Abstract
Objectives
Few studies have addressed optimal follow-up for HIV-infected women after cervical treatment. This study aimed to compare performance of three available tests to detect post-treatment cervical disease in HIV-infected women in Kenya.
Design
This is a prospective cohort study.
Methods
At least six months following cryotherapy, 517 HIV-infected women were evaluated concurrently with VIA, Pap smear, and HR-HPV testing. Women positive by any test (≥LSIL for Pap) were scheduled for colposcopy and biopsy. Among 248 with histological confirmation (and 174 assumed to be truly negative for CIN2+ after testing negative by all three tests), the ability of each test alone, or in combination, to detect CIN2+ was calculated to determine their utility in post-treatment follow-up.
Results
The median age of women was 35 years, 68% were WHO stage 1–2, with a median CD4 count of 410 cells/uL, and 87% were on combination antiretroviral therapy. At a median of 6.3 months post-treatment, 64% had an abnormal screen by VIA, Pap, and/or HR-HPV. Among women with histological confirmation, 72 (30%) had persistent/recurrent CIN2+. As single tests, Pap correctly classified the most cases (83%) and had the highest specificity (91% (88%, 95%); sensitivity 44% (35, 53%)), whereas HR-HPV had the highest sensitivity (85% (75%, 96%); specificity 54% (49%, 58%)). VIA was not sensitive (27% (18%, 36%)) for the detection of post-treatment CIN2+ (specificity 82% (79%, 86%)).
Conclusions
With the goal to minimize the number of false negatives (e.g. not miss CIN2+ post-treatment) in this population that is high-risk due to both prior cervical disease and HIV infection, HR-HPV based algorithms are recommended.
Keywords: VIA, Pap smear, HPV, screening, post-treatment, HIV, cryotherapy, sensitivity
Introduction
Over half of HIV-infected persons in Kenya are women. Although HIV-infected women are living longer and healthier lives with increasing access to highly active antiretroviral therapy (ART), they remain at increased risk for cervical cancer[1–3]. Overall, cervical cancer is the most common cause of cancer death affecting women in Kenya[4], and women with HIV have three times the risk of cervical precancer and cancer than uninfected women[5]. Fortunately, many studies have demonstrated that a single-visit “screen-and-treat” approach using visual inspection with acetic acid (VIA) with immediate cryotherapy is a safe, acceptable and effective strategy for cervical cancer prevention in low-resource settings[6–8]. Despite the fact that women with prior cervical abnormalities are at higher risk for recurrent/persistence cervical intraepithelial neoplasia (CIN)[9–13], very few studies have addressed the best way to follow-up HIV-infected women after cryotherapy.
As implementation of “see-and-treat” programs continue to increase across sub-Saharan Africa, there is a need to identify feasible and effective approaches to ensure that these women remain disease free after treatment with cryotherapy. This is essential to ensure long-term programmatic reductions in cervical cancer morbidity and mortality. Therefore, this study aims to determine and compare the accuracy of VIA, conventional Pap smear, and HR-HPV testing as six month post-treatment follow-up tools to detect histologically-confirmed CIN grade 2, CIN 3, or cancer among HIV-infected women who had undergone VIA/cryotherapy.
Methods
Study population and design
To study the utility of different screening tests after cervical treatment, women were recruited from September, 2011 to June, 2013 from four AMPATH-supported Cervical Cancer Screening clinics within Kenyan Ministry of Health sites: Moi Teaching and Referral Hospital, and Mosoriot, Turbo and Chulaimbo Health Centers. Women were approached if they were attending clinic, as recommended per the standard of care, for post-treatment examination. Women eligible for the original screening study, from which women were enrolled into the present study, must have been HIV positive, be age 18–55 years old, generally healthy, and undergone cryotherapy at least six months ago due to a positive VIA screen. Women were excluded if they were pregnant or had been pregnant within the previous three months, had current mucopurulent discharge, had active vaginal bleeding, or had a syndromic sexually transmitted infection (STI) diagnosis in the two weeks before enrollment. Women with genital tract infection underwent syndromic treatment and were eligible to enroll three weeks after treatment if the infection cleared.
Study nurses and assistants certified in human subjects’ research informed potential participants that they would be evaluated for recurrent and/or persistent cervical abnormalities using three screening tests (VIA, Pap smear, and HR-HPV DNA testing) and, if positive by any, they would be asked to have a colposcopy to obtain a cervical biopsy for diagnosis and treatment. Written informed consent was obtained from eligible study participants before they were enrolled. The study was reviewed and approved by Institutional Review Boards at Moi University, Indiana University, University of Toronto, and the Miriam Hospital.
Study procedures
At enrollment (a median of 6.3 months after initial VIA+/cryotherapy), data on demographics, past medical history and risk factors for CIN were collected using an interviewer-administered questionnaire. HIV diagnosis, CD4 count, ART status, and WHO stage were abstracted from clinic records. Then, women underwent a gynecologic examination that included, in this order: collection of a Pap smear for conventional cytology, sampling for HR-HPV DNA using an endocervical cytobrush, and VIA. The criteria for VIA were taken from A Practice Manual on Visual Screening for Cervical Neoplasia. WHO 2003. The Pap smears were collected from the endo- and ecto-cervix simultaneously using a plastic cervibroom that was smeared on a slide and immediately fixed. Pap smears were read at Moi University College of Health Sciences and classified according to the Bethesda classification system. The Digene Hybrid Capture II (Qiagen) test was used to detect 14 high-risk HPV genotypes, according to manufacturer’s protocol in the AMPATH reference laboratory.
Women with a positive VIA result underwent colposcopy-directed biopsies within two weeks by a trained gynecologist. Women with a negative VIA result returned in 4–6 weeks to obtain results of Pap and HR-HPV testing. If either the Pap smear was abnormal (low grade squamous intraepithelial lesion (LSIL) or worse) or the HR-HPV test was positive, patients underwent colposcopy-directed biopsies. Thus, women with any abnormal result from VIA, Pap smear, or HR-HPV testing were directed to undergo colposcopy and biopsy. Women negative by all three tests (e.g. those that were VIA- and Pap <LSIL, and no HR-HPV detected) were not referred for biopsy, and for the sake of clinical management and our analyses, these women were considered to be truly negative for the outcome of CIN2+.
A cervical biopsy was obtained for gold-standard histological confirmation, and if no lesion was apparent on colposcopy, a biopsy was collected randomly at either the 6 or 12 o’clock position of the cervix. The pathology readings were done at Moi University College of Health Sciences, with a random 10% sample of biopsy and pap smears sent to Brown University to be re-read by a single pathologist, revealing consistent diagnoses in >80% of cases. Women with CIN 2+ were counseled and referred to Moi Teaching and Referral Hospital for treatment with protocols based on recommendations from the American Society for Colposcopy and Cervical Pathology (ASCCP) and the International Agency for Research on Cancer/WHO. Women with less than CIN2 were counseled to return in six months for follow-up, per local standardized screening protocols. For women who failed to attend follow-up visits, the study nurses and assistants made at least three attempts to contact them via phone, tagging of medical charts, and home visits before considering them lost-to-follow-up.
Statistical Analysis
Study enrollment and follow-up were summarized using the recommended Consolidated Standards of Reporting Trials (CONSORT)[14]. Women’s demographic characteristics, HIV-related clinic data, and self-reported risk factors were summarized, along with post-treatment screening test results for VIA, Pap smear and HR-HPV. For those with biopsy results, screening results were further cross-tabulated by the histology findings.
By our study design, histology results were systemically missing for those with triple negative test results and for calculation of test characteristics, these were assumed to be truly negative for the outcome of CIN2+. Data were missing for Pap smears that were inadequate for cytological diagnosis (n=39), for women lost before histological confirmation (n=79), and for those with inadequate specimens for histological diagnosis (n=4). For these missing data, multiple imputations were performed using sequential conditional models (aka chain models), which imputed data by steps to mirror the prospective nature of the study[15, 16]. Missing covariates (CD4 and WHO stage) were first imputed by their mode. Then, a conditional model was developed to impute the missing Pap smear results, assuming they were missing at random, conditional on patient characteristics and the results of VIA and HR-HPV testing[17]. A conditional model was also used to impute missing histology results using the same set of variables and the results of VIA, HR-HPV and Pap smear. After creating 10 imputed datasets, Hosmer-Lemeshow tests were used to confirm the goodness of fit. Imputed data were used in all subsequent analyses.
The associations between baseline factors and post-treatment CIN2+ (vs. <CIN2+) were examined using Poisson regression to estimate unadjusted and fully adjusted prevalence ratios. Because of the limited sample size, only age, CD4, WHO stage, ART, age at first sex, and contraception were included in the multivariable model. History of STI was not included because the self-reported data were relatively sparse (only ~10% answered “yes”) and inclusion led to unstable model estimates. Next, the sensitivity, specificity, negative/positive predictive value (NPV/PPV), and rate of correct classification were calculated for the three post-treatment tests individually and considering all “AND” (denoted by &) and “OR” (denoted by |) combinations. For example, an “AND” combination of VIA and Pap-smear (VIA & PAP) meant that a subject was positive only if both are positive; while an “OR” combination (VIA | PAP) meant that the subject was positive if either test is positive. All 95% confidence intervals of the summary statistics were calculated using the standard error formula for analysis using multiply imputed datasets[17]. “Non-overlapping confidence intervals” can be used as a criterion for concluding statistical significance among different post-treatment test strategies (alpha=0.05). All statistical analyses were conducted using Stata 14.
Results
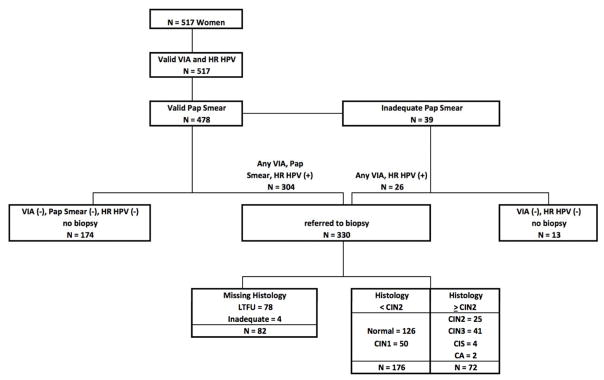
Of the 678 women eligible for the study, 517 consented and enrolled, at a median age of 35 years (IQR: 31–40). The majority of women were married (54%), had completed a primary (51%) or secondary (36%) education, and were self-employed (51%; Table 1). The majority of women had a CD4 count ≥350 cells/uL (62%) and were on antiretroviral therapy (ART; 87%). Of the 478 women with complete screening results (8% of Pap smears were inadequate for diagnosis; Figure 1), 304 were referred for colposcopy due to at least one abnormal result by VIA, Pap, or HR-HPV. The majority (n=26) of the 39 women with an inadequate Pap smear tested positive by VIA and/or HR-HPV so were also referred to colposcopy, for a total of 330 referrals. Of the women referred for colposcopy-directed biopsy, 24% were missing histology results due to loss to follow-up (n=78) or inadequate biopsy for diagnosis (n=4). For the 13 women with an inadequate Pap who were VIA and HR-HPV negative, Pap smear and histology results were imputed and included in calculations of test characteristics.
Table 1.
Baseline characteristics of 517 HIV-infected women at post-treatment follow-up.
| No. | % | |
|---|---|---|
| Age | ||
| 18 to 29 yrs | 108 | 21% |
| 30 to 34 yrs | 133 | 26% |
| 35 to 39 yrs | 146 | 28% |
| 40 or older | 130 | 25% |
| Marital Status | ||
| Single | 103 | 20% |
| Married | 281 | 54% |
| Divorced/widowed/separated | 133 | 26% |
| Education Level | ||
| None | 22 | 4% |
| Primary | 264 | 51% |
| Secondary | 185 | 36% |
| College/university | 46 | 9% |
| Occupation | ||
| Unemployed | 124 | 24% |
| Self-Employed | 264 | 51% |
| Employed | 129 | 25% |
| CD4 count (missing n = 1) | ||
| < 200 | 67 | 13% |
| 200–349 | 130 | 25% |
| 350–499 | 138 | 27% |
| ≥500 | 181 | 35% |
| WHO Stage (missing n = 1) | ||
| 1 | 211 | 41% |
| 2 | 138 | 27% |
| 3–4 | 167 | 32% |
| ART (Yes/No) | ||
| No | 67 | 13% |
| Yes | 450 | 87% |
| Age at first sexual activity | ||
| Younger than 18 | 185 | 36% |
| 18 or older | 168 | 33% |
| Refuse to answer/Not applicable | 164 | 32% |
| History of STI (Missing n = 19) | ||
| No | 445 | 89% |
| Yes | 52 | 11% |
| Unknown | 1 | 0% |
| Current contraceptive use | ||
| None | 246 | 48% |
| Condoms only | 99 | 19% |
| Injectable (Depo-Provera) | 117 | 23% |
| Others | 55 | 10% |
Figure 1.
Flow diagram of participant follow-up and results.
At a median time of 6.3 months after VIA/cryotherapy (range 2.8–21.7 months), 78% of women had <LSIL on Pap smear, 81% were negative by VIA, and 43% were HR-HPV negative (Table 2). Overall, 36% were negative by all three tests. Conversely, 4% were positive by all three tests, and 64% were positive by any test. Among the 248 women with histology, 29% had CIN2+ (n=25 CIN2, n=41 CIN3, n=4 carcinoma in situ, n=2 cancers) at a median of 2.9 months after a positive VIA/Pap/HR-HPV test (and after a median of 11 months total after initial VIA/cryotherapy). Of the 72 CIN2+ cases, 46% had been considered normal on cytology, 69% had been considered negative by VIA, and 14% had tested negative for HR-HPV. Nearly all CIN2+ cases (92%) tested positive by any (e.g. at least one) test whereas only 10% tested positive by all three tests. There was no clear association of age and CD4 count on prevalence of CIN2+, although there was a trend of lower prevalence among those with an older age of sexual debut (Table 3; adjusted Prevalence Ratio (PR): 0.57; 95% confidence interval (CI): 0.32–1.03).
Table 2.
Results of the post-treatment VIA/Pap smear/HR-HPV and subsequent histological confirmation.
| Overall (N=517) | Total histology (N=248) | Histology | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal (N=126) | CIN I (N=50) | CIN II (N=25) | CIN III (N=41) | CIS (N=4) | Cancer (N=2) | |||
| Cytology | ||||||||
| Inadequate | 39(7.5%) | 17 | 9(52.9%) | 2(11.8%) | 2(11.8%) | 4(23.5%) | 0 (0%) | 0 (0%) |
| Normal | 398(77.0%) | 173 | 98(56.6%) | 42(24.3%) | 9 (5.2%) | 20(11.6%) | 3(1.7%) | 1(0.6%) |
| ASCUS | 7 (1.4%) | 3 | 1(33.3%) | 0 (0%) | 1(33.3%) | 1(33.3%) | 0 (0%) | 0 (0%) |
| LSIL | 26 (5.0%) | 18 | 5 (27.8%) | 2(11.1%) | 6(33.3%) | 5 (27.8%) | 0(0%) | 0(0%) |
| HSIL | 45(8.7%) | 35 | 12(34.3%) | 4(11.4%) | 7(20%) | 11(31.4%) | 0(0%) | 1 (2.9%) |
| Cancer | 2(0.4%) | 2 | 1 (50%) | 0 (0%) | 0(0%) | 0(0%) | 1(50%) | 0(0%) |
| VIA | ||||||||
| Positive | 100(19.3%) | 81 | 40(49.4%) | 19(23.5%) | 9(11.1%) | 12(14.8%) | 0(0%) | 1(1.2%) |
| Negative | 417 (80.7%) | 167 | 86(51.5%) | 31(18.6%) | 16 (9.6%) | 29(17.4%) | 4 (2.4%) | 1 (0.6%) |
| HR-HPV positive | ||||||||
| Positive | 275(53.2%) | 214 | 107(50%) | 45(21%) | 20(9.3%) | 36(16.8%) | 4(1.9%) | 2(0.9%) |
| Negative | 242 (42.8%) | 34 | 19(55.9%) | 5 (14.7%) | 5(14.7%) | 5(14.7%) | 0(0%) | 0 (0%) |
| VIA/PAP/HPV | ||||||||
| --/--/-- | 174(36.4%) | 0 | - | - | - | - | - | - |
| --/--/+ | 163(34.1%) | 122 | 69(56.6%) | 28(23%) | 7(5.7%) | 14 (11.5%) | 3 (2.5%) | 1 (0.8%) |
| --/+/-- | 20(4.2%) | 10 | 4 (40%) | 0(0%) | 3 (30%) | 3(30%) | 0 (0%) | 0 (0%) |
| --/+/+ | 32(6.7%) | 26 | 9(34.6%) | 2 (7.7%) | 5 (19.2%) | 9 (34.6%) | 1(3.8%) | 0(0%) |
| +/--/-- | 26(5.4%) | 17 | 12 (70.6%) | 5 (29.4%) | 0 (0%) | 0 (0%) | 0(0%) | 0(0%) |
| +/--/+ | 42(8.8%) | 37 | 18(48.6%) | 9 (24.3%) | 3(8.1%) | 7(18.9%) | 0(0%) | 0(0%) |
| +/+/-- | 4(0.8%) | 4 | 1(25%) | 0(0%) | 1 (25%) | 2(50%) | 0(0%) | 0(0%) |
| +/+/+ | 17(3.6%) | 15 | 4 (26.7%) | 4(26.7%) | 4(26.7%) | 2(13.3%) | 0(0%) | 1(6.7%) |
| Any test positive | 304(63.6%) | 231 | 117 (50.6%) | 48(20.8%) | 23 (10%) | 37 (16%) | 4(1.7%) | 2(0.9%) |
| All tests positive | 17(3.6%) | 15 | 4 (26.7%) | 4(26.7%) | 4(26.7%) | 2(13.3%) | 0(0%) | 1(6.7%) |
Table 3.
Characteristics associated with post-treatment CIN2+ detection at follow-up.
| Unadjusted PR (95% CI) a | Fully adjusted PR (95% CI) b | |
|---|---|---|
| Age | ||
| 18–29 yrs | 1 | 1 |
| 30–34 yrs | 0.52 (0.23 ,1.20) | 0.58 (0.29, 1.17) |
| 35–39 yrs | 0.94 (0.46 ,1.89) | 1.01 (0.58, 1.77) |
| ≥ 40 yrs | 0.73 (0.35 ,1.53) | 0.99 (0.53, 1.85) |
| CD4 | ||
| < 200 | 1 | 1 |
| 200–349 | 0.54 (0.22 ,1.36) | 0.61 (0.30, 1.25) |
| 350–499 | 0.34 (0.14 ,0.85) | 0.39 (0.18, 0.83) |
| ≥ 500 | 0.75 (0.33 ,1.70) | 0.80 (0.42, 1.51) |
| WHO Stage | ||
| 1 | 1 | 1 |
| 2 | 0.84 (0.41 ,1.74) | 0.90 (0.50, 1.64) |
| 3–4 | 1.36 (0.75 ,2.48) | 1.22 (0.77, 1.94) |
| ART | ||
| No | 1 | 1 |
| Yes | 0.98 (0.44 ,2.18) | 0.72 (0.37, 1.41) |
| Age at first sex | ||
| Younger than 18 | 1 | 1 |
| 18 or older | 0.55 (0.28 ,1.09) | 0.57 (0.32, 1.03) |
| Refuse to answer/Not applicable | 0.92 (0.52 ,1.61) | 0.92 (0.58, 1.47) |
| Contraception | ||
| No | 1 | 1 |
| Yes | 1.70 (1.02 ,2.85) | 1.66 (1.03, 2.66) |
based on data collected for the study.
based on data collected for the study with multiple imputation.
For use of a single test, Pap correctly classified the most cases (83%; 95%CI: 79%, 87%) and had the highest specificity (91%; 95%CI: 88%, 95%) and highest PPV (52%; 95%CI: 42%, 62%) but a relatively low sensitivity (44%; 95%CI: 35%, 53%; Table 4). HR-HPV had the highest sensitivity for detection of CIN2+ at 85% (95%CI: 75%, 96%) and the highest NPV (94%; 95%CI: 90%, 99%). However, HR-HPV had the lowest single test specificity (54%; 95%CI: 49%, 58%), relatively low PPV (29%; 95%CI: 23%, 34%), and correctly classified the least amount of cases (59%; 95%CI: 55%, 64%). VIA had the lowest sensitivity of the three tests (27%; 95%CI: 18%, 36%), and moderate specificity (82%; 95%CI: 79%, 86%), correct classification (72%; 95%CI: 68%, 77%), and NPV (84%; 95% CI: 80%, 88%). Positive predictive value of VIA was low at 25% (95%CI: 17%, 33%). When excluding those with missing histology due to inadequate Pap (and negative VIA/HR-HPV), results were unchanged (see online Appendix 1).
Table 4.
Characteristics of VIA/Pap/HR-HPV testing for post-treatment follow-up for detection of CIN2+.a
| Sensitivity | Specificity | Correctly Classified | PPV | NPV | |
|---|---|---|---|---|---|
| VIA | 0.27 (0.18, 0.36) | 0.82 (0.79, 0.86) | 0.72 (0.68, 0.77) | 0.25 (0.17, 0.33) | 0.84 (0.80, 0.88) |
| PAP | 0.44 (0.35, 0.53) | 0.91 (0.88, 0.95) | 0.83 (0.79, 0.87) | 0.52 (0.42, 0.62) | 0.88 (0.84, 0.92) |
| HR HPV | 0.85 (0.75, 0.96) | 0.54 (0.49, 0.58) | 0.59 (0.55, 0.64) | 0.29 (0.23, 0.34) | 0.94 (0.90, 0.99) |
| VIA & PAP | 0.13 (0.08, 0.17) | 0.97 (0.95, 0.99) | 0.82 (0.78, 0.86) | 0.50 (0.32, 0.68) | 0.84 (0.80, 0.87) |
| VIA & HR HPV | 0.22 (0.15, 0.29) | 0.89 (0.86, 0.93) | 0.77 (0.74, 0.81) | 0.31 (0.21, 0.41) | 0.84 (0.80, 0.88) |
| PAP & HR HPV | 0.31 (0.24, 0.38) | 0.94 (0.92, 0.97) | 0.83 (0.79, 0.86) | 0.54 (0.44, 0.65) | 0.86 (0.83, 0.90) |
| VIA & PAP & HR HPV | 0.09 (0.05, 0.13) | 0.98 (0.96, 0.99) | 0.82 (0.78, 0.86) | 0.45 (0.26, 0.65) | 0.83 (0.79, 0.87) |
| VIA | PAP | 0.59 (0.48, 0.69) | 0.76 (0.72, 0.81) | 0.73 (0.69, 0.77) | 0.35 (0.28, 0.41) | 0.89 (0.85, 0.94) |
| VIA | HR HPV | 0.90 (0.80, 1.00) | 0.47 (0.42, 0.51) | 0.54 (0.50, 0.59) | 0.27 (0.22, 0.32) | 0.96 (0.90, 1.00) |
| PAP | HR HPV | 0.99 (0.89, 1.00) | 0.51 (0.46, 0.55) | 0.59 (0.55, 0.64) | 0.30 (0.25, 0.35) | 0.99 (0.95, 1.00) |
| VIA | PAP | HR HPV | 1.00 (0.91, 1.00) | 0.44 (0.39, 0.48) | 0.54 (0.49, 0.58) | 0.28 (0.23, 0.32) | 1.00 (1.00, 1.00) |
Analysis assumed triple negatives are truly negatives and imputed those with otherwise missing histology
Appendix Table 1.
Analysis of post-treatment test characteristics for detection of CIN2+ based on complete case.a
| Sensitivity | Specificity | Correctly Classified | PPV | NPV | |
|---|---|---|---|---|---|
| VIA | 0.31 (0.21, 0.40) | 0.83 (0.79, 0.87) | 0.61 (0.56, 0.65) | 0.27 (0.19, 0.35) | 0.85 (0.81, 0.89) |
| PAP | 0.47 (0.39, 0.54) | 0.93 (0.90, 0.96) | 0.71 (0.67, 0.75) | 0.56 (0.48, 0.65) | 0.90 (0.86, 0.94) |
| HR HPV | 0.86 (0.75, 0.97) | 0.57 (0.52, 0.62) | 0.50 (0.46, 0.55) | 0.29 (0.24, 0.34) | 0.95 (0.90, 1.00) |
| VIA & PAP | 0.15 (0.10, 0.20) | 0.97 (0.95, 1.00) | 0.70 (0.66, 0.74) | 0.53 (0.36, 0.69) | 0.85 (0.82, 0.89) |
| VIA & HR HPV | 0.25 (0.17, 0.33) | 0.89 (0.85, 0.92) | 0.64 (0.59, 0.68) | 0.32 (0.22, 0.41) | 0.85 (0.81, 0.89) |
| PAP & HR HPV | 0.33 (0.26, 0.40) | 0.94 (0.91, 0.97) | 0.70 (0.66, 0.74) | 0.54 (0.43, 0.64) | 0.88 (0.84, 0.92) |
| VIA & PAP & HR HPV | 0.11 (0.06, 0.15) | 0.98 (0.96, 1.00) | 0.70 (0.66, 0.74) | 0.47 (0.28, 0.65) | 0.85 (0.81, 0.89) |
| VIA | PAP | 0.65 (0.55, 0.75) | 0.77 (0.73, 0.82) | 0.62 (0.57, 0.66) | 0.37 (0.31, 0.43) | 0.92 (0.88, 0.96) |
| VIA | HR HPV | 0.92 (0.81, 1.00) | 0.51 (0.46, 0.56) | 0.47 (0.43, 0.52) | 0.28 (0.23, 0.32) | 0.97 (0.92, 1.00) |
| PAP | HR HPV | 1.00 (0.89, 1.00) | 0.55 (0.50, 0.59) | 0.51 (0.47, 0.55) | 0.31 (0.27, 0.36) | 1.00 (0.95, 1.00) |
| VIA | PAP | HR HPV | 1.00 (0.89, 1.00) | 0.50 (0.45, 0.55) | 0.48 (0.43, 0.52) | 0.29 (0.25, 0.33) | 1.00 (0.95, 1.00) |
Analysis assumed triple negatives are truly negatives but excludes those with otherwise missing histology
For use of multiple tests, any combination that required more than one positive test resulted in the lowest of the sensitivities and the highest specificities (e.g. VIA+ and Pap >LSIL resulted in 13% sensitivity (95%CI: 8%, 17%) and 97% specificity (95%CI: 95%, 99%)). On the other hand, requiring a positive test by only one of the two tests maximized sensitivity and negative predictive values for all HR-HPV based combinations. For example, net sensitivity (of the test combination) increased to 90% (95%CI: 80%, 100%) when considering those positive by either HR-HPV or VIA (47% specificity; 54% correctly classified; 96% NPV), and to 99% sensitivity (95%CI: 89%, 100%) when including those positive by either HR-HPV or Pap (51% specificity; 59% correctly classified; 99% NPV) compared to HR-HPV testing alone (85% sensitive).
Discussion
To continue to improve the health and lives of HIV-infected women, providing antiretroviral treatment is not enough. Gains from HIV programs may be diminished if we neglect to address other important comorbidities, particularly the high incidence and mortality due to cervical cancer. In this study, we sought to determine optimal follow-up of women after abnormal VIA cervical screening and cryotherapy treatment. Although we found that Pap smears correctly classified the most women with regard to CIN2+, testing for the presence of HR-HPV DNA had the significantly highest sensitivity as a single test, albeit with the significantly lowest specificity. Only moderate increases in sensitivity were gained by including positivity by Pap or VIA along with HR-HPV positivity. We observed a considerably high rate of post-treatment positive screening and histological confirmation of many CIN 2+ cases, further highlighting the need for HIV-specific guidelines along the entire cervical cancer prevention spectrum.
Screening for cervical disease after cryotherapy focuses on a distinct subset of women and thus requires unique considerations, as compared to primary screening in the general HIV population. As shown extensively in the literature, these women are at higher risk of post-treatment disease because of their prior CIN and/or treatment failure, on top of their already elevated risk due to HIV[9–13]. Thus, a priority for post-treatment follow-up should be to maximize the sensitivity of screening, in order to minimize the likelihood of missing a case. This prioritization suggests that HR-HPV testing is optimal for post-treatment follow-up. Consistent with the inclusion of HR-HPV testing for post-treatment follow-up in several high-resource countries (summarized here[18]), we found HR-HPV was the most sensitive test for CIN2+ detection in our sub-Saharan African setting. Capacity for molecular testing is increasing[19, 20], and HPV testing in this smaller subpopulation may be more feasible than in general screening, where the number false positive tests that require triage might overwhelm the health system or result in unnecessary procedures. However, with a PPV just slightly higher than that of VIA, our data suggests that treatment based one HR-HPV positive test would result in 71% over-treatment for CIN2+.
Importantly, our study also showed that if HR-HPV testing is not feasible even among this small subset of women, concurrently combining Pap and VIA (positive by either Pap or VIA) is an adequate alternative. Although the sensitivity is noticeably and statistically lower than for HR-HPV testing, this combination had the highest sensitivity for non-HPV based algorithms, with the low sensitivity offset by gains in specificity. However, use of dual Pap and VIA also has unique resource requirements and limitations, not too dissimilar to HR-HPV testing (e.g. specialized training and re-training for optimal performance, potential loss to follow-up awaiting the Pap result, and so on)[21]. Our results indicate that VIA alone, despite the benefit of a single visit approach in a population with high loss to follow-up, is not adequate for post-treatment screening since it would have missed nearly three-quarters of CIN2+ cases. Pap smear would have also missed about 50% of cases with a single post-treatment screening.
We observed high post-treatment screening positivity (64%) at a median of 6.3 months and a high rate of CIN2+ detection (29%) at 11 months post-treatment. These findings are consistent with a study of HIV-infected women from Kenya, which found 23% of women had residual CIN 2/3 at 6 months after cryotherapy[22]. Neither the use of antiretroviral therapy nor CD4 counts were associated with post-treatment disease in that study nor in the present study. In exploring whether those with CIN2+ received biopsy after a longer follow-up period and had more time to progress, we found that they actually had a shorter median time to biopsy after screening (2.9 months; range 0–13) as compared to those without CIN2+ detected (3.8 months; range 0–21). Thus, given the relatively short, although variable, time to biopsy, these cases likely represent persistent lesions after treatment, raising the question of whether VIA did not adequately distinguish the subset of lesions requiring LEEP or whether cryotherapy is not an appropriate treatment modality in general for HIV-infected women[23]. Further research is ongoing to investigate the cause, and improve the prevention, of these post-treatment CIN2+ cases, including retraining of the nurses to triage women appropriately to cryotherapy or LEEP.
A strength of our study is that all women with one or two negative tests were referred for a biopsy. Given the high net sensitivity of using three tests in combination, it was not ethically justified to send a random sample of triple negative women to colposcopy-directed biopsy. Thus, for the sake of clinical management and in our analyses, these women were considered to be truly negative for the outcome of CIN2+. The potential for verification bias in our study, which can exaggerate the sensitivity of a test, is likely very minimal since only those women negative by all three tests were not referred to histology. Furthermore, our results are consistent with the literature, including a large study in the Netherlands reporting the same 95% sensitivity for HR-HPV and Pap co-testing in post-treatment follow-up for CIN2+[24].
This study had relatively high loss to follow-up of women needing biopsy. Despite rigorous contact tracing, nearly a quarter of women did not attend their colposcopy appointment. Additionally, nearly a quarter of biopsy specimens or results were lost at the department of pathology. Multiple imputation methods were used to address this limitation of our study, although the data may not have been missing at random, potentially limiting the generalizability of our findings. As shown in the appendix, the complete case analysis and the results that included imputed data for missing Pap smears and histology, were nearly identical. Unfortunately, these sources of missing data reflect the real-world challenges of using subjective tests and multiple visit algorithms in clinical settings globally. Another consideration of our study that highlights challenges in cervical cancer prevention, is the variability in time between abnormal screening and biopsy collection. This can affect the observed sensitivity and specificity since the indicating lesions can naturally regress, or new lesions can form and progress differently across the wide range of follow-up times.
Although several studies have demonstrated that VIA screening followed by immediate cryotherapy is feasible, acceptable, and relatively effective for cervical cancer prevention, few studies have assessed optimal follow-up post-treatment, particularly among HIV-infected women in low-resource settings. As implementation of these see-and-treat programs increase, it is paramount to identify effective procedures to ensure that women are and remain disease-free after treatment. In comparing all screening test combinations, use of HR-HPV DNA testing maximized the likelihood of detecting post-treatment disease, alone or in combination with another test. As challenges remain in many global settings in implementing HR-HPV testing for primary screening or for triage, our results suggest that starting to use this technology among this relatively small, yet high-risk, subset can effectively identify post-treatment disease. In addition, the negative predictive value of HR-HPV testing has the important benefit of safely allowing extended intervals for follow-up in settings where re-screening is low and loss to follow-up is high.
Acknowledgments
Funding: This project was supported by the NIH Fogarty International Center (D43TW000237) and the Providence/Boston CFAR (P30AI042853). A.F. Rositch was supported by the Johns Hopkins University CFAR (1P30AI094189). The funding sources did not play a role in writing the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to declare.
Contributions: Study conception (OO, ACD, SCU ); study design (OO, TL, SCU); study conduct (OO, ACD, PI, JO, SW, DC, LP, SCU); data analysis and interpretation (OO, TL, ACD SCU, AFR ); Drafting the manuscript (OO, ACD, AFR ); Revision and approval of final manuscript (all authors).
References
- 1.Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: current evidence and directions for future research. Infect Agent Cancer. 2010;5:8. doi: 10.1186/1750-9378-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham AG, D’Souza G, Jing Y, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62(4):405–13. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Denslow SA, Rositch AF, Firnhaber C, Ting J, Smith JS. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS. 2014;25(3):163–77. doi: 10.1177/0956462413491735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster WR, Hubbell FA, Largent J, et al. Feasibility of management of high-grade cervical lesions in a single visit: a randomized controlled trial. JAMA. 2005;294(17):2182–7. doi: 10.1001/jama.294.17.2182. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R, Rajkumar R, Esmy PO, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96(5):738–43. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294(17):2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 9.Strander B, Hallgren J, Sparen P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. Bmj. 2014;348:f7361. doi: 10.1136/bmj.f7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tebeu PM, Major AL, Mhawech P, Rapiti E. The recurrence of cervical intraepithelial neoplasia in HIV-positive women: a review of the literature. Int J STD AIDS. 2006;17(8):507–11. doi: 10.1258/095646206778145703. [DOI] [PubMed] [Google Scholar]
- 11.Huchko MJ, Leslie H, Maloba M, Bukusi EA, Cohen CR. Factors associated with recurrence of cervical intraepithelial neoplasia 2+ after treatment among HIV-infected women in Western Kenya. J Acquir Immune Defic Syndr. 2014;66(2):188–92. doi: 10.1097/QAI.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forhan SE, Godfrey CC, Watts DH, Langley CL. A systematic review of the effects of visual inspection with acetic acid, cryotherapy, and loop electrosurgical excision procedures for cervical dysplasia in HIV-infected women in low- and middle-income countries. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S350–6. doi: 10.1097/QAI.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell H, Medley G, Carlin JB. Risk of subsequent cytological abnormality and cancer among women with a history of cervical intraepithelial neoplasia: a comparative study. Cancer causes & control : CCC. 1990;1(2):143–8. doi: 10.1007/BF00053165. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Schulz KF, Altman DG, Group C. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Clinical oral investigations. 2003;7(1):2–7. doi: 10.1007/s00784-002-0188-x. [DOI] [PubMed] [Google Scholar]
- 15.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, N.J: Wiley-Interscience; 2004. [Google Scholar]
- 16.Buuren Sv. Flexible imputation of missing data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 17.Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken, N.J: Wiley; 2002. [Google Scholar]
- 18.Cuschieri K, Bhatia R, Cruickshank M, Hillemanns P, Arbyn M. HPV testing in the context of post-treatment follow up (test of cure) J Clin Virol. 2016;76(Suppl 1):S56–61. doi: 10.1016/j.jcv.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Firnhaber C, Mayisela N, Mao L, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PLoS One. 2013;8(1):e53494. doi: 10.1371/journal.pone.0053494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chibwesha CJ, Frett B, Katundu K, et al. Clinical Performance Validation of 4 Point-of-Care Cervical Cancer Screening Tests in HIV-Infected Women in Zambia. Journal of lower genital tract disease. 2016;20(3):218–23. doi: 10.1097/LGT.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman JS, Cespedes MS, Cu-Uvin S, et al. An Insight Into Cervical Cancer Screening and Treatment Capacity in Sub Saharan Africa. Journal of lower genital tract disease. 2016;20(1):31–7. doi: 10.1097/LGT.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vuyst H, Mugo NR, Franceschi S, et al. Residual disease and HPV persistence after cryotherapy for cervical intraepithelial neoplasia grade 2/3 in HIV-positive women in Kenya. PloS one. 2014;9(10):e111037. doi: 10.1371/journal.pone.0111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirenje ZM, Rusakaniko S, Akino V, Munjoma M, Mlingo M. Effect of HIV Disease in Treatment Outcome of Cervical Squamous Intraepithelial Lesions Among Zimbabwean Women. Journal of lower genital tract disease. 2003;7(1):16–21. doi: 10.1097/00128360-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Uijterwaal MH, Kocken M, Berkhof J, et al. Posttreatment assessment of women at risk of developing high-grade cervical disease: proposal for new guidelines based on data from the Netherlands. Journal of lower genital tract disease. 2014;18(4):338–43. doi: 10.1097/LGT.0000000000000012. [DOI] [PubMed] [Google Scholar]