Abstract
A successful antitumoral response requires immunological activation as well as an antigenic pool capable of stimulating both the innate and the adaptive immune system. Recent advances in immunotherapy have been aimed at boosting the activation status of the innate and adaptive immune system, including cytokine administration, monoclonal antibodies engineered to target high yield elements in oncogenic signaling pathways, cancer vaccines, and checkpoint inhibitors. Herein, we examine the ways that radiation therapy induced cell death provides a pool of stimulus antigen, and draw parallels from the immunobiology of autoimmunity to explore how the immunogenicity of antigen derived from radiation-induced cell death might augment the antitumoral response. We also review basic research into the ability of different radiation dose fractionation schedules to induce an antitumoral response. After a discussion of basic immunotherapeutic principles, we review the published literature in the field of non-small cell lung cancer (NSCLC) and examine the ways that combining radiation and immunotherapy have begun to change the therapeutic terrain. We provide a summary of ongoing clinical trials aimed at combining immunotherapy and radiation therapy in NSCLC while emphasizing the need for identification of biomarkers with predictive power and the assessment of efficacy as a function of fractionation strategy.
Keywords: Radiotherapy, immunotherapy, non-small cell lung cancer (NSCLC), checkpoint inhibition
Immunotherapy and radiotherapy: theoretical and practical synergy
The intuitive appeal of perturbing the immune system to generate an effective anti-tumor response is so profound that the idea emerged contemporaneously with the field of cellular immunology itself. In 1884, Elie Metchnikoff discovered macrophages (1) and Anton Chekov noted a connection between erysipelas and cancer remission (2). Less than a decade later, William Coley was injecting patients with a cocktail of Streptococcus pyogenes and Serratia marcescens (3-5). Over a century of subsequent empirical inquiry has uncovered a plethora of interacting signal transduction cascades within a multitude of interacting cell types. We are faced with not only understanding this system, but with purposefully manipulating it for the advancement of human health. Despite formidable immunological complexity, immunotherapy has yielded recent gains in overall survival and disease-free progression in a variety of cancers, most notably: melanoma (6-10), non-small cell lung cancer (NSCLC) (11-14), and renal cell carcinoma (RCC) (15-17). These therapies are designed to work by increasing the activation levels of the immune system in response to the antigenic load generated by the tumor in question.
At the most reductive level, harnessing the immune system to attack a tumor consists of two components that are amenable to manipulation: the stimulus and the subsequent response. The word and concept of “immunotherapy” invites a particular focus on the latter, but manipulation of the stimulus (in this case, the antigenic load provided by the tumor) may be equally powerful. The most obvious way to influence the quality or quantity of antigenic load is by inducing preferential killing of tumor cells, either systemically with chemotherapy, or locally with radiation therapy. Increasing the antigenic load and facilitating immune activation with optimal kinetics may achieve a synergistic anti-tumor response, producing an effect on the immune system more definitive and durable than either approach alone. In this review we will provide a brief overview of the conceptual and empirical underpinnings that make radiotherapy and immunotherapy such promising therapeutic partners before turning our attention specifically to oligometastatic lung cancer and summarizing current experience with the combined approach of radiotherapy and immunotherapy in this particular patient population.
Augmented immunological activation
Like the brain, the immune system generates complex output in response to input that varies in character from the simple to the multiplex. Every fate choice, line of cellular communication, and metabolic activation state becomes a branch point in an intricate effector response that might be modified to produce an improved clinical outcome. Over the past several decades, we have attempted to influence the cytokine milieu, kick-start the innate and adaptive arms of the immune system with vaccines and their adjuvants, and prevent T cell exhaustion with immune checkpoint inhibitors (as depicted in Figure 1). A full discussion of the history, breadth, and efficacy of these approaches is beyond the scope of this review, though we will touch on relevant highlights here, with particular attention paid to NSCLC.
Figure 1.
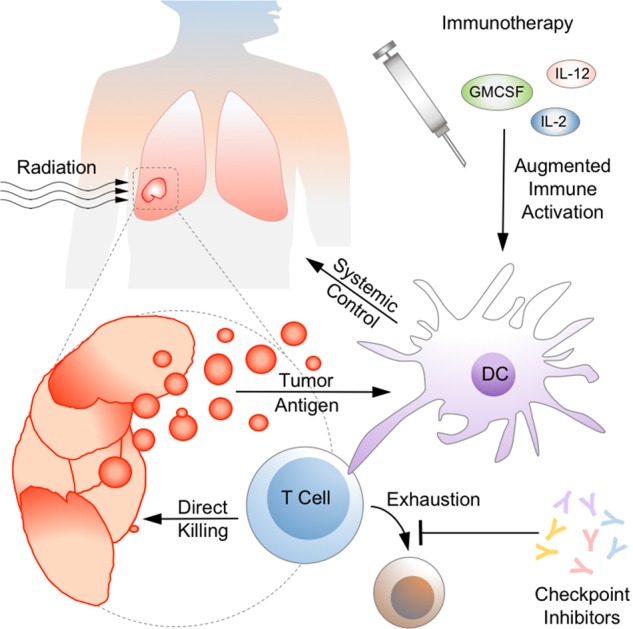
Synergy between radiation therapy and immune therapy in NSCLC. Radiation therapy (top left) releases and alters antigen as targeted tissue dies, influencing immunogenicity in ways we are only beginning to understand. Cellular contents activate the innate immune system, represented here by a dendritic cell (DC), which then leads to an antitumoral response by the adaptive immune system. Immunotherapeutic agents such as cytokines and vaccines (pictured top right), augment immune activation by acting on antigen presenting cells or T cells. Checkpoint inhibitors maintain T-cell activation by blocking inhibitory signaling pathways. Activated T-cells act to improve both systemic and local control. NSCLC, non-small cell lung cancer.
Cytokines
Administration of purified cytokines began in the 1980s with IL-2 (18). Dramatic clinical responses in a modest percentage of patients with melanoma (19) and RCC (20) led to FDA approval of IL-2 for the treatment of these diseases, though unfortunately, IL-2—monotherapy did not provide a significant benefit in patients with NSCLC (21). Other gamma-chain cytokines, such as IL-7, IL-15, and IL-21 have theoretical promise in stimulating antitumoral T-cell activation (22), though they have yet to fulfill their bench promise of bedside clinical gains. Cytokines that are major players in innate responses have also been shown to augment the antitumoral response. IL-12 is a particularly attractive immunomodulator due to its ability to activate cytocidal innate and adaptive responses, but its efficacy in early clinical trials was disappointing and it carried attendant toxicities (23). Interest in IL-12 continues with alternative administration strategies designed to increase intratumoral levels while circumventing systemic exposure (24). GM-CSF has shown promise in murine models of melanoma, and it has consequently been evaluated as a monotherapy and as part of multipronged approaches, with mixed results (25).
Vaccination
Cancer vaccination is a strategy with attractive theoretical underpinnings, and many ongoing trials are using peptide vaccines, cellular vaccines, or viral-based approaches to stimulate the adaptive immune system to identify and attack tumor cells (26). Of particular relevance to NSCLC was the Phase III MAGRIT trial, which evaluated a peptide vaccine containing MAGE-A3, an antigen expressed in 35% of lung cancers. Unfortunately, vaccination failed to confer any benefit to overall survival (27). Cellular vaccines, composed of cancer cell lines, are intended to provide a selection of antigen that is broad as well as a more authentic stimulus, which allows for cross-presentation by dendritic cells, though in NSCLC there have yet to be any significant improvements in overall survival (28). A viral-based vaccine designed to stimulate an immune response against the antigen Mucin 1 (MUC1), expressed in NSCLC, has shown an improvement in progression-free survival, but has not yet demonstrated a difference in overall survival, though final results from the phase III trial have not yet been published (29).
Checkpoint inhibitors
The most promising immunotherapeutic interventions have come in the form of checkpoint inhibitors, so called because they remove the biochemical brakes on immunological activation. Two such inhibitory pathways have been targeted in T cells: the signaling cascade initiated by cytotoxic T lymphocyte antigen 4 (CTLA-4), and the signaling cascade initiated by the receptor known as programmed death 1 (PD-1) and its ligand, PD-L1, both of which function in T cells. CTLA-4 was first identified as a homolog to CD28, another member of the Ig superfamily known to be essential to the two-signal model of T cell activation (30). Mice deficient in CTLA-4 had a dramatically proinflammatory phenotype (31) and blockade of this pathway enhanced antitumoral immunity in murine tumor models (32). Two monoclonal antibodies have been developed that serve as CTLA-4 antagonists: ipilimumab and tremelimumab. The first success with CTLA-4 blockade came in a trial of ipilimumab used as a second-line agent in melanoma, which showed an advantage in overall survival (7). The beneficial effects of ipilimumab extended to other cancers, including NSCLC, where a regimen of ipilimumab and paclitaxel increased overall survival when compared with paclitaxel alone (11). As of yet, tremelimumab has not been approved by the FDA for use in treating any cancer as initial trials in melanoma failed to demonstrate significant survival benefit (33).
PD-1 and PD-L1, a receptor and ligand respectively, control T cell exhaustion, maintain tissue tolerance, and initiate resolution of inflammation (34,35). Mice deficient in PD-1 do not spontaneously develop flagrant autoimmune disease, though they have a predisposition toward developing spontaneous glomerulonephritis on the B6 background and dilated cardiomyopathy on the BALB/c background (36). Two monoclonal antibodies that target PD-1 have been approved by the FDA for NSCLC: nivolumab and pembrolizumab. Nivolumab has been approved for use in second-line NSCLC based on the results of a phase III trial comparing nivolumab to docetaxel which showed a benefit in overall survival (12,13). Pembrolizumab was also found to confer an overall survival benefit in NSCLC patients who failed other therapies and whose tumors expressed PD-L1 (37). Atezolimumab and durvalumab are two of several antibodies under development that target PD-L1 rather than its receptor. Atezolimumab was initially approved by the FDA for its promise in bladder cancer (38), and a recently completed phase II study has demonstrated an increase in overall survival in patients with previously treated NSCLC (39).
Biomarkers
Checkpoint inhibitors have been notable for the durability and magnitude of the clinical responses they effect in certain subpopulations of patients. There is consequently a great deal of interest in identifying biomarkers that, used as screening tools, would signify a higher pre-test probability of response in a given patient. The B7 family of cell surface proteins consists of related ligands for CTLA-4 that are expressed by many different cancers, including NSCLC (40). Perhaps because of the wide variety of B7 family members that are expressed on host antigen presenting cells at baseline, no surface marker has yet been identified capable of predicting response to ipilimumab (41).
Significantly more progress has been made in predicting responses to PD-1 blockade. PD-1 transduces an inhibitory signal after binding its ligand; therefore, patients with tumors expressing PD-L1 would potentially be good candidates for therapy with nivolumab, pembrolizumab, atezolimumab and durvalumab. PD-L2, which leads to inhibitory signaling through PD-1 (42), is also expressed by tumor cells. Tumoral overexpression of PD-L2 may render these cancers particularly sensitive to nivolumab and pembrolizumab, while anti-PD-L1 antibodies might fail to provide significant clinical benefit. PD-L1 and PD-L2 status have been determined as part of several trials, and some data is beginning to emerge on the utility of these two molecules as predictive markers. A trial evaluating the use of PD-1 blockade in NSCLC demonstrated that patients with PD-L1 expressing tumors responded to treatment while those without PD-L1 expressing tumors did not (43).
The picture has been complicated by subsequent studies, which have revealed a subset of PD-1 negative tumors that respond to PD-1 blockade (44). Alternative predictive strategies are therefore needed. Venturing beyond surface markers, genetic analysis of the mutational burden in tumors from patients with NSCLC has demonstrated that a high mutational load predicts a positive response to PD-1 blockade (45). Immunohistological characteristics of pre-treatment tumors in patients with melanoma have demonstrated that a preponderance of CD8+, PD-1+ T cells near or within the tumor correlates with robust T cell infiltration and response to anti-PD-1 therapy (46).
Augmented antigenic immunogenicity
When it comes to tumor cells, the manner of death may be as important as death itself when immunological activation is on the line. Recent insights into cellular death pathways have transformed the idea of a binary live/dead fate into interacting signal cascades influenced by cell intrinsic and extrinsic factors. The baseline burden of dying cells is estimated to be on the order of billions of events per day (47,48), and any defect in clearance of this material—whether from deficiencies in complement (49), mutations in Fcγ receptors (50), disruption of phagocytosis (51,52), inability to break down DNA (53)—leads to autoimmunity. Insights into aberrant immune activation and the pathogenesis of autoimmune disease are directly responsible for the development of checkpoint inhibitors. The potential synergy between antigen load and immunological activation is illustrated in Figure 1. Proinflammatory cell death triggers the innate immune system to stimulate an adaptive antitumoral response while checkpoint inhibitors sustain that activation by preventing T cell exhaustion.
Forms of cell death and their relative immunogenicity
Here we will describe three forms of cell death in the order of putative increasing immunogenicity: apoptosis, necrosis/necroptosis, and pyroptosis. Apoptosis is an intrinsically or extrinsically mediated proteolytic cascade that transforms a dying cell into consumable packets that fall away like so many leaves. Dendritic cells take up the debris and present it to T cells. In the absence of costimulatory innate signals, this process promotes and maintains peripheral tolerance (54,55). The canonical contrast to apoptosis is necrosis, a disaster of cytoplasmic swelling, plasma membrane rupture, and organelle degradation that was originally thought to proceed in the absence of intracellular signaling (56). While there is little ambiguity in the fatal mechanical disruption, if necrotic death takes place over the course of hours, there seems to be some room for cellular preparation for the inevitable in the form of a signaling cascade dependent on RIP kinases that is known as necroptosis (57). Pyroptosis is a form of proinflammatory cell death in which pores in the plasma membrane, created by the activity of caspase-1, achieve membrane lysis in seconds and allow undegraded DNA and bioactive cytoplasmic enzymes to spill into the extracellular space (58). This form of cell death has been described in macrophages and other professional phagocytic cells. Our understanding of cellular death pathways is far from complete, and it is worth noting that a binary conceptualization—immunogenic or not—is unlikely to reflect in vivo reality. Immunogenic potential of tumor antigen is perhaps better described as a spectrum determined by the load, kinetics, and manner of cellular death. As we move away from morphology-based descriptions and toward biochemical characterization of cellular demise, the hope is that our ability to predict the relative immunogenicity of tumor antigen liberated by chemotherapy and radiation therapy will improve.
Immunological impacts of chemotherapy
Chemotherapy preferentially affects rapidly dividing cells by inducing death or cell cycle arrest. While this is an effective strategy for killing tumor cells, it hampers the ability of the adaptive immune system to mount an effective response against tumor antigen. In the broadest terms, impaired proliferation in the presence of chemotherapy leads to subpar clonal selection, in turn blunting the specificity of the antitumoral response. Furthermore, the cytocidal action of chemotherapeutic agents has been primarily characterized as apoptotic by in vitro studies, which (given the caveats mentioned above) is primarily a tolerogenic form of cell death (59). The picture rapidly complicates when individual agents or classes of agents are considered, with different drugs interacting to influence the immune system in unexpected ways. As a case in point, imatinib, famous for its specificity, has been shown to activate NK cells to produce IFN-γ in a manner that is independent of mutation status in KIT or PDGFRA when studied in a population of patients with GIST tumors. In these patients, IFN-γ levels correlated with prognosis, suggesting that imatinib-mediated activation of NK cells may be playing a clinically meaningful role (60,61).
Radiation therapy as an immunomodulator
As our understanding of cellular death pathways deepens, we will gain additional tools to assess the role these forms of cell death may play in the tissue response to radiation in vivo. The ability of ionizing radiation to induce apoptosis via the creation of double strand breaks has been studied the most, and is reviewed elsewhere (62). We are only beginning to explore the roles that necroptosis, and pyroptosis may play. Necroptosis has been demonstrated to occur in an anaplastic thyroid cell line exposed to radiation in vitro (63), but the extent to which this occurs in vivo is as yet unknown. Pyroptosis occurs in macrophages in response to multiple signals, including adenosine triphosphate (ATP) (64), which has been demonstrated to be released from cells exposed to ionizing radiation (65). Though it continues to be difficult to study cellular death pathways within the context of a living host, one might predict that if radiation-induced cell death in vivo is capable of providing a stimulatory signal to the immune system one might see anti-tumor effects that occur outside the radiation field. Such an “abscopal”, or “away from the target”, effect was first described in 1953 (66). In recent years there have been a small number of patients who, after receiving an immunotherapeutic agent followed by radiation therapy, have had responses outside the radiation field (67-69). The “abscopal effect” is a putative combination of augmented immunological activation with augmented availability of antigen, which gives it a satisfying theoretical appeal. There is little wonder it has so captured the excitement and attention of the oncology community, with the hope that predictable, reproducible, and durable responses in at least a subset of patients might be achieved.
Immunological correlates of fractionation strategies
In a murine model of melanoma it has been demonstrated that both single fraction and multi-fraction regimens increase the number of tumor-infiltrating lymphocytes that synthesized IFN-γ and lysed tumor cells (70). A subsequent series of experiments in a murine model of breast cancer assessed the ability of fractionated versus single-dose radiotherapy to activate CD8 T cells and elicit an anti-tumor response outside the radiation field found that a fractionated strategy was superior to a single dose. The fractionation regimen consisted of either 8 Gy × 3 fractions or 6 Gy × 5 fractions, both of which would be comparable to a hypofractionated, or stereotactic body radiation therapy (SBRT) regimen (71). A second study that compared an ablative to a conventionally fractionated regimen in a murine model of melanoma demonstrated that a hypofractionated regimen was superior to a conventional regimen in its ability to activate CD8 T cells and trigger the reduction or destruction of distant metastases (72). These findings were supported by a study assessing tumor control in a murine melanoma model as a function of dose and fractionation. The most effective strategy was a hypofractionated regimen. The less robust response in the conventionally fractionated regimen was associated with an increase in regulatory T cells (73). It is tempting to hypothesize that cell death induced by conventional fractionation may be more tolerogenic than death via hypofractionation, but ambiguity remains. A murine model examining tumor-associated macrophages exposed to radiation therapy found that high dose radiation caused impaired T-cell recruitment while low dose radiation led to effective T cell recruitment and tumoral killing (74). A follow-up study demonstrated that low-dose irradiation converted tumor-associated macrophages back to the M1 phenotype, which are better able to coordinate antitumoral T cell responses (75).
Immunotherapy and radiation in oligometastatic and oligoprogressive NSCLC
Initial clinical experiences in the metastatic setting
Given that checkpoint inhibitors have yielded promising results in NSCLC, there has been a great deal of interest in combining radiation therapy and immunotherapy in these patients. A case report documenting an abscopal effect in a patient with metastatic NSCLC who was receiving ipilimumab demonstrated a post-treatment immunological response in the form of infiltrating CD8 T cells within an affected supraclavicular node when compared to an adjacent pre-treatment node removed from the same site. To date there have been no prospective studies combining checkpoint inhibition with radiation therapy for lung cancer. A proof-of-principle trial assessing local radiotherapy in conjunction with the cytokine GM-CSF enrolled 41 patients with metastatic solid tumors, which included 18 patients with NSCLC. An abscopal response was defined as: “a decrease in the longest diameter of at least 30% in any measurable non-irradiation lesion from baseline”. In patients with multiple tumors outside the radiation field, the best response was reported. According to these criteria, abscopal responses occurred in four patients with NSCLC (68). Further studies are being conducted using a combined approach of radiotherapy and immunotherapy in the metastatic setting, and in coming years we should have an improved idea of the magnitude of benefit a combined approach may provide. Ongoing trials that combine immunotherapy with RT in metastatic NSCLC; these are listed in Table 1. The results of a phase Ib trial combining NHS-IL2 with radiotherapy in NSCLC patients who had received first-line palliative chemotherapy have recently been published. Thirteen patients were treated with varying doses of NHS-IL2. Though the trial was not designed to test efficacy, two patients achieved long-term survival, defined as >4 years from first chemotherapy (76).
Table 1. Ongoing trials combining immunotherapy with radiation in NSCLC.
| NCT# | Phase | Description |
|---|---|---|
| Metastatic disease | ||
| NCT02318771 | I | Use of anti-PD-1 + RT in patients with metastatic or recurrent solid tumor |
| NCT02303990 | I | RADVAX: use of pembrolizumab + hypofractionated RT in metastatic melanoma or NSCLC |
| NCT02400814 | I | Use of MPDL3280A (anti-PD-1) with stereotactic ablative radiotherapy in patients with stage IV NSCLC |
| NCT02444741 | I/II | Use of dose escalated ipilimumab and SBRT in patients with metastatic solid tumors |
| NCT02221739 | II | Use of ipilimumab and RT in patients with metastatic NSCLC |
| NCT02831933 | II | ENSIGN: use of SBRT and gene therapy prior to nivolumab in patients with metastatic NSCLC |
| NCT02658097 | II | Use of pembrolizumab following SBRT in patients with previously treated stage IV NSCLC |
| NCT02492568 | II | Use of pembrolizumab following SBRT in patients with previously treated stage IV NSCLC |
| NCT02407171 | II | Use of anti-PD1 MK-3475 (pembrolizumab) and stereotactic body radiotherapy in patients with metastatic melanoma or NSCLC |
| Oligometastatic/oligoprogressive disease | ||
| NCT02621398 | I | Use of pembrolizumab with chemoradiation in stage II/III NSCLC |
| NCT00828009 | II | Use of bevacizumab and BLP25 vaccine in patients with stage III SNCLC who have received chemoradiation |
| NCT02434081 | II | NiCOLAS: use of nivolumab consolidation after standard first line chemoradiation in locally advanced NSCLC |
| NCT02125461 | III | PACIFIC: use of anti-PD1 MEDI4736 (AstraZeneca) following chemoradiation in patients with unresectable stage III NSCLC |
| NCT02768558 | III | RTOG 3505: use of chemoradiation with adjuvant nivolumab in patients with locally advanced NSCLC |
NSCLC, non-small cell lung cancer; PD-1, programmed death 1; SBRT, stereotactic body radiation therapy.
Immunotherapy in the oligometastatic and oligoprogressive settings
While the abscopal effect has inspired intense interest, there are other ways in which immunotherapy and radiation might advantageously be combined. Oligometastatic disease has no consensus definition but is understood to represent a low disease burden, with limited spread. Immunotherapy, if used in this setting, may enhance the efficacy of local control by stimulating the immune system to respond more robustly within the radiation field, perhaps significantly prolonging survival and improving quality of life by giving a boost to the “three Es” of immunoediting—elimination, equilibrium, and escape. In the oligometastatic setting, the first line use of radiation and immunotherapy may increase the magnitude of the initial round of tumoral elimination and prolong and enhance the amount of time the immune system is able to keep growth in check during the equilibrium phase. The combination of radiation and immunotherapy may also have a role in the oligoprogressive state where most sites of disease are responding to therapy but one or two continue to progress. It may be that the combined approach of immunotherapy and radiation directed at the progressing site is capable of preventing or slowing disease escape.
Ongoing trials and future efforts
Subgroup analyses of large trials have indicated a potential synergy between immunotherapy and select groups of patients who had received radiation therapy. In particular, the START trial, which examined the MUC1 liposomal vaccine, showed no significant difference between vaccine versus placebo, but a subgroup of patients who received concurrent radiation therapy did show a statistically significant benefit (77). Perhaps most promising are the trials combining checkpoint inhibitors with radiation therapy. The Phase III double-blinded PACIFIC trial is evaluating maintenance therapy with an anti-PD-1 agent MEDI4736 versus placebo in patients with stage III NSCLC (NCT02125461). We have provided a list of other ongoing trials in Table 1. Further inquiries into the safety and efficacy of combined immunotherapy and radiation therapy in NSCLC are needed, but based on the immunological principles and data reviewed above, there may be certain trajectories that are more fruitful than others. As future trials unfold, the following approaches may be of particularly high yield: (I) prospective investigation into combination therapy should include a gross evaluation of basic immunologic competence, including a quantitative assessment of circulating cellular compartments in the peripheral blood with a particular focus on the CD4 and CD8 T cell compartments, as the ability to mount an effective immune response may be at least correlative if not causative in the efficacy of any immunomodulatory agent; (II) investigation into the biomarkers PD-L1 and PD-L2 should continue, with tumoral expression of these ligands determined for patients receiving anti-PD-1 therapy; (III) given the lack of clarity regarding the immunological benefits of conventional versus hypofractionation, these two strategies should be prospectively compared in the presence of immunomodulatory agents.
Concluding remarks
The potential synergy of immunotherapy and radiation therapy has begun to blur the boundaries between systemic and local control. As synthesized in Figure 1, radiation releases and alters antigen as targeted tissue dies, influencing immunogenicity in ways we are only beginning to characterize, comprehend, and predict. The innate and adaptive immune systems work together to mount responses against tumor cells, aided by immunotherapeutic agents that provide stimulatory signals or circumvent checkpoints that prevent sustained T cell activation. Activated T cells act systemically, but also may play a potentially important role in augmenting radiation-induced local control in the oligometastatic or oligoprogressive setting. The confluence of basic science advances in immunology, radiobiology, and oncology have made this a particularly promising time for translational research. Anton Chekhov, one of the earliest physicians to point out the connection between infection and spontaneous cancer remission, said also: “If you say in the first chapter that there is a rifle hanging on the wall, in the second or third chapter it absolutely must go off.” This dictum is known as “Chekov’s Gun,” and is meant to be a tool of narrative fiction. But if immunotherapy is that rifle, we have been looking at it for a long time.
Acknowledgements
The authors would like to thank Noah Capurso M. D. for assistance in editing the manuscript.
Footnotes
Conflicts of Interest: RH Decker receives research support from Merck & Co., Inc. AM Campbell has no conflicts of interest to declare.
References
- 1.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol 2008;38:3257-64. 10.1002/eji.200838855 [DOI] [PubMed] [Google Scholar]
- 2.Gresser I. A. CkM.D., and Coley’s toxins. N Engl J Med 1987;317:457. 10.1056/NEJM198708133170716 [DOI] [PubMed] [Google Scholar]
- 3.Coley WB. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med 1910;3:1-48. [PMC free article] [PubMed] [Google Scholar]
- 4.Nauts HC, Swift WE, Coley BL. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res 1946;6:205-16. [PubMed] [Google Scholar]
- 5.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 1991;(262):3-11. [PubMed] [Google Scholar]
- 6.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 10.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Wang XL, Deng QL, et al. The efficacy and safety of immunotherapy in patients with advanced NSCLC: a systematic review and meta-analysis. Sci Rep 2016;6:32020. 10.1038/srep32020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Drake CG, Sznol M, et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J Clin Oncol 2015;33:2013-20. 10.1200/JCO.2014.58.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol 2016;34:833-42. 10.1200/JCO.2015.63.7421 [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485-92. 10.1056/NEJM198512053132327 [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. 10.1200/JCO.1999.17.7.2105 [DOI] [PubMed] [Google Scholar]
- 20.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688-96. 10.1200/JCO.1995.13.3.688 [DOI] [PubMed] [Google Scholar]
- 21.Grande C, Firvida JL, Navas V, et al. Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs 2006;17:1-12. 10.1097/01.cad.0000182748.47353.51 [DOI] [PubMed] [Google Scholar]
- 22.Pulliam SR, Uzhachenko RV, Adunyah SE, et al. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunol Lett 2016;169:61-72. 10.1016/j.imlet.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer RJ, Rakhit A, Thompson JA, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res 2001;21:257-63. 10.1089/107999001750169934 [DOI] [PubMed] [Google Scholar]
- 24.Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ 2015;22:237-46. 10.1038/cdd.2014.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman HL, Ruby CE, Hughes T, et al. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2014;2:11. 10.1186/2051-1426-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014;11:24-37. 10.1038/nrclinonc.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:822-35. 10.1016/S1470-2045(16)00099-1 [DOI] [PubMed] [Google Scholar]
- 28.Giaccone G, Bazhenova LA, Nemunaitis J, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 2015;51:2321-9. 10.1016/j.ejca.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 29.Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol 2016;17:212-23. 10.1016/S1470-2045(15)00483-0 [DOI] [PubMed] [Google Scholar]
- 30.Harper K, Balzano C, Rouvier E, et al. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol 1991;147:1037-44. [PubMed] [Google Scholar]
- 31.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541-7. 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- 32.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 33.Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 2016;34:539-73. 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- 34.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-7. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 35.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141-51. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 36.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 38.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 39.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 40.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013;14:157-63. 10.1016/j.cllc.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 43.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipson EJ, Forde PM, Hammers HJ, et al. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol 2015;42:587-600. 10.1053/j.seminoncol.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ATHENS JW , HAAB OP, RAAB SO, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 1961;40:989-95. 10.1172/JCI104338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important? BMJ 2001;322:1536-8. 10.1136/bmj.322.7301.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology 2002;205:395-406. 10.1078/0171-2985-00141 [DOI] [PubMed] [Google Scholar]
- 50.Ramos PS, Brown EE, Kimberly RP, et al. Genetic factors predisposing to systemic lupus erythematosus and lupus nephritis. Semin Nephrol 2010;30:164-76. 10.1016/j.semnephrol.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004;304:1147-50. 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]
- 52.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol 2010;22:740-6. 10.1016/j.coi.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Napirei M, Karsunky H, Zevnik B, et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 2000;25:177-81. 10.1038/76032 [DOI] [PubMed] [Google Scholar]
- 54.Liu K, Iyoda T, Saternus M, et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med 2002;196:1091-7. 10.1084/jem.20021215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiraz Y, Adan A, Kartal Yandim M, et al. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol 2016;37:8471-86. 10.1007/s13277-016-5035-9 [DOI] [PubMed] [Google Scholar]
- 56.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 2006;1757:1371-87. 10.1016/j.bbabio.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 57.Vandenabeele P, Declercq W, Van Herreweghe F, et al. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal 2010;3:re4. 10.1126/scisignal.3115re4 [DOI] [PubMed] [Google Scholar]
- 58.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99-109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zitvogel L, Tesniere A, Apetoh L, et al. Immunological aspects of anticancer chemotherapy. Bull Acad Natl Med 2008;192:1469-87; discussion 1487-9. [PubMed] [Google Scholar]
- 60.Borg C, Terme M, Taïeb J, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004. Aug;114(3):379-88. 10.1172/JCI21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ménard C, Blay JY, Borg C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res 2009;69:3563-9. 10.1158/0008-5472.CAN-08-3807 [DOI] [PubMed] [Google Scholar]
- 62.Golden EB, Pellicciotta I, Demaria S, et al. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012;2:88. 10.3389/fonc.2012.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nehs MA, Lin CI, Kozono DE, et al. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery 2011;150:1032-9. 10.1016/j.surg.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 64.Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol 2016;26:R568-72. 10.1016/j.cub.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 65.Ohshima Y, Tsukimoto M, Takenouchi T, et al. gamma-Irradiation induces P2X(7) receptor-dependent ATP release from B16 melanoma cells. Biochim Biophys Acta 2010;1800:40-6. 10.1016/j.bbagen.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 66.MOLE RH Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 67.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. 10.1016/S1470-2045(15)00054-6 [DOI] [PubMed] [Google Scholar]
- 69.Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516-23. 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 71.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 75.Prakash H, Klug F, Nadella V, et al. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis 2016;37:301-13. 10.1093/carcin/bgw007 [DOI] [PubMed] [Google Scholar]
- 76.van den Heuvel MM, Verheij M, Boshuizen R, et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med 2015;13:32. 10.1186/s12967-015-0397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]


