through endosome recycling or exosome pathways. These studies raise intriguing questions for future investigation. In particular, it will be interesting to determine how MOMIPP, and its non-cytotoxic analog, MOPIPP, might affect the amyloidogenic processing of βAPP and the release of Aβ.
In the instances of methuosis that have been described to date, dysfunctional macropinosome trafficking and extreme cytoplasmic vacuolization are obvious morphological hallmarks that precede the loss of cell viability (Maltese and Overmeyer 2014). Nevertheless, it has been difficult to establish an unequivocal cause-effect relationship between vacuolization and cell death because pharmacological agents (amiloride, cytochalasin D) or genetic manipulations (Rac1 dominant-negative or knockdown) that block macropinocytosis can compromise cell viability. The comparisons between MOMIPP and meta-MOMIPP (a very closely related analog that induces neither vacuolization nor cell death), provide correlative evidence suggesting that vacuolization of macropinosome and endosomal compartments and the attendant defects in endolysosomal trafficking contribute to cytotoxicity. However, the results obtained with another analog, MOPIPP, present us with a conundrum. By all of the morphological criteria examined in Figures 1–3, MOPIPP causes accumulation of cytoplasmic vacuoles that are similar in size, number and composition to those elicited by MOMIPP. Yet, GBM cells treated with MOPIPP continue to proliferate (Trabbic et al. 2014) and do not exhibit substantial loss of viability (Fig. 1 b & c). In fact, there is little difference between MOPIPP, a robust vacuole-inducing compound, and meta-MOMIPP, an analog that does not induce any vacuoles, in terms of cell viability. These results argue against the notion that accumulation of vacuoles simply causes cells to explode by direct physical disruption of membrane integrity. It now seems more likely that, despite the general morphological similarities of the vacuoles induced by MOMIPP and MOPIPP, there are underlying mechanistic differences in the effects of the two compounds on pathways critical for cell viability. In this regard, it is interesting to note that although both compounds have similar inhibitory effects on cathepsin maturation (Fig. 7) and autophagosome-lysosome fusion (Fig. 4), MOMIPP clearly has a more severe impact on endosomal sorting and lysosomal degradation of ligand-activated EGFR and LDLR (Figs. 5&6). These observations suggest that the differential cytotoxicity of MOMIPP versus MOPIPP may be related to a “threshold effect” on critical receptor-mediated endocytic signaling or nutrient uptake pathways, beyond which cell death pathways are triggered. Interestingly, another class of vacuole-inducing compounds (e.g., vacuolin-1) that induces vacuolization by blocking lysosomal exocytosis without impairing endocytic or secretory trafficking does not induce cell death (Cerney et al., 2004). This tends to support the notion that dysfunction of endosomal and macropinosomal internalization pathways may have a greater impact on cell viability than simple endosomal swelling or altered lysosome recycling. A more detailed comparison of the cellular effects of different vacuole-inducing compounds can be found in reviews by Aki et al. (2012) and Maltese and Overmeyer (2014).
Although the specific mechanisms whereby disruption of endolysosomal trafficking of receptors may induce apoptotic or non-apoptotic cell death remain poorly understood, there are a few published studies that provide clues as to how this might occur. In one example, the vacuole-inducing anti-cancer compound, 5-benzylglycinyl-amiloride, has been reported to cause necrotic cell death in glioblastoma cells by redirecting endosomes containing the LRP-1 receptor and its associated urokinase plasminogen activator system to perinuclear mitochondrial regions, where release of mitochondrial AIF (apoptosis-inducing factor) is triggered (Pasupuleti et al. 2015). In another study, leelamine, a tricyclic diterpene that induces caspase-independent death in melanoma cells, was shown to impair endosomal trafficking and autophagic flux, with consequent accumulation of cholesterol in endosomal compartments and disruption of multiple pro-survival receptor tyrosine kinase signaling pathways (Kuzu et al. 2014). With these observations in mind, ongoing studies in our laboratory are focused on further defining the potential downstream effects of MOMIPP-mediated disruption of endolysosomal trafficking on cellular metabolism, stress responses, and mediators of non-apoptotic cell death. For this task, the availability of closely related non-lethal vacuole-inducing analogs like MOPIPP should prove to be very useful for delineating the critical molecular pathways required for cytotoxicity of the indole-based chalcones.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 CA115495) and by the Helen and Harold McMaster Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge Andrew Trunk and Bryan DeWitt for early exploratory work on this project.
Abbreviations
The abbreviations used are
- MOMIPP
3-(5-methoxy-2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one
- MOPIPP
3-(5-methoxy-2-propyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one
- GBM
glioblastoma multiforme
- LAMP-1
lysosome-associated membrane protein-1
- LC3
microtubule-associated protein light chain 3
- EGFR
epidermal growth factor receptor
- LDLR
low density lipoprotein receptor
- MVE
multivesicular endosome
- βAPP
β-amyloid precursor protein
- DMEM
Dulbecco s modified Eagle s medium
- HBSS
Hank s balanced salt solution
- FBS
fetal bovine serum
- PVDF
polyvinylidene fluoride
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- TGN
trans-Golgi network
- ESCRT
endosomal sorting complex required for transport
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest in regard to the contents of this article.
Author Contributions
NEM carried out all of the experiments, analyzed the results and wrote the initial draft of the paper. JHO, worked with NEM on the design, execution and interpretation of the experiments. WAM conceived the idea for the project, directed the design of the experiments and wrote the paper with NEM.
References
- Aki T, Nara A, Uemura K. Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biol Toxicol. 2012;28:125–31. doi: 10.1007/s10565-012-9212-3. [DOI] [PubMed] [Google Scholar]
- Bhanot H, Young AM, Overmeyer JH, Maltese WA. Induction of non-apoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol Cancer Res. 2010;8:1358–74. doi: 10.1158/1541-7786.MCR-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, et al. The small chemical vacuolin-1 inhibits Ca2+ -dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2004;5:883–8. doi: 10.1038/sj.embor.7400243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso GL, Gandy SE, Buxbaum JD, Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer β/A4 amyloid precursor protein. Proc Natl Acad Sci USA. 1992;89:2252–6. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Moore AN. Inhibitors of endocytosis, endosome fusion, and lysosomal processing inhibit the intracellular proteolysis of the amyloid precursor protein. Neurosci Lett. 1993;164:183–6. doi: 10.1016/0304-3940(93)90887-q. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harbor Perspect Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbruck R, Desel C, von Figura K, Hille-Rehfeld A. Proteolytic processing of cathepsin D in prelysosomal organelles. Eur J Cell Biol. 1994;64:7–14. [PubMed] [Google Scholar]
- Dugan JM, deWit C, McConlogue L, Maltese WA. The ras-related GTP binding protein, Rab1B, regulates early steps in exocytic transport and processing of β-amyloid precursor protein. J Biol Chem. 1995;270:10982–9. doi: 10.1074/jbc.270.18.10982. [DOI] [PubMed] [Google Scholar]
- Dunn WAJ. Studies on the mechanisms of autophagy: Maturation of the autophagic vacuole. J Cell Biol. 1990;110:1935–45. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WAJ. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–43. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–72. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- Edgar JR, Willen K, Gouras GK, Futter CE. ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-beta accumulation. J Cell Sci. 2015;128:2520–8. doi: 10.1242/jcs.170233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL. Maturation of autophagic vacuoles in Mammalian cells. Autophagy. 2005;1:1–10. doi: 10.4161/auto.1.1.1270. [DOI] [PubMed] [Google Scholar]
- Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–43. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–8. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophy Res Commun. 1988;151:40–7. doi: 10.1016/0006-291x(88)90556-6. [DOI] [PubMed] [Google Scholar]
- Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–56. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harbor Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer's disease beta-secretase. J Biol Chem. 2000;275:33729–37. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Johnson EE, Overmeyer JH, Gunning WT, Maltese WA. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J Cell Sci. 2006;119:1219–32. doi: 10.1242/jcs.02833. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N. Posttranslational processing and modification of cathepsins and cystatins. J Signal Transduct. 2010;2010 doi: 10.1155/2010/375345. Article ID 375345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–71. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Kitambi SS, Toledo EM, Usoskin D, Wee S, Harisankar A, Svensson R, et al. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell. 2014;157:313–28. doi: 10.1016/j.cell.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Eskelinen EL, Deretic V. Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes... wait, I'm confused. Autophagy. 2014;10:549–51. doi: 10.4161/auto.28448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornienko A, Mathieu V, Rastogi SK, Lefranc F, Kiss R. Therapeutic agents triggering nonapoptotic cancer cell death. J Med Chem. 2013;56:4823–39. doi: 10.1021/jm400136m. [DOI] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, et al. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–32. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Kuzu OF, Gowda R, Sharma A, Robertson GP. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol Cancer Ther. 2014;13:1690–703. doi: 10.1158/1535-7163.MCT-13-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence B, Brown W. Autophagic vacuoles rapidly fuse with pre-existing lysosomes in cultured hepatocytes. J Cell Sci. 1992;102:515–26. doi: 10.1242/jcs.102.3.515. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–13. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–9. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- Maltese WA, Overmeyer JH. Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am J Pathol. 2014;184:1630–42. doi: 10.1016/j.ajpath.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese WA, Overmeyer JH. Non-apoptotic cell death associated with perturbations of macropinocytosis. Front Physiol. 2015;6:38. doi: 10.3389/fphys.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese WA, Wilson S, Tan Y, Suomensaari S, Sinha S, Barbour R, et al. Retention of the Alzheimer's amyloid precursor fragment C99 in the endoplasmic reticulum prevents formation of amyloid beta-peptide. J BiolChem. 2001;276:20267–79. doi: 10.1074/jbc.M007238200. [DOI] [PubMed] [Google Scholar]
- McConlogue L, Castellano F, deWit C, Schenk D, Maltese WA. Differential effects of a Rab6 mutant on secretory versus amyloidogenic processing of Alzheimer's b-amyloid precursor protein. J Biol Chem. 1996;271:1343–8. doi: 10.1074/jbc.271.3.1343. [DOI] [PubMed] [Google Scholar]
- Minna E, Romeo P, De CL, Dugo M, Cassinelli G, Pilotti S, et al. miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma. Oncotarget. 2014;5:2513–28. doi: 10.18632/oncotarget.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Murrow L, Malhotra R, Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17:300–10. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer JH, Kaul A, Johnson EE, Maltese WA. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. MolCancer Res. 2008;6:965–77. doi: 10.1158/1541-7786.MCR-07-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer JH, Young AM, Bhanot H, Maltese WA. A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol Cancer. 2011;10:69. doi: 10.1186/1476-4598-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Pasternak SH, Callahan JW, Mahuran DJ. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer's disease: reexamining the spatial paradox from a lysosomal perspective. J Alzheimer's Dis. 2004;6:53–65. doi: 10.3233/jad-2004-6107. [DOI] [PubMed] [Google Scholar]
- Pasupuleti N, Grodzki AC, Gorin F. Mis-trafficking of endosomal urokinase proteins triggers drug-induced glioma nonapoptotic cell death. Mol Pharmacol. 2015;87:683–96. doi: 10.1124/mol.114.096602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao MX, Bai JW, Zhang PF, Zhang YZ. PCSK9 regulates apoptosis in human neuroglioma u251 cells via mitochondrial signaling pathways. Int J Clin Exp Pathol. 2015;8:2787–94. [PMC free article] [PubMed] [Google Scholar]
- Poirier S, Mayer G, Poupon V, McPherson PS, Desjardins R, Ly K, et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J Biol Chem. 2009;284:28856–64. doi: 10.1074/jbc.M109.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185:305–21. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Reyes EM, Salipur FR, Shams M, Forsthoefel MK, Bates PJ. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol Oncol. 2015;9:1392–405. doi: 10.1016/j.molonc.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnboutt S, Stoorvogel W, Geuze HJ, Strous GJ. Identification of subcellular compartments involved in biosynthetic processing of cathepsin D. J Biol Chem. 1992;267:15665–72. [PubMed] [Google Scholar]
- Robinson MW, Overmeyer JH, Young AM, Erhardt PW, Maltese WA. Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J Med Chem. 2012;55:1940–56. doi: 10.1021/jm201006x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti E, Calamai M, Goulbourne CN, Zhang L, Hong C, Lin RR, et al. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Mol Cell Biol. 2013;33:1503–14. doi: 10.1128/MCB.01716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–40. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Sisodia SS. β-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA. 1992;89:6075–9. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Kane SE, Gal S, Mason RW, Gottesman MM. Glycosylation of procathepsin L does not account for species molecular-mass differences and is not required for proteolytic activity. Biochem J. 1989;262:931–8. doi: 10.1042/bj2620931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–8. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Tam JH, Seah C, Pasternak SH. The amyloid precursor protein is rapidly transported from the Golgi apparatus to the lysosome and where it is processed into beta-amyloid. Molecular Brain. 2014;7:54. doi: 10.1186/s13041-014-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabbic CJ, Dietsch HM, Alexander EM, Nagy PI, Robinson MW, Overmeyer JH, et al. Differential induction of cytoplasmic vacuolization and methuosis by novel 2-indolyl-substituted pyridinylpropenones. ACS Med Chem Lett. 2014;5:73–7. doi: 10.1021/ml4003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabbic CJ, Overmeyer JH, Alexander EM, Crissman EJ, Kvale HM, Smith MA, et al. Synthesis and biological evaluation of indolyl-pyridinyl-propenones having either methuosis or microtubule disruption activity. J Med Chem. 2015;58:2489–512. doi: 10.1021/jm501997q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer JB, Palade GE. Effects of Brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. Eur J Cell Biol. 1991;54:38–54. [PubMed] [Google Scholar]
- Unni AM, Lockwood WW, Zejnullahu K, Lee-Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife. 2015;4:e06907. doi: 10.7554/eLife.06907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, et al. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989;57:115–26. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, et al. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer's disease. Int J Biochem Cell Biol. 2004;36:2531–40. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Molecular Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]