A recent publication by Pillai et al. in the field of cardiac tissue mineralization has evoked a multitude of echoes in the press about turning hearts into bone or stone (1). Whilst the ‘turning into stone’ metaphor is not particularly novel, it has been effective in gaining public interest into this issue (2,3). Hardening the heart or turning hearts into stone, and ultimately healing it to transform them back into soft and compassionate organs of flesh, occurs in the literature through the centuries. Metaphors like this can be found from the Bible (4) to the Qur’an (5), from Shakespeare (6) to Hauff (7) and Wilde (8), all referring to turning a soft and tender heart into a rigid and unfeeling object. This analogy sits surprisingly well with the biological situation explored by Pillai et al. (1).
The heart’s movement is incredibly complex and its deformable extracellular matrix (ECM) skeleton supports a range of degrees of freedom of local deformation that is not available from rigid skeletons. To function properly, the heart itself requires the right balance of active and passive mechanical properties for soft tissue deformation (9). Even small regions of increased rigidity, like fibrotic scars or mineralized cardiac tissue, can compromise cardiac performance—not only by replacing active, force-generating myocytes, but also passively by disturbing intricate motion patterns, decreasing overall effectiveness of pump action.
Physiologically, mineralization takes place in bone and teeth development and continues as bone remodeling in the adult. Several diseases are linked to pathological mineralization or calcification of normally soft tissues, including generalized arterial calcification of infancy (GACI), Monckeberg's sclerosis, fibrodysplasia ossificans progressiva, or chondrocalcinosis (10,11). These diseases are associated with ‘calcium deposits’, containing calcium hydroxyapatite (the bone mineral), basic calcium phosphate, and/or calcium pyrophosphate dihydrate (CPPD). The resulting change in tissue stiffness, ectopic bone formation and deformability impedes local cell function and mechanical tissue properties. GACI and Monkeberg’s sclerosis are phenotypically linked by calcium hydroxyapatite deposition, mainly in the vascular medial layer, e.g., from loss of function mutations in ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) and of ATP-binding cassette transporter subtype 6, respectively (12). Both these membrane proteins play a role in regulating extracellular mineral deposition, ultimately by producing pyrophosphate (PPi) which inhibits calcium hydroxyapatite crystal formation (13). Correspondingly, a genetic cause for chondrocalcinosis is a gain of function mutation in the progressive ankylosis protein homolog, a transmembrane protein responsible for transporting PPi into the extracellular space, leading to high extracellular PPi concentrations and subsequent formation of CPPD and basic calcium phosphate. While PPi suppresses hydroxyapatite deposition and inhibits connective tissue calcification, hydrolysis of PPi via tissue non-specific alkaline phosphatases results in accumulation of phosphate, which is a component of hydroxyapatite crystal deposition (14). In contrast to genetic causes for disturbed extracellular PPi, phosphate and calcium equilibrium, fibrodysplasia ossificans progressiva is caused by mutation of the activing receptor type IA receptor—a member of the bone morphogenetic protein type I receptor family—which leads to accumulation of hydroxyapatite in tendons, skeletal muscles and ligaments (15). Furthermore, conditions like end-stage renal disease and diabetes mellitus can also be linked to ectopic tissue calcification. Soft tissue calcification is not only disease-related, but it occurs naturally with age, and in regions of injury and/or fibrosis where altered or excess ECM provides a calcifiable substrate (10,16).
In soft tissues, pathological mineralization has recently been shown to recapitulate some aspects of physiological mineralization by osteoblasts related to metabolic, genetic, and inflammatory processes at injury sites, but the exact mechanisms and participating cell types are still under investigation. In vascular calcification, several cell types are suspected of adopting osteogenic fates, including myofibroblasts, transdifferentiated smooth muscle cells, and endothelial cells undergoing epithelial-to-mesenchymal transition, all potentially contributing to ectopic mineralization through calcific deposits (11).
Cardiac fibroblasts form an intriguing, albeit ill-characterized and -defined cell population, whose patho-physiological relevance and roles as therapeutic targets extends well beyond their classic function as ECM house-keepers (17). Prior to this study, Pillai et al. had demonstrated cardiac fibroblast plasticity in the event of heart injury, when fibroblasts can adopt an endothelial like cell fate and contribute to cardiac neo-vascularization (18). Additionally, cardiac fibroblasts are responsible for fibrotic scar formation after heart injury, possibly providing a calcifiable matrix of collagen I in scar tissue (10,19). Keeping the parallels to other soft tissue calcification mechanisms and the plasticity of cardiac fibroblasts in mind, Pillai and colleagues systematically investigated cardiac fibroblasts as a cell type potentially responsible for pathological cardiac calcification via adoption of an osteogenic fate (1).
The authors first tested the ability of isolated cardiac fibroblasts from mouse and human hearts to adopt an osteogenic cell-like fate in vitro. Promoting an osteogenic signature, based on genes that are differentially expressed during osteogenic differentiation like Runx2 or osteocalcin, they showed the potential of cardiac fibroblasts to take on an osteogenic fate by exposure to a classical osteogenic differentiation medium, and to mineralize ECM with calcific deposits in vitro. They excluded other possibly responsible cell types, like endothelial cells or pericytes, which have been suggested to participate in soft tissue calcification processes (11,13), by isolating the respective cell types and subjecting them to the same treatment. Adoption of an osteogenic fate in cardiac fibroblasts was stable over 14 days, even after removal of the osteogenic differentiation cell culture medium. Whilst this illustrates the ability of osteogenic differentiation medium (20) to promote an osteogenic cell fate in fibroblasts in vitro, in vivo osteogenic differentiation is thought to be induced by bone morphogenic protein signaling or chronic inflammation (21). This was assessed in vivo, comparing mice of strains that are prone to calcification in the heart after injury (e.g., C3H) with others that are not (e.g., B6). The authors used three different myocardial injury models: administration of high-dose steroids to induce cardiomyocyte necrosis, cryo-ablation, and coronary artery ligation. All of these led to calcified patches of injured tissue in the C3H background, but not in B6, highlighting the relevance of background genotypic heterogeneity for the different phenotypes seen in response to injury—similar to human, in whom not all cardiac scar tissue calcifies (22). Calcium deposits were observed in regions of fibrosis, as had been shown before (11). Using genetic lineage tracing, osteogenic differentiation of cardiac fibroblasts was exposed, while cardiomyocytes did not express osteogenic markers. To further assess the connection between osteogenic differentiation and calcification by cardiac fibroblasts, the authors explanted cardiac fibroblasts from uninjured and injured hearts and implanted them subcutaneously in wild type C3H mice. There, only cells isolated from injured hearts remained positive for osteogenic markers and induced ectopic neo-calcification, again underlining the stable phenotype conversion of these cells.
Taken together with the non-calcifying injury model in B6 strains, in whom fibroblasts did not show osteogenic differentiation, the work by Pillai et al. not only identifies cardiac fibroblasts as a plausible culprit for cardiac calcification in vivo, but it also highlights that osteogenic differentiation is not necessarily a generic response to injury. In addition, the work is a suitable reminder of the need for caution when extrapolating findings from individual murine models—both to other murine strains and, perhaps more importantly, to human patho-physiology. Indeed, genome-wide association studies in humans have identified different association signatures for coronary artery calcification in myocardium from different ethnic backgrounds (23). This finding is in line with the finding by Pillai et al., where different murine strains varied in their susceptibility to calcification.
Having identified a cell type responsible for calcification in C3H mice, the authors looked for pathways involved in mineralization that could form possible drug targets. Gene expression studies showed upregulation of ENPP1 in injured hearts, and this upregulation was more pronounced in calcification-prone C3H mice, compared to B6, suggesting this enzyme may be a druggable target. However, ENPP1 breaks down ATP to AMP and PPi, with PPi normally seen as a potent inhibitor of calcium hydroxyapatite formation (21). Using Raman spectroscopy, the authors confirmed that calcification was indeed mediated by calcium hydroxyapatite, rather than CPPD, suggesting that PPi may not directly contribute to formation of crystalline deposits. A possible explanation is further hydrolysis of PPi to phosphate as the cause for calcification, although non-specific alkaline phosphatase (an enzyme responsible for such breakdown) was not found to be differentially expressed. This finding is a direct parallel to ‘normal’ osteogenesis events, where osteoblasts deposit calcium hydroxyapatite to mineralize bone matrix, with the phosphate produced via this axis.
Since clinical outcomes are worse in patients with calcified cardiac scar tissue (24), Pillai et al. tested two different small-molecule inhibitors of ENPP1 to see whether calcification upon cardiac injury could be prevented. Both inhibitors, administered before cardiac injury, reduced cardiac calcification. Additionally, more traditional compounds, bisphosphonates (Etidronate, an inhibitor of bone mineralization) that have been used successfully to treat of other forms of ectopic calcification (e.g., GACI) (25), prevented cardiac scar calcification.
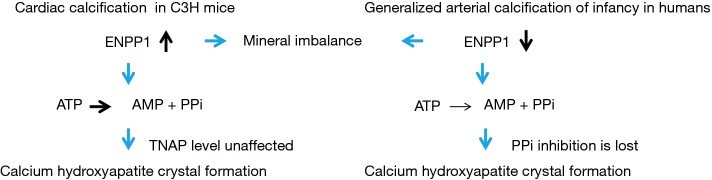
Once calcified, reversal was not observed. Prevention of the calcific phenotype by drug administration before a cardiac injury improved cardiac function afterwards, again highlighting the negative effect of calcification on cardiac outcome. Interestingly, the proposed mechanism of cardiac calcification in C3H mice, acting through upregulation of ENPP1, is different from genetic forms of vascular calcification like GACI where a loss of function mutation in ENPP1 is being held responsible for mineral deposition (Figure 1). Both involve dys-regulation of calcium deposition, but the nature of deposition, the interplay with other proteins involved in mineral balance (CPPD or calcium hydroxyapatite), and the pathogenesis of osteogenic differentiation in the different disease backgrounds need further investigation. The same holds for ENPP1 as a drug target, as general inhibitors of this enzyme may give rise to side effects on the physiological dynamics of calcification processes in other tissues. Perhaps a risk stratification may become possible, where patients can be grouped into high-risk (C3H-like) and low-risk (B6-like), to guide selection of pharmacological interventions after cardiac injury. This, together with an identification of other factors involved in the connection of inflammation, fibrosis and calcification (11) may help to prevent, if not correct, cardiac mineralization. Since calcification events in the murine models were linked to cardiac cell apoptosis/necrosis after injury, possible causal links to cell debris and apoptotic bodies would also be of interest (10).
Figure 1.
Schematic illustration of two putative mechanisms of soft tissue calcification.
Overall, the study by Pillai et al. is exemplary in its breadth and depth, offering thorough evidence for cardiac fibroblasts trans-differentiation into an osteogenic lineage that can drive calcific deposit formation in C3H mouse heart. Expanding these findings to other models that mimic human cardiovascular physiology more closely (such as rabbit or mini-pig) and determining the reasons for which different murine strains respond differentially to cardiac injury may offer important steps towards solving the clinical problem of ectopic calcification. In addition, means to promote reversal of ectopic calcification are needed, as administration of a drug prior to soft tissue damage is limited in its clinical applicability.
Pillai et al. have moved the field ahead by a significant margin, promoting the hope that the Rolling Stones may have it wrong, and the scientific community will find ways to ‘break this heart of stone’ challenge.
Acknowledgements
Support by the European Research Council Advanced Grant CardioNECT is gratefully acknowledged.
Provenance: This is an invited Editorial commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pillai IC, Li S, Romay M, et al. Cardiac Fibroblasts Adopt Osteogenic Fates and Can Be Targeted to Attenuate Pathological Heart Calcification. Cell Stem Cell 2017;20:218-232.e5. 10.1016/j.stem.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ScienceDaily. How the heart turns into bone. Retrieved February 15 2017. Available online: www.sciencedaily.com/releases/2016/11/161117151804.htm.
- 3.Ivey KN. A Heart of Stone: Cardiac Fibroblasts Turn to Bone in Calcified Hearts. Cell Stem Cell 2017;20:151-2. 10.1016/j.stem.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 4.The Bible : Old and New Testament, Einheitsübersetzung (German). Herder, Munich, 1999. Ezekiel 36:22-8. [Google Scholar]
- 5.Ali AY. The Qur’an. Tahrike Tarsile Qur’an, Flushing, 2013. [Google Scholar]
- 6.Shakespeare W, McDonald R. The Tragedy of Othello, the Moor of Venice. Penguin Books, New York, 2001. [Google Scholar]
- 7.Hauff W. Das Kalte Herz (Vollständige Ausgabe). e-artnow; 2015.
- 8.Wilde O, Jackson R, Small I, et al. The Complete Works of Oscar Wilde. Oxford University Press, Oxford, 2000. [Google Scholar]
- 9.Rostand SG, Sanders C, Kirk KA, et al. Myocardial calcification and cardiac dysfunction in chronic renal failure. Am J Med 1988;85:651-7. 10.1016/S0002-9343(88)80237-7 [DOI] [PubMed] [Google Scholar]
- 10.Kirsch T. Determinants of pathological mineralization. Curr Opin Rheumatol 2006;18:174-80. 10.1097/01.bor.0000209431.59226.46 [DOI] [PubMed] [Google Scholar]
- 11.Demer LL, Tintut Y. Inflammatory, Metabolic, and Genetic Mechanisms of Vascular Calcification. Arterioscler Thromb Vasc Biol 2014;34:715-23. 10.1161/ATVBAHA.113.302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 2012;90:25-39. 10.1016/j.ajhg.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res 2011;109:578-92. 10.1161/CIRCRESAHA.111.247965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polewski MD, Johnson KA, Foster M, et al. Inorganic pyrophosphatase induces type I collagen in osteoblasts. Bone 2010;46:81-90. 10.1016/j.bone.2009.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 2009;30:379-90. 10.1002/humu.20868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugashetti R, Shinkai K, Ruben BS, et al. Calcium may preferentially deposit in areas of elastic tissue damage. J Am Acad Dermatol 2011;64:296-301. 10.1016/j.jaad.2010.01.046 [DOI] [PubMed] [Google Scholar]
- 17.Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 2016;15:620-38. 10.1038/nrd.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubil E, Duan J, Pillai IC, et al. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature 2014;514:585-90. 10.1038/nature13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lighthouse JK, Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol 2016;91:52-60. 10.1016/j.yjmcc.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 1997;64:295-312. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 2006;99:1044-59. 10.1161/01.RES.0000249379.55535.21 [DOI] [PubMed] [Google Scholar]
- 22.Shackley BS, Nguyen TP, Shivkumar K, et al. Idiopathic massive myocardial calcification: a case report and review of the literature. Cardiovasc Pathol 2011;20:e79-83. 10.1016/j.carpath.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Assimes TL, Knowles JW, Basu A, et al. Susceptibility locus for clinical and subclinical coronary artery disease at chromosome 9p21 in the multi-ethnic ADVANCE study. Hum Mol Genet 2008;17:2320-8. 10.1093/hmg/ddn132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stallion A, Rafferty JF, Warner BW, et al. Myocardial calcification: a predictor of poor outcome for myocarditis treated with extracorporeal life support. J Pediatr Surg 1994;29:492-4. 10.1016/0022-3468(94)90074-4 [DOI] [PubMed] [Google Scholar]
- 25.Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med 2012;22:145-9. 10.1016/j.tcm.2012.07.011 [DOI] [PubMed] [Google Scholar]