Dermal adipocytes are involved in the protection of skin infection, in hair growth and wound healing (1). Significant modulation of dermal adipose tissue (dWAT) can be observed in aged skin, and loss of dermal adipocytes is a hallmark of fibrotic lesions. Therefore, identification of molecular mechanisms governing adipose progenitor proliferation and self-renewal is critical to counteract depletion of adipocytes occurring with aging in the skin. Moreover, understanding adipose progenitor maintenance in general might also be important for other adipose depots.
PDGFR appears recently as a critical functional marker of adipose progenitors. The PDGF family consists of two receptor genes, PDGFRα and PDGFRβ and four ligand genes, PDGFA, B, C and D. PDGF-AA and PDGF-CC exclusively act through PDGFRα, while PDGF-DD activates PDGFRβ. The PDGF ligands exert their function by causing dimerization and auto-phosphorylation of the PDGF receptors. This, in turn, results in activation of a multitude of intracellular signaling cascades (2). PDGFRα+ cells exist throughout the body at various developmental stages of development. In adult, PDGFRα expression is more confined in specific progenitor populations, including adipose progenitors (3,4). In a recent paper, Rivera-Gonzalez and colleagues showed that the maintenance of adipose progenitors resident in the skin is under the control of PDGFA/PI3K-AKT signaling pathway (5).
In mammals, two types of adipocytes coexist, i.e., brown and white, which are both involved in energy balance regulation while having opposite functions (6-8). White adipose tissue (WAT) is dispersed throughout the body and is mainly involved in energy storage. The two largest depots of white adipose tissues in human and rodents, are subcutaneous and visceral WAT (sWAT and vWAT, respectively). These two types of WAT differ in important aspects during pathophysiological increase in fat mass, i.e., obesity. Whereas increased subcutaneous fat depots present little or no cardiovascular risk, increased white visceral fat depots correlates with the adverse metabolic outcomes of obesity. Visceral and subcutaneous adipose progenitors display distinct intrinsic capacities to proliferate and to undergo differentiation into mature adipocytes (9). However, intrinsic differences in signaling pathways that may account for their specific behaviour are not completely understood. In contrast to WAT, brown adipose tissue (BAT) is specialized in energy expenditure. Activated BAT consumes metabolic substrate and burns fat to produce heat via the uncoupling protein (UCP)-1 (10). Brite/beige, adipocytes were recently described as brown-like adipocytes and represent a third type of adipocytes recruited in WAT (11-13). Lineage tracing studies in mice revealed that the different colours of adipocytes derived from distinct embryonic origins (14). Adipose progenitors are maintained in adult tissues where they assure adipocytes renewal. However, adipocyte renewal is low in vivo (15) making the identification of circuits regulating adipose progenitor maintenance in the different fat depots poorly understood.
Dermal adipocytes emerged as another type of adipocytes. They are defined as the upper part of the subcutaneous white adipose tissue, and represent a thin layer of adipocytes that encase mature hair follicles. They play distinct roles compared to other adipocyte depots, as they are involved in protection against skin infection, in wound healing and in the hair cycling (1). Dermal adipose progenitors (dAPs) are maintained in the dermis and participate to adipocyte regeneration during the hair cycle and following wounding (16,17). The dermal adipose tissue (dWAT) volume decreases with aging and skin that has been chronically photo-damaged displays a reduction in the volume of dWAT caused by the replacement of adipocytes by fibrotic structures. This adipocyte-fibroblast transition might be connected with lineage tracing experiments that revealed that dermal fibroblasts and adipocytes derived from a common progenitor. However, signaling pathways mediating dAP proliferation, as well as those involved in the adipocyte-fibroblast transition in the skin remains to be identified.
In contrast to other fat depots, dermal adipocytes demonstrate high turnover rates making the skin a powerful platform to identify regulators of adipose progenitor self renewal and proliferation.
Using an inducible adipocyte-labelling mouse model, Rivera-Gonzalez and colleagues (5) characterized dAPs as Lin−; CD29+; CD34+; CD24+ (hereafter named CD24+), and showed that proliferation of the dAPs generated around 20% of new dermal adipocytes during the hair cycle. It was previously showed that aging and multiple round of hair depilation dramatically reduced the skin thickness and the number of dermal adipocytes. Rivera-Gonzalez et al. showed that the pool of dAPs was also reduced in these conditions. The authors have previously shown that PDGFA and its receptor PDGFRalpha are expressed by APs in different white depots (16,18). In the new paper, they investigated the role of PDGFA in dAP maintenance by deleting Pdgfa specifically from dermal mesenchymal cells in mice (PDGFA cKO). Interestingly, whereas dWAT was similar in wild-type and in mutant mice during the resting phase, a reduction of 20% in dermal adipocytes during the hair follicle growth was observed in PDGFa cKO mice. The quantification of APs revealed a specific reduction of APs in the skin of mutant mice with no difference in the number of APs in the vWAT and sWAT between wild type and mutant mice. By means of Pdgfa-null dAP transplantation in the skin of wild type mice, the authors demonstrated that PDFGA signaling directly in the dAPs was required for their maintenance. Then, RNA sequencing allowed the authors to identify a gene expression program specifically induced in dAPs upon PDGFA treatment through activation of PI3K/Akt signalling. dAP proliferation and gene expression induced by PDGFA were blocked in the presence of the PI3K/Akt inhibitor LY294002. Previously the Rodeheffer group showed that AKT kinases are expressed in AP present in vWAT and sWAT (19). PI3K/AKT signaling is activated during expansion of vWAT and inhibition of PI3K signaling specifically blocked AP proliferation in vWAT but not in sWAT (19). Altogether, these data demonstrated that the PDGFA-PI3K/Akt signaling axis mediates the proliferation of APs, and strongly suggest that this axis plays a critical role in the depletion of dAPs with aging. Could re-activation of the PDGFA-PI3K/Akt axis in aged skin restore dAP proliferation and self-renewal? This is an exiting hypothesis to counteract skin disorders. However, the specificity of PDGFA-PI3K/Akt responses in dAPs need to be dissected because several reports pointed out a role of PDGFA signaling pathway in pathological fibrosis (20). Interestingly, a bipotent progenitor able to undergo differentiation into adipocytes and myofibroblasts has been characterized in human and rodent skeletal muscles, and this fibro-adipogenic progenitor is identified as PDGFRa+ (21). PDGFRa is now recognized as a marker of fibro-adipogenic progenitor that plays at a central role in muscle development when activated at “physiological levels” but leading to fibrosis occurring in pathological skeletal muscles when its activation is exacerbated. The effects of PDGFA on the fate of adipose progenitor are summarized in Figure 1. Very recently, multiple transcriptional variants of Pdgfra have been described in muscle fibro-adipogenic progenitors, one of them acting as a decoy to prevent over activation of PDGF signaling and its pathological consequences (22).
Figure 1.
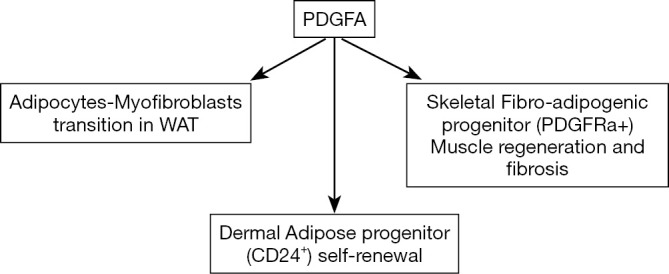
Physiological and pathological effects of PDGFR signalling activation. PDGFA acts on adipose progenitors (CD24+) resident in the skin to stimulate their proliferation and self-renewal. The role of this pathway in the adipocyte-myofibroblast transition in white adipose tissue has also been described. PDGFA also acts on fibro-adipogenic progenitor (PDGFRa+) resident in skeletal muscles to both participate to muscle regeneration of healthy tissue and to be responsible to fibrosis if muscle diseases.
Therefore, there is a necessity in understanding in details the PDGFA signaling in adipose progenitor maintenance and also in the adipocyte-myofibroblast transition for the development of therapies against age-related loss of adipocytes.
Acknowledgements
This study was supported by the French government (ANR) through the “Investments for the future” LABEX Signalife, program reference ANR-11-LABEX-0028-01.
Provenance: This is an invited Editorial commissioned by Editor-in-Chief Zhizhuang (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kruglikov IL, Scherer PE. Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends Endocrinol Metab 2016;27:1-10. 10.1016/j.tem.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev 2014;25:273-83. 10.1016/j.cytogfr.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Uezumi A, Fukada S, Yamamoto N, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143-52. 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- 4.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153-63. 10.1038/ncb2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera-Gonzalez GC, Shook BA, Andrae J, et al. Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Cell Stem Cell 2016;19:738-751. 10.1016/j.stem.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444-52. 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- 7.Cohen P, Spiegelman BM. Cell biology of fat storage. Mol Biol Cell 2016;27:2523-7. 10.1091/mbc.E15-10-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Pan R, Pfeifer A. Fat tissues, the brite and the dark sides. Pflugers Arch 2016;468:1803-7. 10.1007/s00424-016-1884-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab 2005;288:E267-77. 10.1152/ajpendo.00265.2004 [DOI] [PubMed] [Google Scholar]
- 10.Enerbäck S. Human brown adipose tissue. Cell Metab 2010;11:248-52. 10.1016/j.cmet.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366-76. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol 2015;55:207-27. 10.1146/annurev-pharmtox-010814-124346 [DOI] [PubMed] [Google Scholar]
- 13.Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153-64. 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta 2014;1842:340-51. [DOI] [PMC free article] [PubMed]
- 15.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783-7. 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 16.Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011;146:761-71. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013;140:1517-27. 10.1242/dev.087593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302-8. 10.1038/ncb2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffery E, Church CD, Holtrup B, et al. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015;17:376-85. 10.1038/ncb3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 2009;16:303-13. 10.1016/j.devcel.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis 2014;5:e1186. 10.1038/cddis.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller AA, van Velthoven CT, Fukumoto KD, et al. Intronic polyadenylation of PDGFRα in resident stem cells attenuates muscle fibrosis. Nature 2016;540:276-9. 10.1038/nature20160 [DOI] [PMC free article] [PubMed] [Google Scholar]


