Abstract
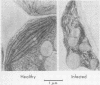
Chloroplasts isolated from powdery mildew-infected (Erysiphe polygoni DC) sugar beet leaves (Beta vulgaris L) showed a reduction in the rate of electron transport and in the accompanying ATP formation in noncyclic photophosphorylation (water as electron donor, NADP as electron acceptor) and little or no change in the rate of ATP formation in cyclic photophosphorylation catalyzed by phenazine methosulfate. The inhibition of noncyclic photophosphorylation appeared to lead in the parent leaves to a decreased rate of photosynthetic CO2 assimilation and a shift in products resulting in a relative increase of amino acids. These changes were accompanied by alterations in chloroplast ultrastructure and by a reduction in the activity of enzymes necessary for the formation of organic acids (phosphoenolpyruvate carboxylase and malate dehydrogenase). These results are similar to the findings of Montalbini and Buchanan (1974 Physiol. Plant Pathol. 4: 191-196) with chloroplasts from rust-infected Vicia faba leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Matthews R. E. Changes in the proportions of early products of photosynthetic carbon fixation induced by TYMV infection. Virology. 1972 Apr;48(1):255–258. doi: 10.1016/0042-6822(72)90132-8. [DOI] [PubMed] [Google Scholar]
- Livne A. Photosynthesis in Healthy and Rust-Affected Plants. Plant Physiol. 1964 Jul;39(4):614–621. doi: 10.1104/pp.39.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyarosy A. C., Buchanan B. B., Schürmann P. Effect of a systemic virus infection on chloroplast function and structure. Virology. 1973 Oct;55(2):426–438. doi: 10.1016/0042-6822(73)90184-0. [DOI] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B., Arnon D. I. Role of cyclic photophosphorylation in photosynthetic carbon dioxide assimilation by isolated chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):111–124. doi: 10.1016/0005-2728(72)90143-0. [DOI] [PubMed] [Google Scholar]
- Waygood E. R., Pao L. Y., Godavari H. R. Stimulation of phosphoenolpyruvate carboxylase activity in rust-infected wheat leaves. Experientia. 1974 Sep 15;30(9):986–988. doi: 10.1007/BF01938961. [DOI] [PubMed] [Google Scholar]