Abstract
Background
To review the outcome of using radiofrequency ablation (RFA) for patients with close resection margin during hepatectomy.
Methods
From Oct 2004 to Sept 2013, 862 patients received hepatectomy for hepatocellular carcinoma (HCC) in the Department of Surgery, Queen Mary Hospital in Hong Kong. Fourteen patients received additional RFA because of close resection margin (<1 cm) during the operation for HCC. The result of 28 patients with close liver resection margin was selected for comparison. The two groups of patients were matched in terms of tumor size, tumor number, stage of disease and magnitude of resection.
Results
In the RFA group (n=14), the median age of the patients was 58.5 (range, 25–78 years). The median tumor size was 2.25 cm (range, 1.2–12 cm). In the resection alone group (n=28), the median age for the patients was 61 (range, 36–79 years). The median tumor size was 2.7 cm (range, 1–11 cm). There was no difference in terms of liver function assessment between the two groups. There was no RFA related complication recorded during the study period. There was no hospital mortality in both groups. The 1- and 3-year disease free survival was 38.3% and 25.5% respectively in the RFA group vs. 57.4% and 39.3% respectively in the liver resection alone group (P=0.563). The 1- and 3-year overall survival was 81.5% and 69.8% respectively in the RFA group vs .88.4% and 59.9% respectively in the liver resection alone group (P=0.83).
Conclusions
RFA to hepatectomy resection surface in patients with close margin is a safe treatment option but its effectiveness on prevention of local recurrence has yet to be confirmed.
Keywords: Close resection margin, hepatocellular carcinoma (HCC), radiofrequency ablation (RFA), case-matched study
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer with around 750,000 new patients every year (1,2). Surgical resection is regarded as the standard treatment with curative intent for HCC as suggested by Asia Pacific Association for the Study of Liver Disease guideline (3). The operative mortality is less than 5% for major hepatectomy and the 5-year survival is 50% (4). However, not all patients are suitable for surgical resection (5). The presence of multifocal disease, proximity of tumor to major vascular and biliary structures preventing adequate resection margin, advance tumor staging on presentation or insufficient liver remnant volume are all contra-indications for major resection (6). Liver transplantation provides an alternative curative treatment for a selected group of HCC patients, but the paucity of liver grafts limits the application of the approach (7,8).
The long-term survival of HCC is unsatisfactory because of high recurrence rate which at 5 year is 75% (9). The recurrence can be divided into local recurrence and intrahepatic metastasis (10). The pathogenesis of local recurrence is thought to be related to the presence of residual tumor cell, leading to regrowth of the tumor and the risk factors include large tumor size, multiple tumor nodules, and vascular invasion (11). On the other hand, intrahepatic metastasis is due to the spread of micrometastasis by means of portal venous dissemination (12). Studies have shown that deposition of microsatellites occur anywhere from 0.5 to 6 cm away from the main tumor (13). The surgical resection margin has no effect on the tendency of the tumor to develop microsatellites (14). Tumor biology, namely Edmundson score of differentiation, size and presence of capsule are predictors of the pattern of microsatellite deposition (15).
As local recurrence is thought to be related to the presence of residual tumor cells, resection margin is a surgical factor which has been evaluated for its influence on the long term outcome after resection, but its precise significance remains to be determined (16). Whenever possible, anatomical resection based on segmental liver anatomy is the preferred technique of choice because it may prevent intrahepatic recurrence by eradicating intrahepatic microscopic tumor foci. Makuuchi’s group was the first to describe anatomical resection which entails removing the entire liver segment, where the tumor resides, together with its vascular and biliary tributaries so that microsatellite tumor foci could be removed concurrently (17). However, anatomical resection is not always possible. Proponents of nonanatomical resection have stressed the importance of preserving the functional liver reserve and preventing liver failure. For most patients undergoing resection for HCC, a majority of them suffers from Hepatitis B virus or C virus related liver cirrhosis. In their case, an aggressive approach is needed to preserve the noncancerous liver parenchyma, otherwise liver failure might ensue. Cirrhotic livers lack the regenerative potential of healthy liver parenchyma. Therefore, although anatomical resection offers the best oncological control, it is not always the best operative option. For subcapsular wedge resection of HCC less than 3 cm and in patients where anatomical resection is not possible, a 1 cm margin is recommended (13).
Besides the issue of the healthy liver preservation, there are also anatomical and technical concerns with securing adequate margin. Tumor location occasionally precludes the achievement of adequate margin. Close proximity to major vascular and biliary structures, and deep location of tumor are such examples. Additionally, there are technical concerns. In cirrhotic livers, intra-operative ultrasound can sometimes fail to provide accurate information (18). The surgeon can misjudge the exact extent and location of the tumor. This is usually discovered during the examination of the specimen after the resection is completed. In such scenarios, a re-resection to establish adequate margin will prolong operation time, and incurred additional blood loss which can be detrimental to the patient. This study aims to investigate the role of radiofrequency ablation (RFA) to HCC resection bed when the margin is intra-operatively found to be suboptimal.
RFA is an ablative surgical technique which produces coagulative necrosis created by alternating high frequency electric current (19,20). Heat is created by the movement of the ions within the tissues as they are agitated by the current. At high temperature, proteins are denatured and microvasculature is destroyed by thrombosis (21). Proximity to large blood vessel plays a role in the heat transmission. Blood flow protects the vessel wall from damage but also act a heat sink and cools nearby tissue, limiting the effect of RFA (22). A major safety concern for the use of RFA in HCC is the potential damage to nearby bile ducts caused by the heat generated by this device. The damage results in causes stenosis and distal obstruction. A method to counter potential biliary injury is intraductal cooling of the central bile duct (23).
Methods
Between October of 2004 and September 2013, 862 patients underwent hepatectomy for HCC with macroscopically complete resection of tumor in the Department of Surgery at the University of Hong Kong at Queen Mary Hospital. Fourteen patients received additional RFA because of close/involved resection margin found intraoperatively. Another 28 patients with similarly close resection margin but did not received additional RFA was selectively matched for comparisons. These two groups of patients were matched according to the tumor size, the number of tumors, pathological stage of their disease and the magnitude of the resection.
All patients were regularly followed up at our outpatient clinic and were monitored for recurrence by serum alpha-fetoprotein level assessment and contrast CT scan every 4–6 months. A computerized database has been established for collection of clinical and pathological data of all patients, including size of tumor, resection margin as assessed by pathologists. Any post-operative recurrence was entered into the database after confirmation of diagnosis.
Surgical margin was defined as the shortest macroscopic distance from the edge of the tumor to the line of transection with close being less than 1 cm. Both groups were matched according tumor, patient and biochemical parameter. For tumor factors, size, number, Edmundson grade of differentiation pathological TMN staging and resection margins were matched. For patient factors, age, gender, hepatitis B/C virus status, the medical comorbidities, Child-Pugh grade of liver cirrhosis and indocyanine green retention test were matched. Biochemically, the pre-operative liver and renal function, the alpha-fetal protein were matched. The peri-operative data including blood loss, duration of operation, the type of resection and length of hospital admission were all taken into account.
Statistical analysis
The computer software SPSS, version 21.0, from IBM SPSS Statistics was used for statistical analyses.
Comparisons between the two groups were performed using the Pearson chi-square test for nominal variables and the Mann Whitney U test was used for continuous variables. Cumulative survival and recurrence rates were evaluated by the Kaplan-Meier method and compared by the log-rank test. All statistical analyses were performed using statistical software (SPSS version 21.0. from IBM SPSS statistics, Chicago, IL, USA). P value of less than 0.05 was considered statistically significant.
Results
Among the 862 patients, 14 with close resection margin, which was defined as less than 1 cm, received additional RFA to the surgical margin intra-operatively. These 14 patients were compared with another 28 patients with close margin who did not receive RFA. Both groups are extensively matched.
The detailed of the close proximity to margin is listed in Table 1. Historic controls of similar situations without RFA were selected for comparison in a ratio of 1:2. They matched in tumour number, tumour size and stage of disease.
Table 1. The clinicopathologic data of these two groups of patients.
| Tumor characteristics | Control (n=28, %) | RFA ablation on margin (n=14, %) | P value |
|---|---|---|---|
| Resection margin (cm) [median (range)] | 0.977 | ||
| Involved (i.e., 0 cm) | 7 (25.0) | 4 (28.6) | |
| 0.05 cm | 1 (3.6) | 0 (0) | |
| 0.1 cm | 4 (14.3) | 2 (14.3) | |
| 0.2 cm | 6 (21.4) | 4 (28.6) | |
| 0.3 cm | 2 (7.1) | 0 (0) | |
| 0.4 cm | 3 (10.7) | 1 (7.1) | |
| 0.5 cm | 1 (3.6) | 1 (7.1) | |
| 0.8 cm | 2 (7.1) | 1 (7.1) | |
| 1.0 cm | 2 (7.1) | 1 (7.1) | |
| TNM staging [1997] 5th edition | 0.357 | ||
| I | 4 (14.3) | 4 (28.6) | |
| II | 10 (35.7) | 3 (21.4) | |
| IIIA | 10 (35.7) | 3 (21.4) | |
| IVA | 4 (14.3) | 4 (28.6) | |
| Tumor size [median (range)] | 1 | ||
| ≤3 cm | 20 (71.4) | 10 (71.4) | |
| >3 cm | 8 (28.6) | 4 (28.6) |
There were no significant differences in any of the tumor factors, including resection margin, TMN stage, and size.
The patients’ demographic conditions are listed in Table 2 in detail.
Table 2. The data comparing these two groups regarding patient factors including age, gender, hepatitis status, comorbidities, presence of ascites, Child-Pugh grade, ICG retention at 15 min, biochemistry, and tumor number.
| Patients’ characteristics | Control (n=28) | RFA ablation on margin (n=14) | P value |
|---|---|---|---|
| Age [median (range)] | 61 (36.0–79.0) | 58.5 (25.0–78.0) | 0.362 |
| Sex (male:female) | 24:4 | 12:2 | 1.000 |
| Hepatitis B (positive) | 23 (82.1%) | 13 (92.9%) | 0.640 |
| Hepatitis C (positive) | 1 (3.6%) | 0 (0%) | 1.000 |
| Comorbid disease (yes, %) | 16 (57.1%) | 3 (21.4%) | 0.028 |
| Heart | 12 (42.9%) | 3 (21.4%) | 0.172 |
| Lung | 7 (25.0%) | 1 (7.1%) | 0.331 |
| Renal | 2 (7.1%) | 1 (7.1%) | 1.000 |
| DM | 5 (17.9%) | 0 (0%) | 0.238 |
| Gastrointestinal | 3 (10.7%) | 3 (21.4%) | 0.640 |
| Ascite | – | ||
| Absent | 28 (100.0%) | 14 (100.0%) | |
| Child-Pugh grade | 1.000 | ||
| A | 27 (96.4%) | 13 (92.9%) | |
| B | 1 (3.6%) | 1 (7.1%) | |
| C | – | – | |
| ICG 15 min (%) | 12.75 (5.9–36.8) | 14 (7.0–62.2) | 0.396 |
| Pre-operative chemistry data | |||
| Serum bilirubin | 10 (4.0–22.0) | 10 (3.0–21.0) | 0.843 |
| Creatinine | 85.5 (52.0–117.0) | 90 (63.0–131.0) | 0.436 |
| Albumin | 40 (29.0–49.0) | 41.5 (27.0–48.0) | 0.189 |
| INR | 1 (0.9–1.3) | 1.1 (0.9–1.3) | 0.420 |
| Platelet count | 157 (46.0–258.0) | 140.5 (82.0–278.0) | 0.501 |
| AFP | 10.5 (2.0–478.0) | 115 (4.0–16,744.0) | 0.004 |
| AST | 37 (18.0–85.0) | 45 (21.0–108.0) | 0.272 |
| ALT | 37 (18.0–96.0) | 30.5 (15.0–117.0) | 0.390 |
| HGB | 14.15 (11–16.9) | 13.3 (11.2–15.4) | 0.198 |
| Size of tumor (cm) [median (range)] | 2.7 (1.0–11.0) | 2.25 (1.2–12.0) | 0.589 |
| No. of tumor(s) | 0.739 | ||
| 1 | 22 (78.6%) | 9 (64.3%) | |
| 2 | 3 (10.7%) | 3 (21.4%) | |
| 3 | 1 (3.6%) | 1 (7.1%) | |
| Multiple | 2 (7.1%) | 1 (7.1%) |
There was no difference between the two groups.
The complication profiles between the two groups are similar. Five patients (18%) had complications in the control group and two patients (14%) had complication in the RFA group (P=1).
Long-term outcomes in terms of overall survival and post-operative recurrence rates were similar between the two groups. The 1-, 3-, 5-year overall survival rates were respectively 82%, 70% and 70% in the RFA group and for the controlled groups these numbers are 88%, 60% and 46% (P=0.83). Analyses after stratification of patient according to tumor size revealed no significant difference between the two groups.
During the follow-up period, recurrence developed 57% in the control group and 50% in the RFA group (P=0.41). There are no significant differences between the two groups in terms of type of recurrence.
The 1-, 3-year disease free survival rate were respectively 38% and 26% for the RFA group. In comparison the numbers are 57% and 39% for the control group. Analyses after stratification of patients according to tumor size revealed no significant difference between the two groups.
Table 3 shows the operative and pathological details of the two groups.
Table 3. Intra-operative and post-operative data of these two groups of patients.
| Intraoperative/postoperative data | Control (n=28) | RFA ablation on margin (n=14) | P value |
|---|---|---|---|
| Blood loss (L) | 0.4 (0.02–3.30) | 0.51 (0.02–1.80) | 0.390 |
| Blood transfusion (L) | 0 (0–2.56) | 0 (0–0.64) | 0.906 |
| Blood transfusion (yes, %) | 3 (10.7%) | 2 (14.3%) | 1.000 |
| Hospital stay (days) | 7 [3–41] | 8 [3–21] | 0.722 |
| Hospital mortality | 0 (0%) | 0 (0%) | – |
| OT duration (min) | 291.5 [129–883] | 239.5 [120–485] | 0.362 |
| Type of operation | 0.853 | ||
| Major | 2 (7.1%) | 2 (14.3%) | |
| Minor | 26 (92.9%) | 12 (85.7%) | |
| Pattern of recurrence | 0.406 | ||
| Nil | 12 (42.9%) | 7 (50.0%) | |
| Intrahepatic | 12 (42.9%) | 3 (21.4%) | |
| Extrahepatic | 1 (3.6%) | 2 (14.3%) | |
| Both recurrence | 3 (10.7%) | 2 (14.3%) | |
| New Edmonson grade | 0.783 | ||
| Well-differentiated | 5 (17.9%) | 4 (28.6%) | |
| Moderately-differentiated | 18 (64.3%) | 8 (57.1%) | |
| Poorly differentiated | 4 (14.3%) | 2 (14.3%) | |
| Not available | 1 (3.6%) | – |
There are no significant differences between the Edmonson grade, blood loss, operation time, type of resection, duration hospital stay and type of recurrence.
Subgroup analyses of tumour cut off value of 3 cm were compared for overall survival rates (Figures 1,2). Subgroup analyses of tumour cut off value of 3 cm were compared for disease free survival rates (Figures 3,4).
Figure 1.
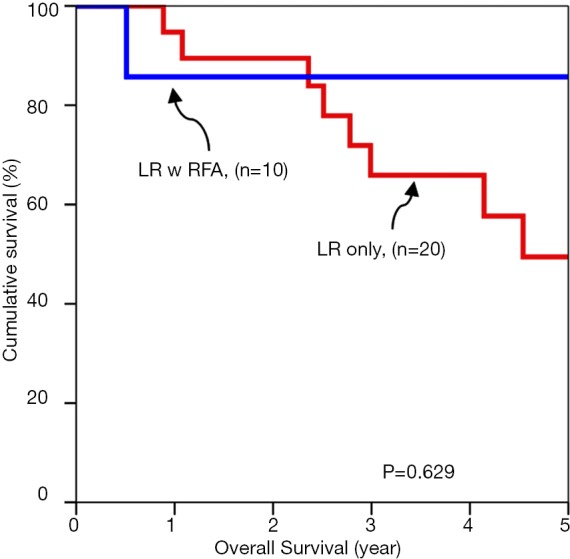
Overall survival of tumors up to 3 cm.
Figure 2.
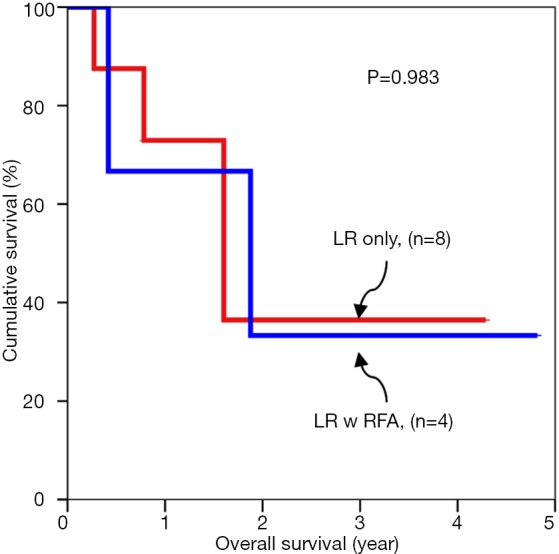
Overall survival for tumors larger than 3 cm.
Figure 3.
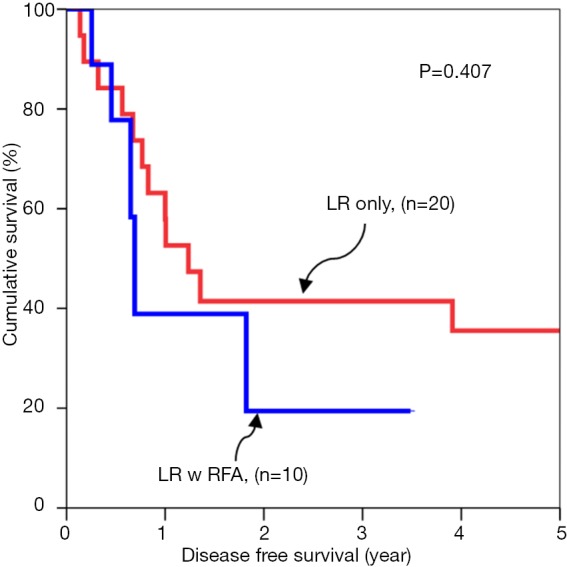
Disease free survival for tumors up to 3 cm.
Figure 4.
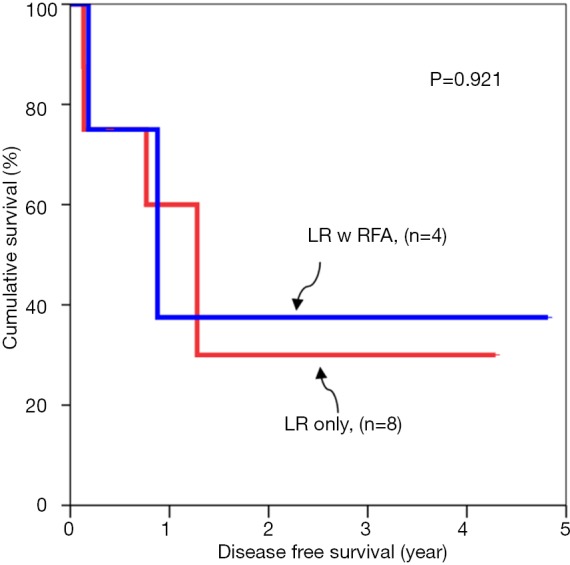
Disease free survival for tumor larger than 3 cm.
Discussion
Resection is the gold standard of treatment for HCC for patients who are fit to undergo operation. Anatomical resection offers the best chance of cure. The removal of associated segmental vascular and biliary tributaries reduces the likelihood of microsatellite tumor deposit and neoplastic emboli in the small portal branches which are connected to the neovascularization of the tumor. However this approach is not always possible. The advent of RFA has provided an alternative option for treating HCC. For small tumors less than 2 cm, the survival benefit using RFA has proven to be on par with resection. However, similar data does not exist for larger tumors, in which case, resection remains the only hope of cure.
In patients with cirrhotic liver from chronic hepatitis virus infection, preservation of functional residual parenchyma is paramount to avoid post-operative liver failure. In such case where anatomical resection is not feasible, an adequate resection margin of 1 cm is usually recommended.
Some studies have shown that the amount of resection margin around small HCC does not influence postoperative recurrence rates. In other words, margin less than 1 cm are occasionally acceptable. Small tumors measuring 1.5 cm or less are usually well differentiated. Well differentiated tumors give rise to less portal microinvasion and microsatellite formation. The degree of tumor differentiation is related to the size of the tumor. Larger tumors are less well differentiated which will result in more portal microinvasion and tumor microsatellites, normally within 1 cm of the main tumor.
Achieving a 1 cm resection margin is, however, often not feasible for large HCCs. Tumor location and other technical factors sometimes preclude surgeons from achieving the desired resection margin. For tumors abutting major vessels, a less than optimal margin is usually accepted in order to preserve the vasculature which is needed to provide adequate supply and drainage to the liver remnant. Technical errors of judgement might also results in inadequate resection margin. Surgical planning error can occur because intra-operative ultrasound localization of tumor can be difficult to interpret in the background of liver cirrhosis. It is only after the delivery of the specimen and during on-table examination that an inadequate margin is discovered. In such cases, re-resection is possible but would results in more blood loss and longer operation duration which many older patients cannot tolerate.
In such scenarios, RFA has been adopted to create a surrogate margin. RFA is an ablative technology which employs alternating high frequency electric current to produce coagulative necrosis (24). The RFA is applied to the surgical resection bed after the discovery of inadequate margin. The probe which transmits the energy creates zone of necrosis. This area serves as a buffering resection margin. Using ablation technology is faster than re-resection. Another advantage is the fact that the treatment modality is also associated with minimal blood loss.
This study examines the benefits and risks of using RFA in cases where the resection margin has been found intra-operatively to be less than satisfactory. The postulation is that RFA will confer additional survival benefit, especially regarding reduction in marginal recurrence. However, the data has failed to produce any concrete evidence to support the hypothesis. The pathophysiology of HCC recurrence is twofold. The cirrhotic liver is premalignant, and this explains the multicentric nature of HCC recurrence. The second type of recurrence is marginal recurrence in which the tumor arises quickly after the operation at the edge of the resected area. The postulation behind this type of pattern is the presence of tumor microsatellites which are usually present within 1cm from the main tumor bulk. In these cases, an inadequate margin during operation would likely result in rapid recurrence. In order to reduce rapid recurrence near the surgical bed after operation, an adequate margin should be achieved. However, as explained earlier, that is not always feasible. The application of RFA to the surgical bed can theoretically create a surrogate margin with the 1 cm zone of necrosis it induces. Despite the logical basis of this assumption, we have failed to prove any survival benefit with this technique in the study.
Nonetheless, the examination of the data proves that the technique is safe and the complication profile is the same as the control group.
The main limitation of this study is the small sample size. Although the scale of the study is small, it is one of the first to examine the use of RFA in tumor with close resection margin. The information we have gain from this study fills a void in the literature. The benefits have not been demonstrated thus far but as numbers accrue, the results might be different. This study is also retrospective in nature which limits the power of its conclusion. However, a randomized controlled trial would be difficult to conduct for this scenario.
Close resection margin in surgery for liver tumor is often encountered. Perhaps, it might be unavoidable. Finding a solution to remedy this problem will be pivotal to reduce local recurrence rates. In this study, the use of RFA has failed to show any concrete benefit but it is proven to be safe. Conceptually, the RFA zone should act as a surrogate resection margin if properly executed; more research is needed to confirm the conclusion of this study and to explain the findings.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis 1999;19:271-85. 10.1055/s-2007-1007117 [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM, Rustgi VK, Hoofnagle JH, et al. NIH conference. Hepatocellular carcinoma. Ann Intern Med 1988;108:390-401. 10.7326/0003-4819-108-3-390 [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-30. 10.1097/00000658-199903000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TC, Lo CM. Resection strategies for hepatocellular carcinoma. Semin Liver Dis 2013;33:273-81. 10.1055/s-0033-1351782 [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999;229:790-9; discussion 799-800. 10.1097/00000658-199906000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont-Bierre E, Compagnon P, Raoul JL, et al. Resection of hepatocellular carcinoma in noncirrhotic liver: analysis of risk factors for survival. J Am Coll Surg 2005;201:663-70. 10.1016/j.jamcollsurg.2005.06.265 [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 8.Ng KK, Lam CM, Poon RT, et al. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol 2003;18:616-29. 10.1046/j.1440-1746.2003.02991.x [DOI] [PubMed] [Google Scholar]
- 9.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993;105:488-94. 10.1016/0016-5085(93)90724-Q [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg 2000;231:544-51. 10.1097/00000658-200004000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. 10.1002/lt.20472 [DOI] [PubMed] [Google Scholar]
- 12.Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology 1989;9:457-60. 10.1002/hep.1840090320 [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Zhang CQ, Zhang YQ, et al. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg 2004;28:376-81. 10.1007/s00268-003-7308-x [DOI] [PubMed] [Google Scholar]
- 14.Sasaki K, Matsuda M, Ohkura Y, et al. Minimum resection margin should be based on tumor size in hepatectomy for hepatocellular carcinoma in hepatoviral infection patients. Hepatol Res 2013;43:1295-303. 10.1111/hepr.12079 [DOI] [PubMed] [Google Scholar]
- 15.Sasaki A, Kai S, Iwashita Y, et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 2005;103:299-306. 10.1002/cncr.20798 [DOI] [PubMed] [Google Scholar]
- 16.Lai EC, You KT, Ng IO, et al. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg 1993;17:786-90; discussion 791. 10.1007/BF01659097 [DOI] [PubMed] [Google Scholar]
- 17.Makuuchi M. Surgical treatment for HCC--special reference to anatomical resection. Int J Surg 2013;11 Suppl 1:S47-9. 10.1016/S1743-9191(13)60015-1 [DOI] [PubMed] [Google Scholar]
- 18.Makuuchi M, Hasegawa H, Yamazaki S. Development on segmentectomy and subsegmentectomy of the liver due to introduction of ultrasonography. Nihon Geka Gakkai Zasshi 1983;84:913-7. [PubMed] [Google Scholar]
- 19.Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic hepatic malignancies. Int J Clin Oncol 2002;7:72-81. [DOI] [PubMed] [Google Scholar]
- 20.Buscarini E, Savoia A, Brambilla G, et al. Radiofrequency thermal ablation of liver tumors. Eur Radiol 2005;15:884-94. 10.1007/s00330-005-2652-x [DOI] [PubMed] [Google Scholar]
- 21.Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist 2001;6:14-23. 10.1634/theoncologist.6-1-14 [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Crocetti L, Cioni D, et al. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol 2004;39:689-97. 10.1097/00004424-200411000-00007 [DOI] [PubMed] [Google Scholar]
- 23.Lam VW, Ng KK, Chok KS, et al. Safety and efficacy of radiofrequency ablation for periductal hepatocellular carcinoma with intraductal cooling of the central bile duct. J Am Coll Surg 2008;207:e1-5. 10.1016/j.jamcollsurg.2008.03.028 [DOI] [PubMed] [Google Scholar]
- 24.Higgins H, Berger DL. RFA for liver tumors: does it really work? Oncologist 2006;11:801-8. 10.1634/theoncologist.11-7-801 [DOI] [PubMed] [Google Scholar]


